Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain
Abstract
:1. Introduction
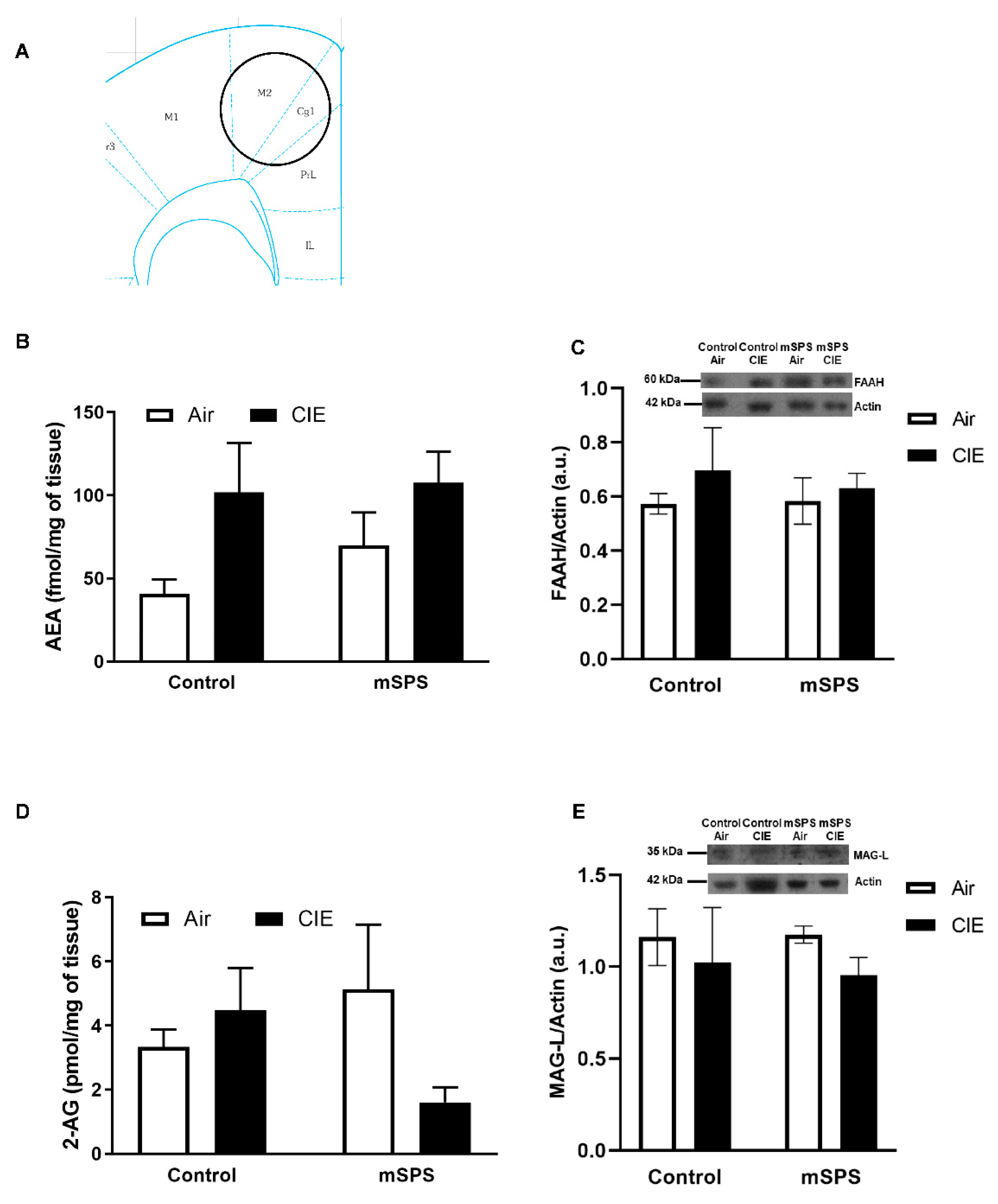
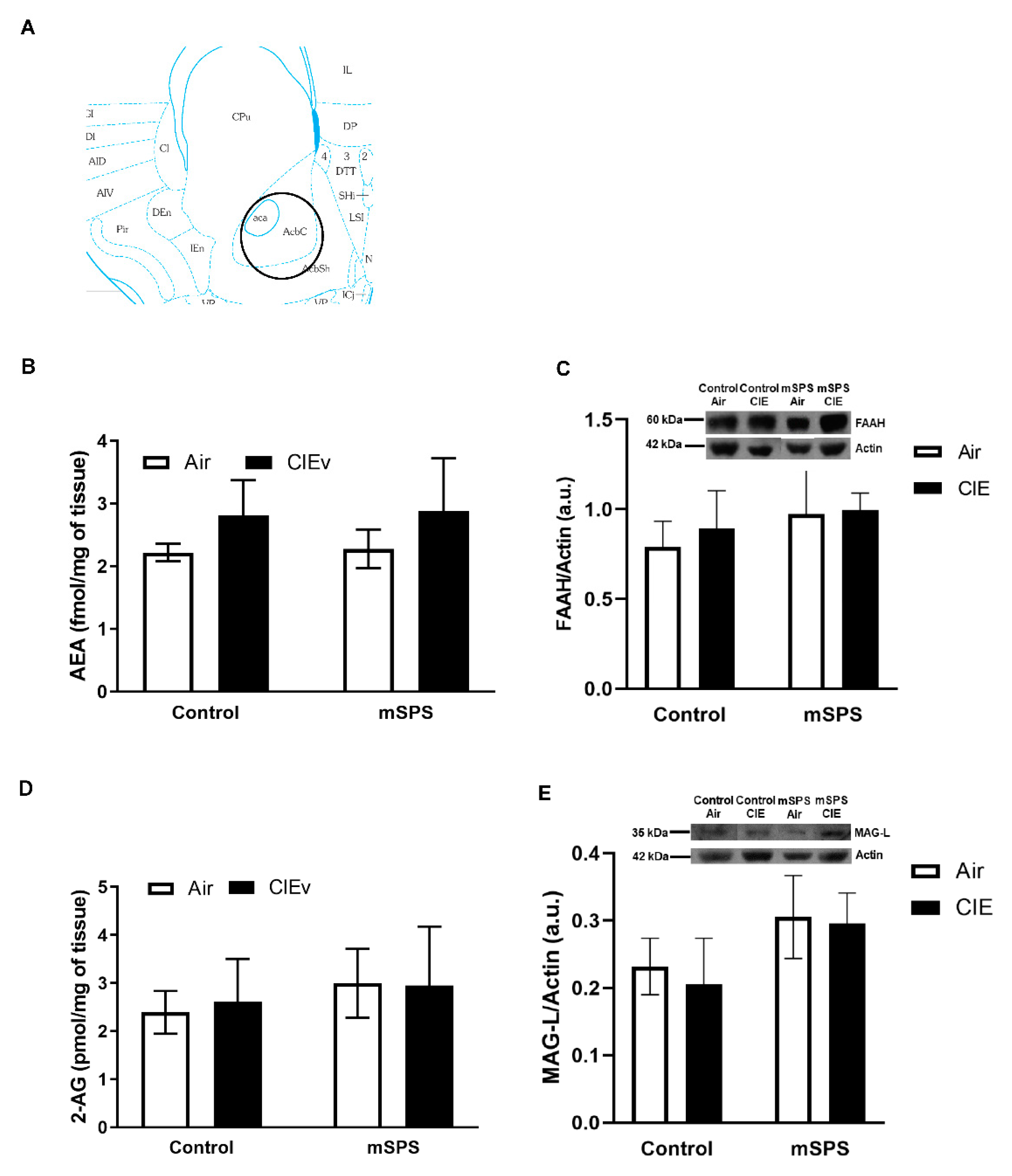
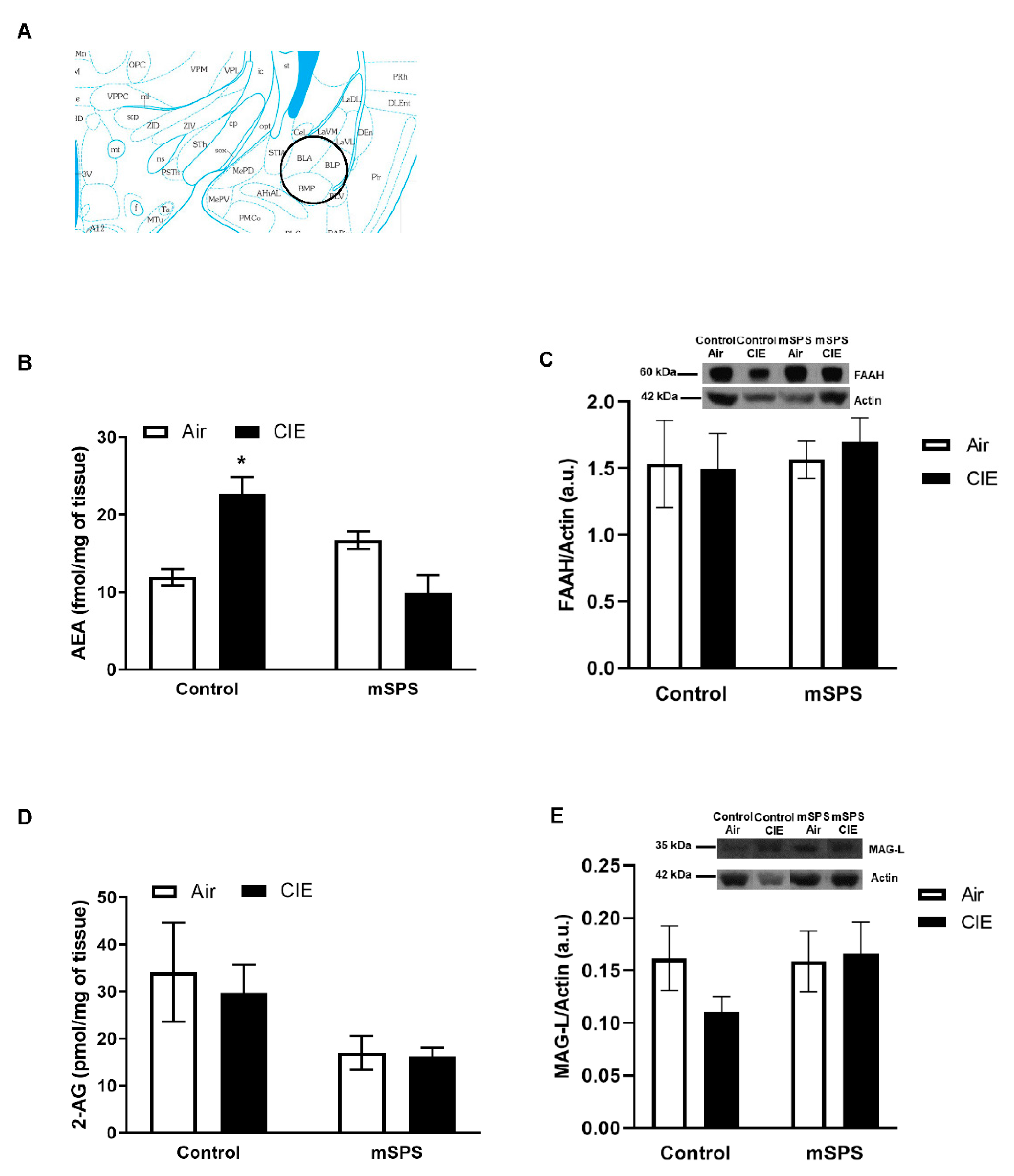
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
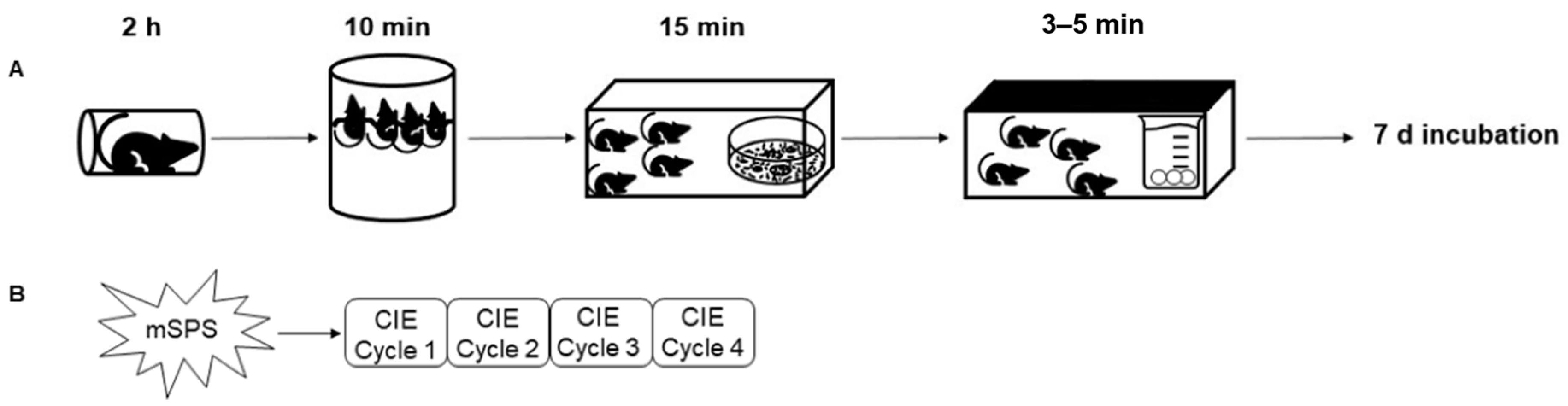
4.2. Mouse Single-Prolonged Stress (mSPS)
4.3. Chronic Intermittent Ethanol (CIE) Vapor Exposure
4.4. Brain Tissue Dissection
4.5. AEA and 2-AG Extraction for UHPLC-MS/MS Analysis
4.6. UHPLC-MS/MS
4.7. Protein Extraction for Immunoblot Analysis
4.8. Immunoblotting
4.9. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roberts, A.L.; Gilman, S.E.; Breslau, J.; Breslau, N.; Koenen, K.C. Race/Ethnic Differences in Exposure to Traumatic Events, Development of Post-Traumatic Stress Disorder, and Treatment-Seeking for Post-Traumatic Stress Disorder in the United States. Psychol. Med. 2011, 41, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Breslau, N.; Peterson, E.L.; Poisson, L.M.; Schultz, L.R.; Lucia, V.C. Estimating Post-Traumatic Stress Disorder in the Community: Lifetime Perspective and the Impact of Typical Traumatic Events. Psychol. Med. 2004, 34, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.; Wilcox, H.C.; Storr, C.L.; Lucia, V.C.; Anthony, J.C. Trauma Exposure and Posttraumatic Stress Disorder: A Study of Youths in Urban America. J. Urban Health: Bull. N. Y. Acad. Med. 2004, 81, 530–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkonigg, A.; Kessler, R.C.; Storz, S.; Wittchen, H.U. Traumatic Events and Post-Traumatic Stress Disorder in the Community: Prevalence, Risk Factors and Comorbidity. Acta Psychiatr. Scand. 2000, 101, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime Prevalence and Age-of-Onset Distributions of Dsm-Iv Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, A.C.; Capone, C.; Short, E.E. Co-Occurring Posttraumatic Stress Disorder and Alcohol Use Disorders in Veteran Populations. J. Dual Diagn. 2011, 7, 285–299. [Google Scholar] [CrossRef]
- Ralevski, E.; Olivera-Figueroa, L.A.; Petrakis, I. Ptsd and Comorbid Aud: A Review of Pharmacological and Alternative Treatment Options. Subst. Abus. Rehabil. 2014, 5, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Petrakis, I.L.; Simpson, T.L. Posttraumatic Stress Disorder and Alcohol Use Disorder: A Critical Review of Pharmacologic Treatments. Alcohol. Clin. Exp. Res. 2017, 41, 226–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrine, S.A.; Eagle, A.L.; George, S.A.; Mulo, K.; Kohler, R.J.; Gerard, J.; Harutyunyan, A.; Hool, S.M.; Susick, L.L.; Schneider, B.L.; et al. Severe, Multimodal Stress Exposure Induces Ptsd-Like Characteristics in a Mouse Model of Single Prolonged Stress. Behav. Brain Res. 2016, 303, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Aikins, D.E.; Strader, J.A.; Kohler, R.J.; Bihani, N.; Perrine, S.A. Differences in Hippocampal Serotonergic Activity in a Mouse Single Prolonged Stress Paradigm Impact Discriminant Fear Acquisition and Retention. Neurosci. Lett. 2017, 639, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Piggott, V.M.; Bosse, K.E.; Lisieski, M.J.; Strader, J.A.; Stanley, J.A.; Conti, A.C.; Ghoddoussi, F.; Perrine, S.A. Single-Prolonged Stress Impairs Prefrontal Cortex Control of Amygdala and Striatum in Rats. Front. Behav. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaby, L.E.; Sadik, N.; Burson, N.A.; Lloyd, S.; O’Donnel, K.; Winters, J.; Conti, A.C.; Liberzon, I.; Perrine, S.A. Repeated Stress Exposure in Mid-Adolescence Attenuates Behavioral, Noradrenergic, and Epigenetic Effects of Trauma-Like Stress in Early Adult Male Rats. Sci. Rep. 2020, 10, 17935. [Google Scholar] [CrossRef] [PubMed]
