Enantioselective and Synergistic Herbicidal Activities of Common Amino Acids Against Amaranthus tricolor and Echinochloa crus-galli
Abstract
:1. Introduction
2. Results
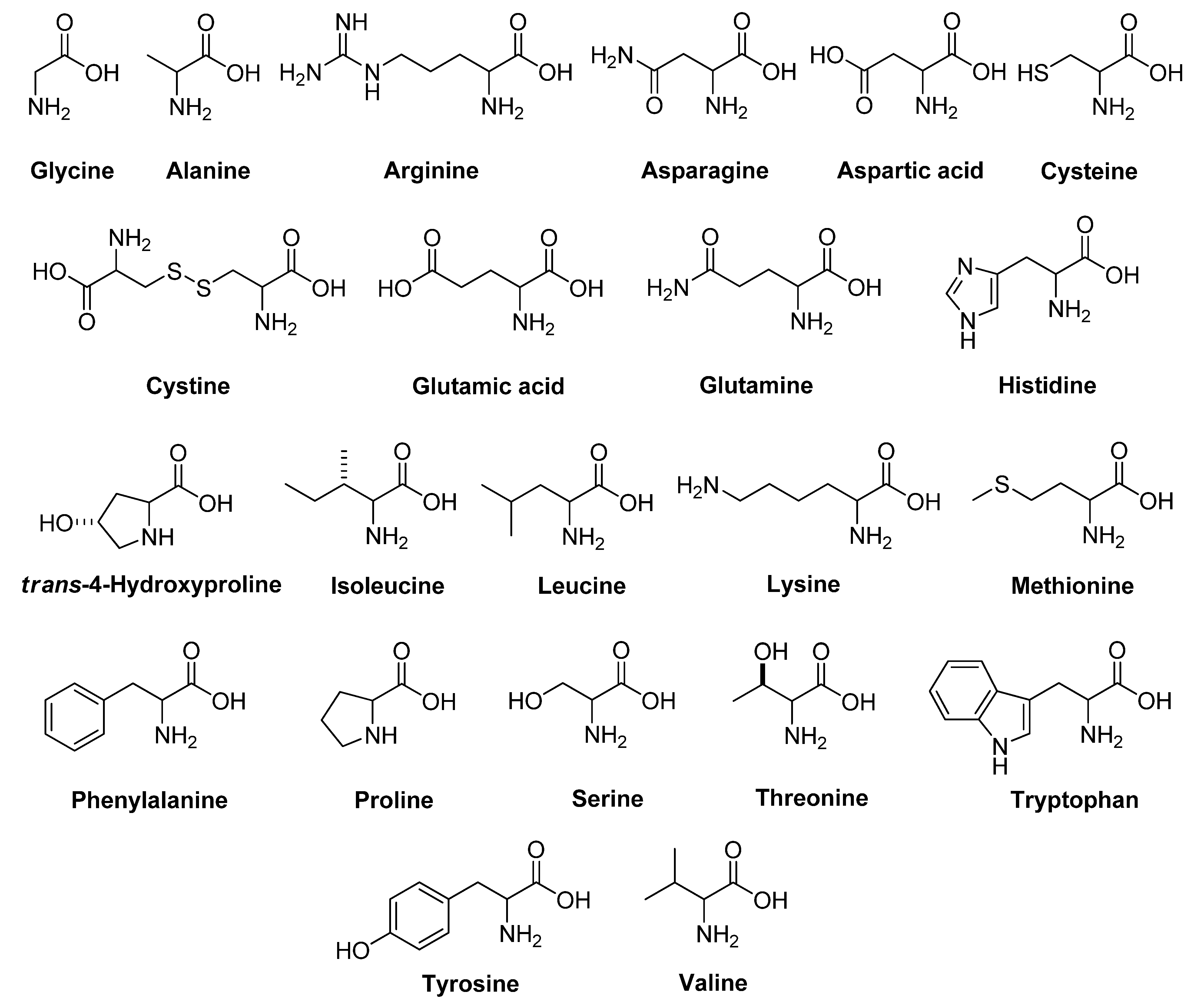
2.1. Herbicidal Activity of Glycine and 21 Common Amino Acids on Chinese Amaranth
2.2. Herbicidal Activity of Glycine and 21 Common Amino Acids on Barnyard Grass
2.3. Synergistic Effects of d- and l-Amino Acids on Tested Plants
2.3.1. Chinese Amaranth
2.3.2. Barnyard Grass
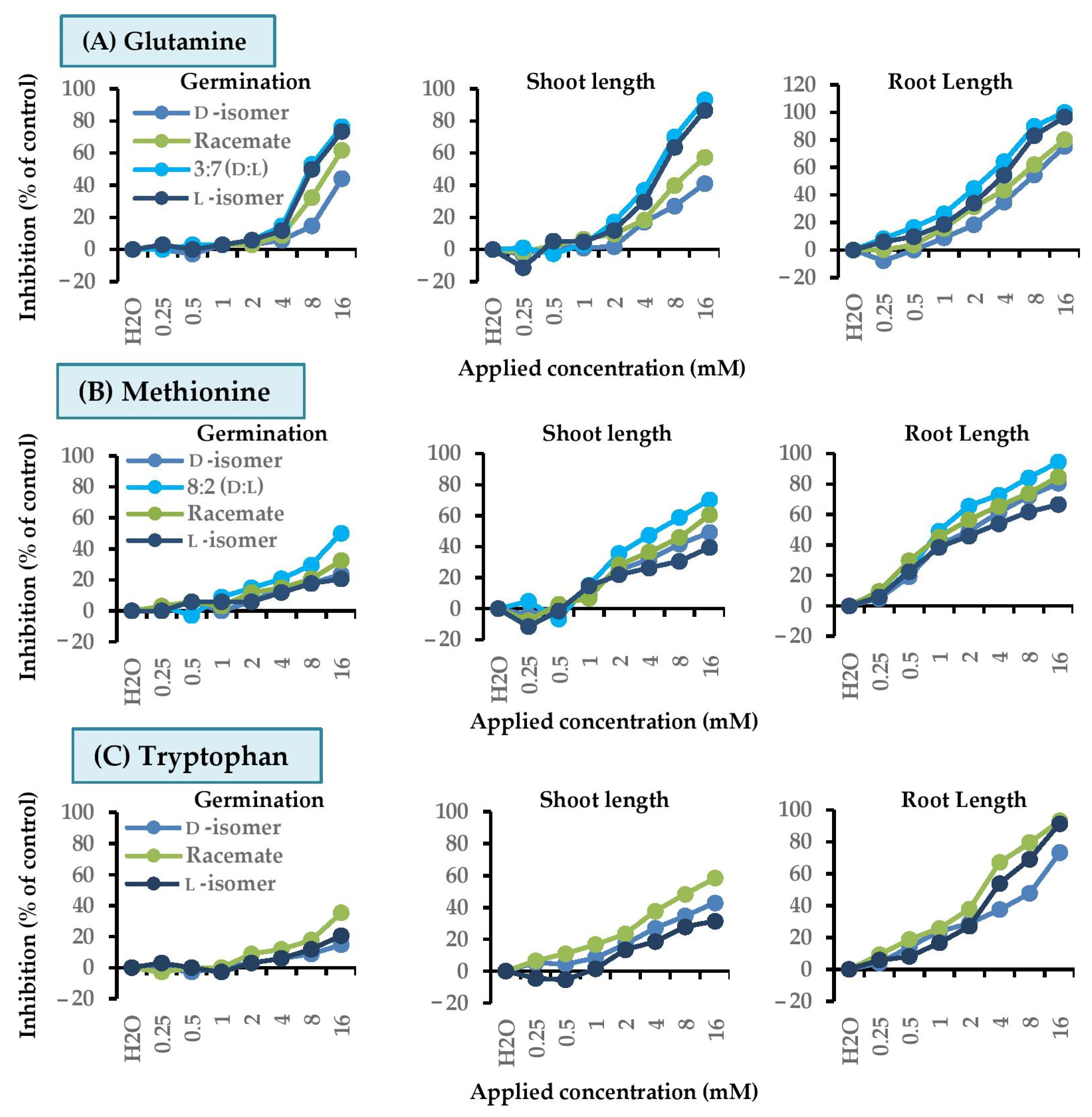
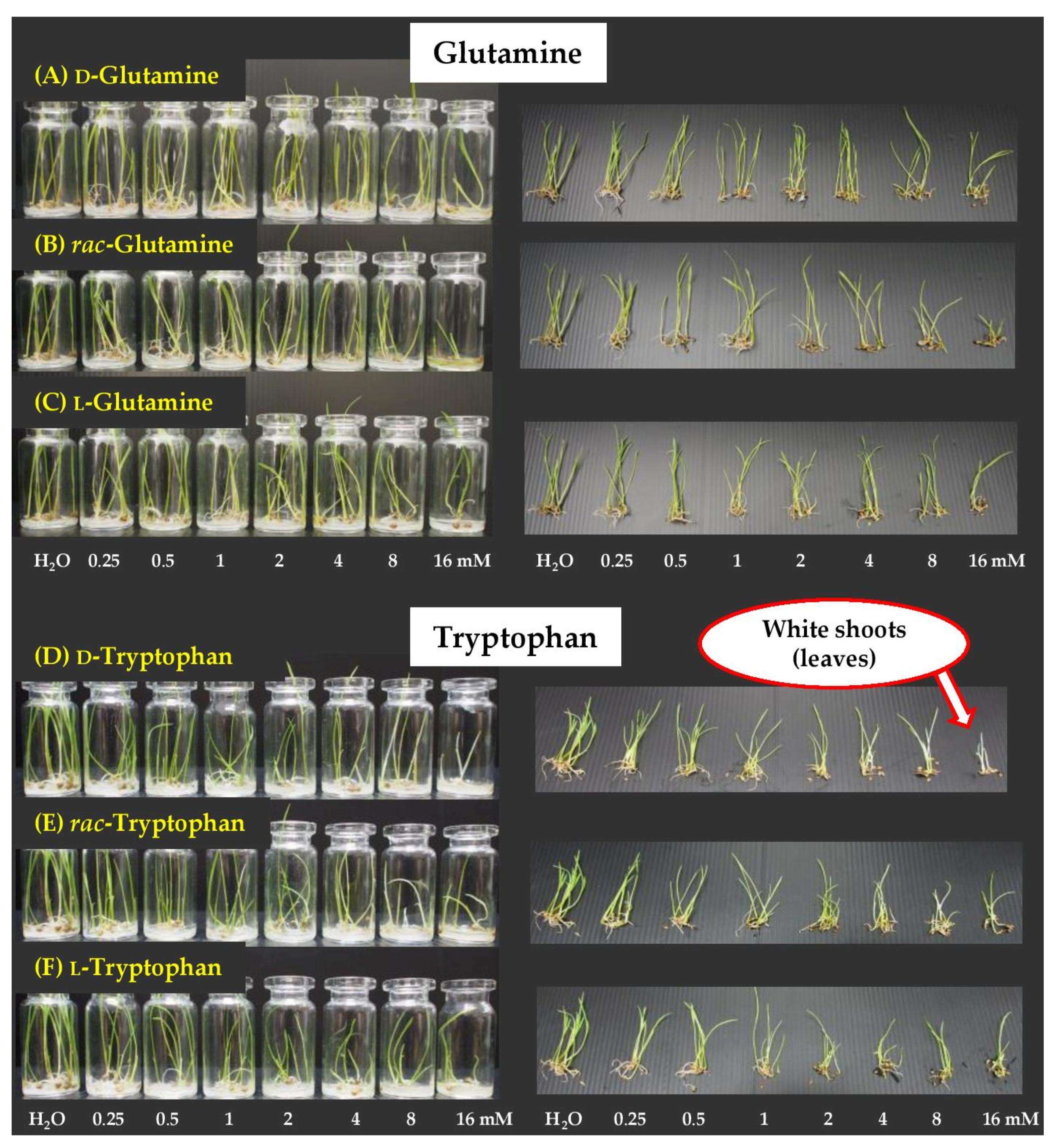
2.4. Dose Responses of Active Amino Acids on Chinese Amaranth
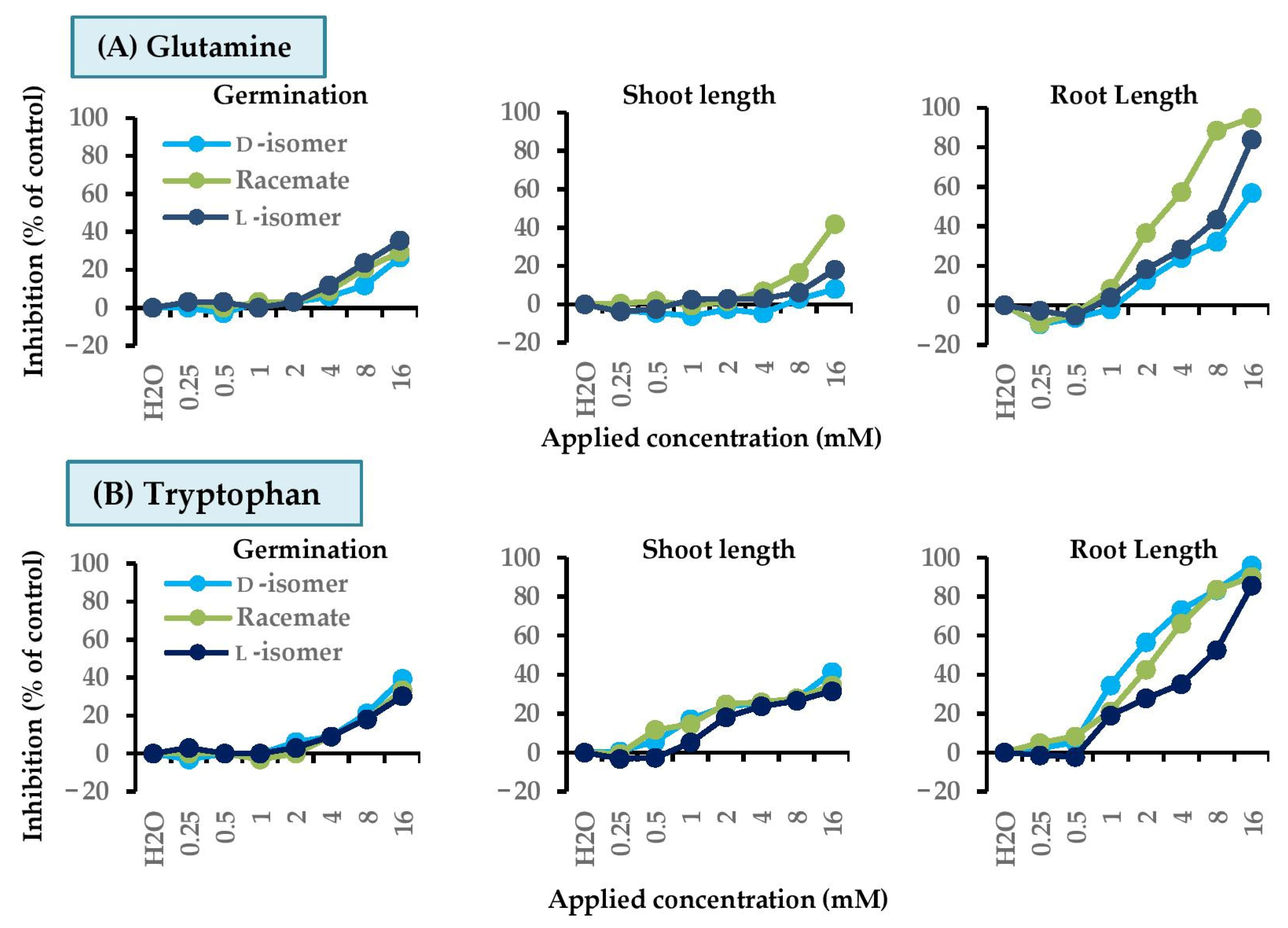
2.5. Dose Responses of Active Amino Acids on Barnyard Grass
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Tested Plants
4.3. Preparation of Aqueous Solutions of Amino Acids at 2 mM
4.4. Preparation of Aqueous Solutions of Amino Acids at 0.25–16 mM
4.5. Bioassay for Seed Germination and Seedling Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gupta, P.K. Chapter 44—Toxicity of herbicides. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 553–567. [Google Scholar]
