Novel Ring Systems: Spiro[Cycloalkane] Derivatives of Triazolo- and Tetrazolo-Pyridazines
Abstract
:1. Introduction
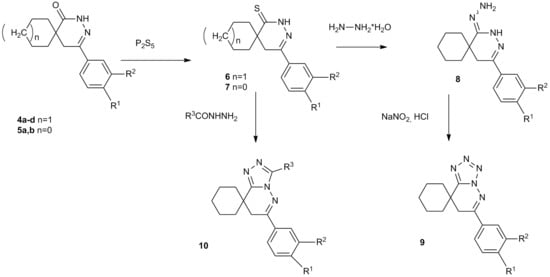
2. Results and Discussion
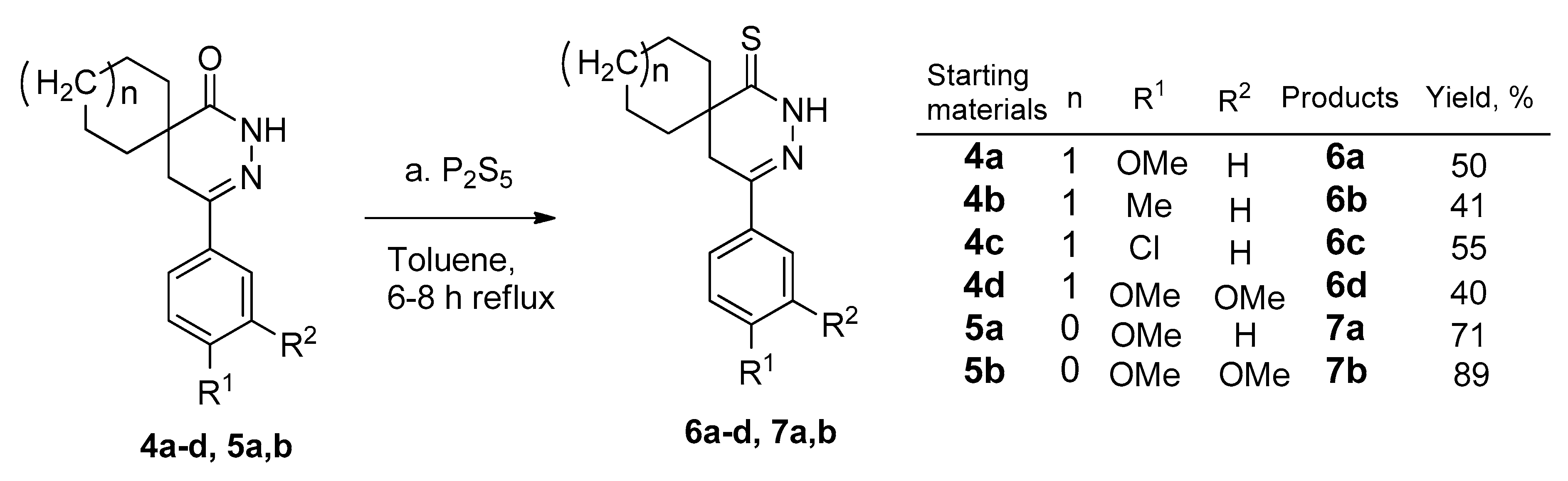
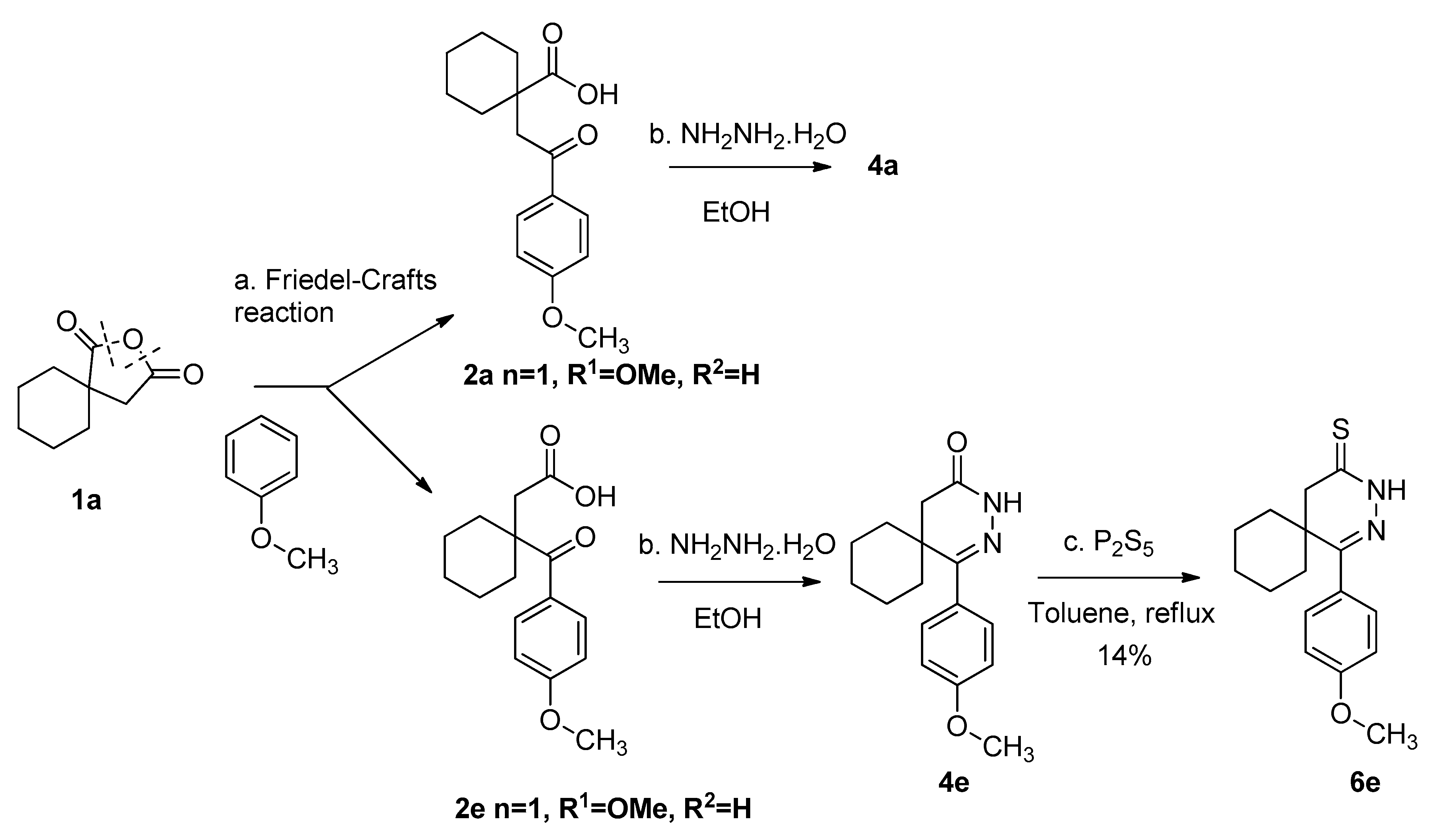
2.1. Synthesis of Pyridazinethiones