- Lisieski, M.J.; Eagle, A.L.; Conti, A.C.; Liberzon, I.; Perrine, S.A. Single-Prolonged Stress: A Review of Two Decades of Progress in a Rodent Model of Post-Traumatic Stress Disorder. Front. Psychiatry 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eagle, A.L.; Knox, D.; Roberts, M.M.; Mulo, K.; Liberzon, I.; Galloway, M.P.; Perrine, S.A. Single Prolonged Stress Enhances Hippocampal Glucocorticoid Receptor and Phosphorylated Protein Kinase B Levels. Neurosci. Res. 2013, 75, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, J.Z. From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits. Front. Neural Circuits 2017, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, A.J.; Mott, D.D. Functional Neuroanatomy of Amygdalohippocampal Interconnections and Their Role in Learning and Memory. J. Neurosci. Res. 2017, 95, 797–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid Signalling in Reward and Addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariyadath, V.; Gowin, J.L.; Stein, E.A. Chapter 8—Resting State Functional Connectivity Analysis for Addiction Medicine: From Individual Loci to Complex Networks. In Progress in Brain Research; Ekhtiari, H., Paulus, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 224, pp. 155–173. [Google Scholar]
- Moal, M.L.; Simon, H. Mesocorticolimbic Dopaminergic Network: Functional and Regulatory Roles. Physiol. Rev. 1991, 71, 155–234. [Google Scholar] [CrossRef] [PubMed]
- Matchynski-Franks, J.J.; Susick, L.L.; Schneider, B.L.; Perrine, S.A.; Conti, A.C. Impaired Ethanol-Induced Sensitization and Decreased Cannabinoid Receptor-1 in a Model of Posttraumatic Stress Disorder. PLoS ONE 2016, 11, e0155759. [Google Scholar] [CrossRef]
- Knox, D.; Perrine, S.A.; George, S.A.; Galloway, M.P.; Liberzon, I. Single Prolonged Stress Decreases Glutamate, Glutamine, and Creatine Concentrations in the Rat Medial Prefrontal Cortex. Neurosci. Lett. 2010, 480, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.S.; Mackie, K. Distribution of the Endocannabinoid System in the Central Nervous System. Handb. Exp. Pharmacol. 2015, 231, 59–93. [Google Scholar] [CrossRef] [PubMed]
- Pava, M.J.; Woodward, J.J. A Review of the Interactions between Alcohol and the Endocannabinoid System: Implications for Alcohol Dependence and Future Directions for Research. Alcohol 2012, 46, 185–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions between Stress and the Endocannabinoid System. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 80–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blankman, J.L.; Cravatt, B.F. Chemical Probes of Endocannabinoid Metabolism. Pharmacol. Rev. 2013, 65, 849–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Batkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; McKinney, M.K.; Cravatt, B.F. Enzymatic Pathways That Regulate Endocannabinoid Signaling in the Nervous System. Chem. Rev. 2008, 108, 1687–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V. Targeting the Endocannabinoid System: To Enhance or Reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef]