- Zimdahl, R.L. Chapter 20—Herbicides and the environment. In Fundamentals of Weed Science, 5th ed.; Zimdahl, R.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 557–590. [Google Scholar]
- Korres, N.E.; Burgos, N.R.; Travlos, I.; Vurro, M.; Gitsopoulos, T.K.; Varanasi, V.K.; Duke, S.O.; Kudsk, P.; Brabham, C.; Rouse, C.E.; et al. Chapter 6—New directions for integrated weed management: Modern technologies, tools and knowledge discovery. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 155, pp. 243–319. [Google Scholar]
- Merfield, C.N. Chapter 5—Integrated weed management in organic farming. In Organic Farming; Chandran, S., Unni, M.R., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 117–180. [Google Scholar]
- Pannacci, E.; Lattanzi, B.; Tei, F. Non-chemical weed management strategies in minor crops: A review. Crop Prot. 2017, 96, 44–58. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [Green Version]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Głąb, L.; Sowiński, J.; Bough, R.; Dayan, F.E. Chapter 2—Allelopathic potential of sorghum (Sorghum bicolor (L.) Moench) in weed control: A comprehensive review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 145, pp. 43–95. [Google Scholar]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Chapter 9—Allelopathy. In Fundamentals of Weed Science, 5th ed.; Zimdahl, R.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 253–270. [Google Scholar]
- Laosinwattana, C.; Teerarak, M.; Charoenying, P. Effects of Aglaia odorata granules on the seedling growth of major maize weeds and the influence of soil type on the granule residue’s efficacy. Weed Biol. Manag. 2012, 12, 117–122. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2013; p. 503. [Google Scholar]
- Voet, D.; Voet, J.G. Biochemistry, 4th ed.; John Wiley and Sons: New York, UY, USA, 2010; p. 1520. [Google Scholar]
- Wade, L. Chapter 24: Amino acids, peptides, and proteins. In Organic Chemistry, 6th ed.; Pearson Prentice Hall Inc.: Hoboken, NJ, USA, 2009; pp. 1153–1199. [Google Scholar]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Flati, V.; Corsetti, G.; Pasini, E.; Dioguardi, F.S.; Eleuteri, A.M. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J. 2017, 284, 1726–1737. [Google Scholar] [CrossRef] [Green Version]