- Crowe, M.S.; Nass, S.R.; Gabella, K.M.; Kinsey, S.G. The Endocannabinoid System Modulates Stress, Emotionality, and Inflammation. BrainBehav. Immun. 2014, 42, 1–5. [Google Scholar] [CrossRef]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Cannabinoid Interventions for Ptsd: Where to Next? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 124–140. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 80–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchicchi, A.; Pistis, M. Anandamide and 2-Arachidonoylglycerol: Pharmacological Properties, Functional Features, and Emerging Specificities of the Two Major Endocannabinoids. Mol. Neurobiol. 2012, 46, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A Comprehensive Profile of Brain Enzymes That Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.N.; Patel, S.; Campolongo, P.; Tasker, J.G.; Wotjak, C.T.; Bains, J.S. Functional Interactions between Stress and the Endocannabinoid System: From Synaptic Signaling to Behavioral Output. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 14980–14986. [Google Scholar] [CrossRef] [PubMed]
- Kunos, G. Interactions between Alcohol and the Endocannabinoid System. Alcohol. Clin. Exp. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Joshi, V.; Shivakumar, M.; Subbanna, S. Distinct Functions of Endogenous Cannabinoid System in Alcohol Abuse Disorders. Br. J. Pharmacol. 2019, 176, 3085–3109. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S. Endocannabinoid System and Alcohol Abuse Disorders. Adv. Exp. Med. Biol. 2019, 1162, 89–127. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.Y. Role of the Endocannabinoid System in the Neurobiology of Suicide. In The Neurobiological Basis of Suicide; Dwivedi, Y., Ed.; CRC Press/Taylor & Francis LLC: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hill, M.N.; Bierer, L.M.; Makotkine, I.; Golier, J.A.; Galea, S.; McEwen, B.S.; Hillard, C.J.; Yehuda, R. Reductions in Circulating Endocannabinoid Levels in Individuals with Post-Traumatic Stress Disorder Following Exposure to the World Trade Center Attacks. Psychoneuroendocrinology 2013, 38, 2952–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauer, D.; Schelling, G.; Gola, H.; Campolongo, P.; Morath, J.; Roozendaal, B.; Hamuni, G.; Karabatsiakis, A.; Atsak, P.; Vogeser, M.; et al. Plasma Concentrations of Endocannabinoids and Related Primary Fatty Acid Amides in Patients with Post-Traumatic Stress Disorder. PLoS ONE 2013, 8, e62741. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; McLaughlin, R.J.; Morrish, A.C.; Viau, V.; Floresco, S.B.; Hillard, C.J.; Gorzalka, B.B. Suppression of Amygdalar Endocannabinoid Signaling by Stress Contributes to Activation of the Hypothalamic-Pituitary-Adrenal Axis. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009, 34, 2733–2745. [Google Scholar] [CrossRef]