- Tamanna, N.; Mahmood, N. Emerging roles of branched-chain amino acid supplementation in human diseases. Int. Sch. Res. Not. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Eleyinmi, A.F. Chemical composition and antibacterial activity of Gongronema latifolium. J. Zhejiang Univ. Sci. B 2007, 8, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Irwansyah, I.; Li, Y.-Q.; Shi, W.; Qi, D.; Leow, W.R.; Tang, M.B.Y.; Li, S.; Chen, X. Gram-positive antimicrobial activity of amino acid-based hydrogels. Adv. Mater. 2015, 27, 648–654. [Google Scholar] [CrossRef]
- Stănilă, A.; Braicu, C.; Stănilă, S. Antibacterial activity of copper and cobalt amino acids complexes. Not. Bot. Horti Agrobot. Cluj Napoca 2011, 39, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Meletis, C.D.; Barker, J.E. Therapeutic uses of amino acids. Altern. Complement. Ther. 2005, 11, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Odia, A.; Esezobor, O.Z. Therapeutic uses of amino acids. In Amino Acid: New Insights and Roles in Plant and Animal; Asao, T., Asaduzzaman, M., Eds.; InTechOpen: London, UK, 2017; pp. 3–14. [Google Scholar]
- Bifari, F.; Ruocco, C.; Decimo, I.; Fumagalli, G.; Valerio, A.; Nisoli, E. Amino acid supplements and metabolic health: A potential interplay between intestinal microbiota and systems control. Genes Nutr. 2017, 12, 27. [Google Scholar] [CrossRef]
- Bertin, C.; Weston, L.A.; Huang, T.; Jander, G.; Owens, T.; Meinwald, J.; Schroeder, F.C. Grass roots chemistry: Meta-tyrosine, an herbicidal nonprotein amino acid. Proc. Natl. Acad. Sci. USA 2007, 104, 16964–16969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Aparicio, M.; Bernard, A.; Falchetto, L.; Marget, P.; Chauvel, B.; Steinberg, C.; Morris, C.E.; Gibot-Leclerc, S.; Boari, A.; Vurro, M.; et al. Investigation of amino acids as herbicides for control of Orobanche minor parasitism in red clover. Front. Plant Sci. 2017, 8, 842. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Reboud, X. Amino Acids for Parasitic Weed Management: Literature Highlight. 2017. ffhal-02190828. Available online: https://hal.archives-ouvertes.fr/hal-02190828/document (accessed on 31 March 2021).