- Gray, J.M.; Vecchiarelli, H.A.; Morena, M.; Lee, T.T.; Hermanson, D.J.; Kim, A.B.; McLaughlin, R.J.; Hassan, K.I.; Kühne, C.; Wotjak, C.T.; et al. Corticotropin-Releasing Hormone Drives Anandamide Hydrolysis in the Amygdala to Promote Anxiety. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 3879–3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubreucq, S.; Matias, I.; Cardinal, P.; Haring, M.; Lutz, B.; Marsicano, G.; Chaouloff, F. Genetic Dissection of the Role of Cannabinoid Type-1 Receptors in the Emotional Consequences of Repeated Social Stress in Mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 1885–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Hill, M.N.; Zhang, L.; Gorzalka, B.B.; Hillard, C.J.; Alger, B.E. Acute Restraint Stress Enhances Hippocampal Endocannabinoid Function Via Glucocorticoid Receptor Activation. J. Psychopharmacol. 2012, 26, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Navarria, A.; Tamburella, A.; Iannotti, F.A.; Micale, V.; Camillieri, G.; Gozzo, L.; Verde, R.; Imperatore, R.; Leggio, G.M.; Drago, F.; et al. The Dual Blocker of Faah/Trpv1 N-Arachidonoylserotonin Reverses the Behavioral Despair Induced by Stress in Rats and Modulates the Hpa-Axis. Pharmacol. Res. 2014, 87, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J.; Meier, S.E.; Shi, L.; Ho, W.S.; Jarrahian, A.; Hillard, C.J. Effects of Acute and Repeated Restraint Stress on Endocannabinoid Content in the Amygdala, Ventral Striatum, and Medial Prefrontal Cortex in Mice. Neuropharmacology 2008, 54, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McLaughlin, R.J.; Pan, B.; Fitzgerald, M.L.; Roberts, C.J.; Lee, T.T.; Karatsoreos, I.N.; Mackie, K.; Viau, V.; Pickel, V.M.; et al. Recruitment of Prefrontal Cortical Endocannabinoid Signaling by Glucocorticoids Contributes to Termination of the Stress Response. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 10506–10515. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Roelke, C.T.; Rademacher, D.J.; Hillard, C.J. Inhibition of Restraint Stress-Induced Neural and Behavioural Activation by Endogenous Cannabinoid Signalling. Eur. J. Neurosci. 2005, 21, 1057–1069. [Google Scholar] [CrossRef]
- Sumislawski, J.J.; Ramikie, T.S.; Patel, S. Reversible Gating of Endocannabinoid Plasticity in the Amygdala by Chronic Stress: A Potential Role for Monoacylglycerol Lipase Inhibition in the Prevention of Stress-Induced Behavioral Adaptation. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 2750–2761. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; McLaughlin, R.J.; Bingham, B.; Shrestha, L.; Lee, T.T.; Gray, J.M.; Hillard, C.J.; Gorzalka, B.B.; Viau, V. Endogenous Cannabinoid Signaling Is Essential for Stress Adaptation. Proc. Natl. Acad. Sci. USA 2010, 107, 9406–9411. [Google Scholar] [CrossRef] [Green Version]
- Vinod, K.Y.; Yalamanchili, R.; Xie, S.; Cooper, T.B.; Hungund, B.L. Effect of Chronic Ethanol Exposure and Its Withdrawal on the Endocannabinoid System. Neurochem. Int. 2006, 49, 619–625. [Google Scholar] [CrossRef]
- DePoy, L.; Daut, R.; Brigman, J.L.; MacPherson, K.; Crowley, N.; Gunduz-Cinar, O.; Pickens, C.L.; Cinar, R.; Saksida, L.M.; Kunos, G.; et al. Chronic Alcohol Produces Neuroadaptations to Prime Dorsal Striatal Learning. Proc. Natl. Acad. Sci. USA 2013, 110, 14783–14788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henricks, A.M.; Berger, A.L.; Lugo, J.M.; Baxter-Potter, L.N.; Bieniasz, K.V.; Petrie, G.; Sticht, M.A.; Hill, M.N.; McLaughlin, R.J. Sex- and Hormone-Dependent Alterations in Alcohol Withdrawal-Induced Anxiety and Corticolimbic Endocannabinoid Signaling. Neuropharmacology 2017, 124, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; McHugh, D.; Fernandez-Ruiz, J.; Bradshaw, H.; Walker, J.M. Short-Term Exposure to Alcohol in Rats Affects Brain Levels of Anandamide, Other N-Acylethanolamines and 2-Arachidonoyl-Glycerol. Neurosci. Lett. 2007, 421, 270–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangieri, R.A.; Hong, K.I.; Piomelli, D.; Sinha, R. An Endocannabinoid Signal Associated with Desire for Alcohol Is Suppressed in Recently Abstinent Alcoholics. Psychopharmacology 2009, 205, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piggott, V.M.; Lloyd, S.C.; Perrine, S.A.; Conti, A.C. Chronic Intermittent Ethanol Exposure Increases Ethanol Consumption Following Traumatic Stress Exposure in Mice. Front. Behav. Neurosci. 2020, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Elsevier, Inc.: San Diego, CA, USA, 2007. [Google Scholar]
- Shin, L.M.; Rauch, S.L.; Pitman, R.K. Amygdala, Medial Prefrontal Cortex, and Hippocampal Function in Ptsd. Ann. New York Acad. Sci. 2006, 1071, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganon-Elazar, E.; Akirav, I. Cannabinoids and Traumatic Stress Modulation of Contextual Fear Extinction and Gr Expression in the Amygdala-Hippocampal-Prefrontal Circuit. Psychoneuroendocrinology 2013, 38, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Carvalho, M.; Clem, R.L. Prefrontal-Amygdala Fear Networks Come into Focus. Front. Syst. Neurosci. 2015, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.L.; Thiruchselvam, R.; Todd, R.; Christoff, K. Emotion and the Prefrontal Cortex: An Integrative Review. Psychol. Bull. 2017, 143, 1033–1081. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrine, S.A.; Ghoddoussi, F.; Michaels, M.S.; Hyde, E.M.; Kuhn, D.M.; Galloway, M.P. Mdma Administration Decreases Serotonin but Not N-Acetylaspartate in the Rat Brain. Neurotoxicology 2010, 31, 654–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.J.; Stuhr, K.L.; Hillard, C.J. Swim Stress Differentially Affects Limbic Contents of 2-Arachidonoylglycerol and 2-Oleoylglycerol. Neuroscience 2012, 204, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.N.; Eiland, L.; Lee, T.T.Y.; Hillard, C.J.; McEwen, B.S. Early Life Stress Alters the Developmental Trajectory of Corticolimbic Endocannabinoid Signaling in Male Rats. Neuropharmacology 2019, 146, 154–162. [Google Scholar] [CrossRef]
- Vaughn, L.K.; Denning, G.; Stuhr, K.L.; de Wit, H.; Hill, M.N.; Hillard, C.J. Endocannabinoid Signalling: Has It Got Rhythm? Br. J. Pharmacol. 2010, 160, 530–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, M.; Viganò, D.; Casico, M.G.; Rubino, T.; Steardo, L.; Parolaro, D.; Di Marzo, V. Differential Diurnal Variations of Anandamide and 2-Arachidonoyl-Glycerol Levels in Rat Brain. Cell. Mol. Life Sci. CMLS 2004, 61, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, B.C.; Kalivas, P.W. Corticostriatal Circuitry in Regulating Diseases Characterized by Intrusive Thinking. Dialogues Clin. Neurosci. 2016, 18, 65–76. [Google Scholar] [PubMed]