- Jeanmart, S. Amino acids as nonselective herbicides. In Bioactive Carboxylic Compound Classes; Lamberth, C., Dinges, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 315–324. [Google Scholar]
- Lamberth, C. Naturally occurring amino acid derivatives with herbicidal, fungicidal or insecticidal activity. Amino Acids 2016, 48, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Boari, A.; Pilgeram, A.L.; Sands, D.C. Exogenous amino acids inhibit seed germination and tubercle formation by Orobanche ramosa (Broomrape): Potential application for management of parasitic weeds. Biol. Control 2006, 36, 258–265. [Google Scholar] [CrossRef]
- Xuan, T.; Elzaawely, A.; Deba, F.; Fukuta, M.; Tawata, S. Mimosine in Leucaena as a potent bio-herbicide. Agron. Sustain. Dev. 2006, 26, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Lamberth, C. Amino acid chemistry in crop protection. Tetrahedron 2010, 66, 7239–7256. [Google Scholar] [CrossRef]
- Kida, T.; Mizuno, H.; Takinami, K.; Matsunaka, S. Relationship between chemical structure and plant growth-regulating activity of amino acid related compounds. Agric. Biol. Chem. 1976, 40, 1551–1557. [Google Scholar] [CrossRef]
- Lin, G.Q.; Li, Y.M.; Chan, A.S.C. Principles and Applications of Asymmetric Synthesis; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 1–69. [Google Scholar]
- Cossy, J.R. 1.1 Introduction: The importance of chirality in drugs and agrochemicals. In Comprehensive Chirality; Carreira, E.M., Yamamoto, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–7. [Google Scholar]
- Crossley, R.J. Chirality and Biological Activity of Drugs; CRC Press: Boca Raton, FL, USA, 1995; Volume 7, p. 208. [Google Scholar]
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; InTechOpen: London, UK, 2015; pp. 165–186. [Google Scholar]
- Liu, W.; Tang, M. Enantioselective activity and toxicity of chiral herbicides. In Herbicides-Mechanisms and Mode of Action; Naguib, M., Hasaneen, A.E.-G., Eds.; IntechOpen: London, UK, 2011; pp. 63–80. [Google Scholar]
- Liu, Y.; Zhang, X.; Liu, C.; Yang, R.; Xu, Z.; Zhou, L.; Sun, Y.; Lei, H. Enantioselective and synergetic toxicity of axial chiral herbicide propisochlor to SP2/0 myeloma cells. J. Agric. Food Chem. 2015, 63, 7914–7920. [Google Scholar] [CrossRef]
- Xie, J.; Tang, W.; Zhao, L.; Liu, S.; Liu, K.; Liu, W. Enantioselectivity and allelopathy both have effects on the inhibition of napropamide on Echinochloa crus-galli. Sci. Total Environ. 2019, 682, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhao, L.; Liu, K.; Guo, F.; Liu, W. Enantioselective effects of chiral amide herbicides napropamide, acetochlor and propisochlor: The more efficient R-enantiomer and its environmental friendly. Sci. Total Environ. 2018, 626, 860–866. [Google Scholar] [CrossRef]
- Ye, J.; Wang, L.; Zhang, Z.; Liu, W. Enantioselective physiological effects of the herbicide diclofop on cyanobacterium Microcystis aeruginosa. Environ. Sci. Technol. 2013, 47, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Cimmino, A.; Evidente, A.; Rubiales, D. Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 2013, 61, 9797–9803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal activities of some allelochemicals and their synergistic behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Inhibitory effects of a variety of aldehydes on Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. Molecules 2018, 23, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal activity of flavokawains and related trans-chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. [Google Scholar] [CrossRef] [Green Version]