- Yager, L.M.; Garcia, A.F.; Wunsch, A.M.; Ferguson, S.M. The Ins and Outs of the Striatum: Role in Drug Addiction. Neuroscience 2015, 301, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Haber, S.N. Corticostriatal Circuitry. Dialogues Clin. Neurosci. 2016, 18, 7–21. [Google Scholar]
- Lovinger, D.M. Neurotransmitter Roles in Synaptic Modulation, Plasticity and Learning in the Dorsal Striatum. Neuropharmacology 2010, 58, 951–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enman, N.M.; Arthur, K.; Ward, S.J.; Perrine, S.A.; Unterwald, E.M. Anhedonia, Reduced Cocaine Reward, and Dopamine Dysfunction in a Rat Model of Posttraumatic Stress Disorder. Biol. Psychiatry 2015, 78, 871–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosse, K.E.; Charlton, J.L.; Susick, L.L.; Newman, B.; Eagle, A.L.; Mathews, T.A.; Perrine, S.A.; Conti, A.C. Deficits in Behavioral Sensitization and Dopaminergic Responses to Methamphetamine in Adenylyl Cyclase 1/8-Deficient Mice. J. Neurochem. 2015, 135, 1218–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarrone, M.; Rossi, S.; Bari, M.; De Chiara, V.; Fezza, F.; Musella, A.; Gasperi, V.; Prosperetti, C.; Bernardi, G.; Finazzi-Agro, A.; et al. Anandamide Inhibits Metabolism and Physiological Actions of 2-Arachidonoylglycerol in the Striatum. Nat. Neurosci. 2008, 11, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Volman, S.F.; Lammel, S.; Margolis, E.B.; Kim, Y.; Richard, J.M.; Roitman, M.F.; Lobo, M.K. New Insights into the Specificity and Plasticity of Reward and Aversion Encoding in the Mesolimbic System. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 17569–17576. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrine, S.A.; Ghoddoussi, F.; Desai, K.; Kohler, R.J.; Eapen, A.T.; Lisieski, M.J.; Angoa-Perez, M.; Kuhn, D.M.; Bosse, K.E.; Conti, A.C.; et al. Cocaine-Induced Locomotor Sensitization in Rats Correlates with Nucleus Accumbens Activity on Manganese-Enhanced Mri. NMR Biomed. 2015, 28, 1480–1488. [Google Scholar] [CrossRef]
- Calhoon, G.G.; Tye, K.M. Resolving the Neural Circuits of Anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sailer, U.; Robinson, S.; Fischmeister, F.P.; Konig, D.; Oppenauer, C.; Lueger-Schuster, B.; Moser, E.; Kryspin-Exner, I.; Bauer, H. Altered Reward Processing in the Nucleus Accumbens and Mesial Prefrontal Cortex of Patients with Posttraumatic Stress Disorder. Neuropsychologia 2008, 46, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Liberzon, I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Daviu, N.; Bruchas, M.R.; Moghaddam, B.; Sandi, C.; Beyeler, A. Neurobiological Links between Stress and Anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.; Deng, X.; Wang, X.; Yuan, Y.; Liu, Z.; Liang, J. Altered Function in Medial Prefrontal Cortex and Nucleus Accumbens Links to Stress-Induced Behavioral Inflexibility. Behav. Brain Res. 2017, 317, 16–26. [Google Scholar] [CrossRef] [PubMed]
- McGrath, A.G.; Briand, L.A. A Potential Role for Microglia in Stress- and Drug-Induced Plasticity in the Nucleus Accumbens: A Mechanism for Stress-Induced Vulnerability to Substance Use Disorder. Neurosci. Biobehav. Rev. 2019, 107, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.J.; Jagannathan, L.; Lowenstein, E.D.; Robinson, T.E.; Becker, J.B.; Morrow, J.D. Single Prolonged Stress Decreases Sign-Tracking and Cue-Induced Reinstatement of Cocaine-Seeking. Behav. Brain Res. 2019, 359, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jaimes, L.; Polis, I.; Parsons, L.H. Regional Influence of Cannabinoid Cb1 Receptors in the Regulation of Ethanol Self-Administration by Wistar Rats. Open Neuropsychopharmacol. J. 2009, 2, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Caillé, S.; Alvarez-Jaimes, L.; Polis, I.; Stouffer, D.G.; Parsons, L.H. Specific Alterations of Extracellular Endocannabinoid Levels in the Nucleus Accumbens by Ethanol, Heroin, and Cocaine Self-Administration. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Svíženská, I.; Dubový, P.; Šulcová, A. Cannabinoid Receptors 1 and 2 (Cb1 and Cb2), Their Distribution, Ligands and Functional Involvement in Nervous System Structures—A Short Review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Weiner, J.L. Neurobiology of Comorbid Post-Traumatic Stress Disorder and Alcohol-Use Disorder. Genes Brain Behav. 2017, 16, 15–43. [Google Scholar] [CrossRef] [Green Version]
- Opitz, B. Memory Function and the Hippocampus. Front. Neurol. Neurosci. 2014, 34, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Kutlu, M.G.; Gould, T.J. Effects of Drugs of Abuse on Hippocampal Plasticity and Hippocampus-Dependent Learning and Memory: Contributions to Development and Maintenance of Addiction. Learn. Mem. (Cold Spring Harb. N. Y.) 2016, 23, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Phillips, L.J.; McGorry, P.D.; Garner, B.; Thompson, K.N.; Pantelis, C.; Wood, S.J.; Berger, G. Stress, the Hippocampus and the Hypothalamic-Pituitary-Adrenal Axis: Implications for the Development of Psychotic Disorders. Aust. New Zealand J. Psychiatry 2006, 40, 725–741. [Google Scholar] [CrossRef]
- Anderson, E.B.; Grossrubatscher, I.; Frank, L. Dynamic Hippocampal Circuits Support Learning- and Memory-Guided Behaviors. Cold Spring Harb. Symp. Quant. Biol. 2014, 79, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Fanselow, M.S.; Dong, H.W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbanna, S.; Shivakumar, M.; Psychoyos, D.; Xie, S.; Basavarajappa, B.S. Anandamide-Cb1 Receptor Signaling Contributes to Postnatal Ethanol-Induced Neonatal Neurodegeneration, Adult Synaptic, and Memory Deficits. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 6350–6366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrirattanakul, S.; López-Valdés, H.E.; Liang, J.; Matsuka, Y.; Mackie, K.; Faull, K.F.; Spigelman, I. Bidirectional Alterations of Hippocampal Cannabinoid 1 Receptors and Their Endogenous Ligands in a Rat Model of Alcohol Withdrawal and Dependence. Alcohol. Clin. Exp. Res. 2007, 31, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Patel, S.; Carrier, E.J.; Rademacher, D.J.; Ormerod, B.K.; Hillard, C.J.; Gorzalka, B.B. Downregulation of Endocannabinoid Signaling in the Hippocampus Following Chronic Unpredictable Stress. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2005, 30, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Herman, M.A.; Roberto, M. The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders. Biol. Psychiatry 2015, 77, 859–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, M.; Chiba, A.A. The Amygdala and Emotion. Curr. Opin. Neurobiol. 1996, 6, 221–227. [Google Scholar] [CrossRef]