- Thi, H.L.; Zhou, H.; Lin, C.-H.; Liu, S.; Berezin, M.Y.; Smeda, R.J.; Fritschi, F.B. Synthesis and plant growth inhibitory activity of N-trans-cinnamoyltyramine: Its possible inhibition mechanisms and biosynthesis pathway. J. Plant Interact. 2017, 12, 51–57. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Qian, H.; Lu, T.; Zhang, Q.; Liu, W. Enantioselective damage of diclofop acid mediated by oxidative stress and acetyl-coA carboxylase in nontarget plant Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 8405–8412. [Google Scholar] [CrossRef]
- Hsiao, Y.-L.; Wang, Y.-S.; Yen, J.-H. Enantioselective effects of herbicide imazapyr on Arabidopsis thaliana. J. Environ. Sci. Health B 2014, 49, 646–653. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Chen, H.; Chen, Z.; Wen, Y. Enantioselective toxicity of chiral herbicide metolachlor to Microcystis aeruginosa. J. Agric. Food Chem. 2019, 67, 1631–1637. [Google Scholar] [CrossRef]
- Forsum, O. On Plant Responses to D-amino Acids. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2016. [Google Scholar]
- Valle, E.; Virtanen, A.I. On the injurious and growth-promoting effects in peas and barley of various amino acids given together with nitrate. Acta Agra. Fenn. 1965, 107, 308–319. [Google Scholar]
- Bollard, E.G. A comparative study of the ability of organic nitrogenous compounds to serve as sole sources of nitrogen for the growth of plants. Plant Soil 1966, 25, 153–166. [Google Scholar] [CrossRef]
- Manabe, H.; Ohira, K. Effects of D-and L-alanine on the growth of suspension-cultured rice, soybean and tobacco cells. J. Soil Sci. Plant Nutr. 1981, 27, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Forsum, O.; Svennerstam, H.; Ganeteg, U.; Näsholm, T. Capacities and constraints of amino acid utilization in Arabidopsis. New Phytol. 2008, 179, 1058–1069. [Google Scholar]
- Cobb, A.H.; Reade, J.P. Herbicides and Plant Physiology; John Wiley & Sons: West Sussex, UK, 2011; p. 296. [Google Scholar]
- Sandmann, G. Bleaching herbicides: Action mechanism in carotenoid biosynthesis, structural requirements and engineering of resistance. In Herbicide Classes in Development; Böger, P., Wakabayashi, K., Hirai, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 43–57. [Google Scholar]
- Borgati, T.F.; Boaventura, M.A.D. Effects of indole amides on lettuce and onion germination and growth. Z. Nat. C 2011, 66, 485–490. [Google Scholar]
- Mendes, M.C.d.S.; Fazolo, B.R.; de Souza, J.M.; de Vasconcelos, L.G.; de Sousa Junior, P.T.; Dall’Oglio, E.L.; Soares, M.A.; Sampaio, O.M.; Vieira, L.C.C. Synthesis and evaluation of indole derivatives as photosynthesis and plant growth inhibitors. Photochem. Photobiol. Sci. 2019, 18, 1350–1358. [Google Scholar] [CrossRef]
- Hilbert, M.; Voll, L.M.; Ding, Y.; Hofmann, J.; Sharma, M.; Zuccaro, A. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol. 2012, 196, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Velini, E.D.; Alves, E.; Godoy, M.C.; Meschede, D.K.; Souza, R.T.; Duke, S.O. Glyphosate applied at low doses can stimulate plant growth. Pest Manag. Sci. 2008, 64, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Velini, E.D.; Trindade, M.L.B.; Barberis, L.R.M.; Duke, S.O. Growth regulation and other secondary effects of herbicides. Weed Sci. 2017, 58, 351–354. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Enantioselective and Synergistic Herbicidal Activities of Common Amino Acids Against Amaranthus tricolor and Echinochloa crus-galli. Molecules 2021, 26, 2071. https://doi.org/10.3390/molecules26072071
Chotsaeng N, Laosinwattana C, Charoenying P. Enantioselective and Synergistic Herbicidal Activities of Common Amino Acids Against Amaranthus tricolor and Echinochloa crus-galli. Molecules. 2021; 26(7):2071. https://doi.org/10.3390/molecules26072071
Chicago/Turabian StyleChotsaeng, Nawasit, Chamroon Laosinwattana, and Patchanee Charoenying. 2021. "Enantioselective and Synergistic Herbicidal Activities of Common Amino Acids Against Amaranthus tricolor and Echinochloa crus-galli" Molecules 26, no. 7: 2071. https://doi.org/10.3390/molecules26072071