- Serrano, A.; Pavon, F.J.; Buczynski, M.W.; Schlosburg, J.; Natividad, L.A.; Polis, I.Y.; Stouffer, D.G.; Zorrilla, E.P.; Roberto, M.; Cravatt, B.F.; et al. Deficient Endocannabinoid Signaling in the Central Amygdala Contributes to Alcohol Dependence-Related Anxiety-Like Behavior and Excessive Alcohol Intake. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 1840–1850. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Committee. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011; p. 256. [Google Scholar] [CrossRef]
- Becker, H.C.; Lopez, M.F. Increased Ethanol Drinking after Repeated Chronic Ethanol Exposure and Withdrawal Experience in C57bl/6 Mice. Alcohol. Clin. Exp. Res. 2004, 28, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wright, R.; Kirchhoff, A.M.; Chester, J.A.; Cooper, B.R.; Davisson, V.J.; Barker, E. Quantitative Lc-Ms/Ms Analysis of Arachidonoyl Amino Acids in Mouse Brain with Treatment of Faah Inhibitor. Anal. Biochem. 2013, 432, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Zoerner, A.A.; Batkai, S.; Suchy, M.T.; Gutzki, F.M.; Engeli, S.; Jordan, J.; Tsikas, D. Simultaneous Uplc-Ms/Ms Quantification of the Endocannabinoids 2-Arachidonoyl Glycerol (2ag), 1-Arachidonoyl Glycerol (1ag), and Anandamide in Human Plasma: Minimization of Matrix-Effects, 2ag/1ag Isomerization and Degradation by Toluene Solvent Extraction. J Chromatogr B Anal. Technol Biomed Life Sci 2012, 883–884, 161–171. [Google Scholar] [CrossRef]
- Hill, M.N.; Karatsoreos, I.N.; Hillard, C.J.; McEwen, B.S. Rapid Elevations in Limbic Endocannabinoid Content by Glucocorticoid Hormones in Vivo. Psychoneuroendocrinology 2010, 35, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ney, L.; Stone, C.; Nichols, D.; Felmingham, K.; Bruno, R.; Matthews, A. Endocannabinoid Reactivity to Acute Stress: Investigation of the Relationship between Salivary and Plasma Levels. Biol. Psychol. 2021, 159, 108022. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piggott, V.M.; Lloyd, S.C.; Matchynski, J.I.; Perrine, S.A.; Conti, A.C. Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain. Molecules 2021, 26, 2086. https://doi.org/10.3390/molecules26072086
Piggott VM, Lloyd SC, Matchynski JI, Perrine SA, Conti AC. Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain. Molecules. 2021; 26(7):2086. https://doi.org/10.3390/molecules26072086
Chicago/Turabian StylePiggott, Veronica M., Scott C. Lloyd, James I. Matchynski, Shane A. Perrine, and Alana C. Conti. 2021. "Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain" Molecules 26, no. 7: 2086. https://doi.org/10.3390/molecules26072086
APA StylePiggott, V. M., Lloyd, S. C., Matchynski, J. I., Perrine, S. A., & Conti, A. C. (2021). Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain. Molecules, 26(7), 2086. https://doi.org/10.3390/molecules26072086