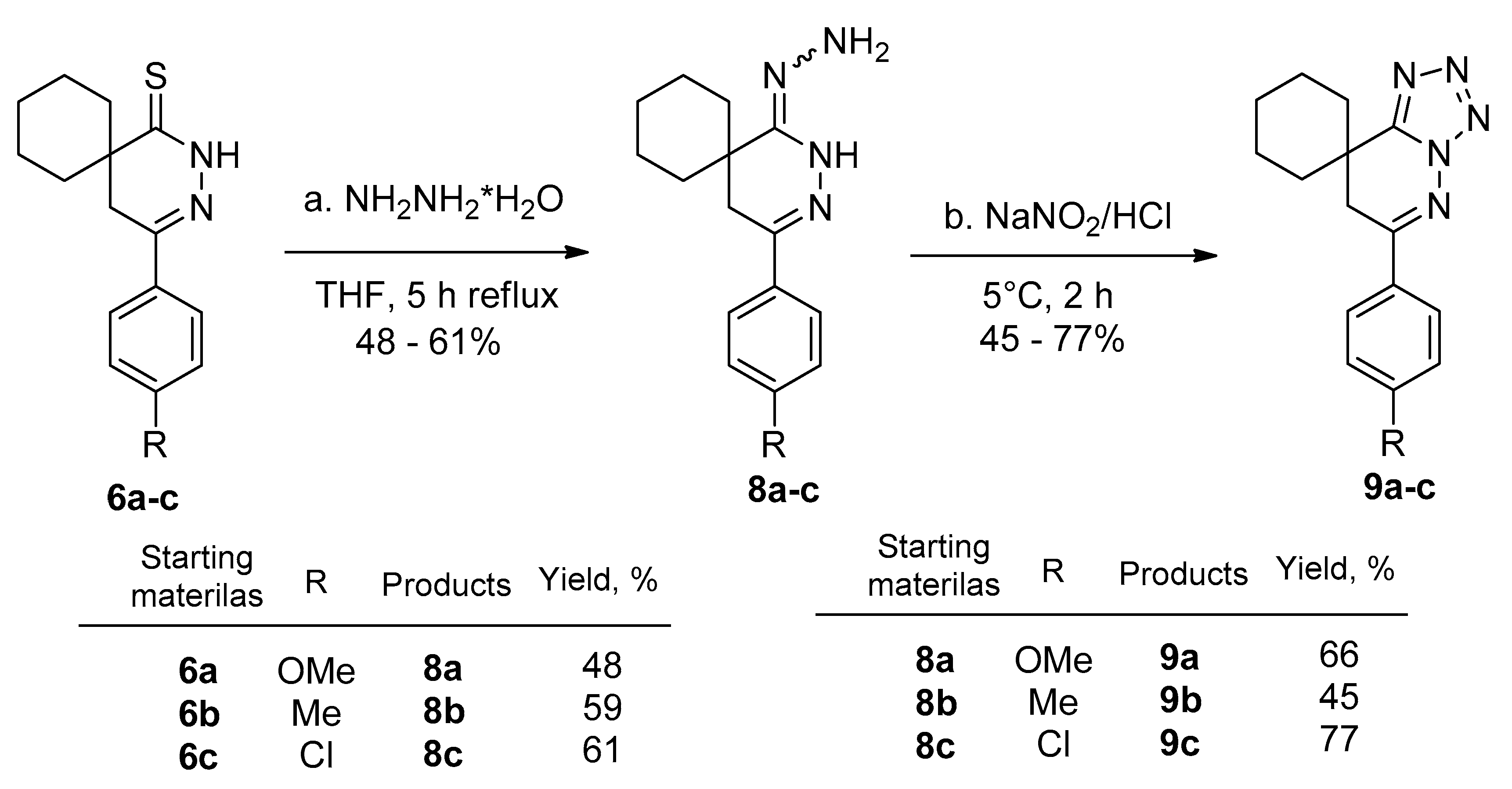
2.2. Preparing the Tetrazole Derivatives through Hydrazones
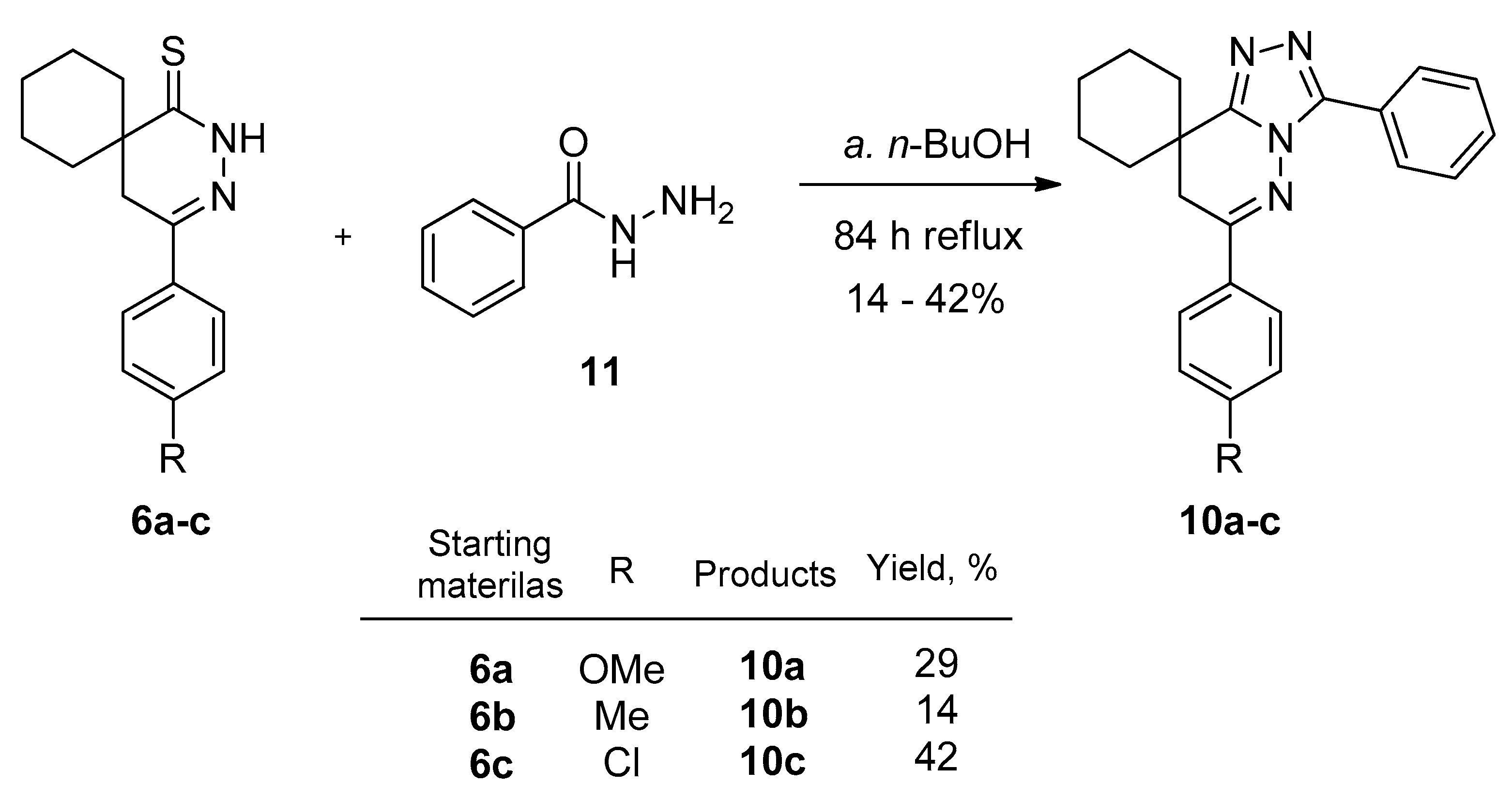
2.3. Preparing the Triazole Derivatives from Thioxo Compounds with Acid Hydrazides
3. Materials and Method
3.1. LC-MS Analysis, TLC, and Preparative TLC
3.2. NMR Spectroscopy
3.3. Mass Spectrometry
3.4. Synthesis of the Starting Materials
3.5. General Procedure for the Synthesis of the Pyridazine-Thiones (6a–e and 7a,b)
3.6. General Procedure for the Preparation of Hydrazones (8a–c)
3.7. Preparation of Tetrazolo-Pyridazines (9a–c)
3.8. General Procedure for the Preparation of 6-(4-Substituted-phenyl)-3-phenyl-7H-spiro[[1,2,4]triazolo[4,3-b]pyridazin-8,1′-cyclohexanes] (10a–c)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wermuth, C.G. Are pyridazines privileged structures? Med. Chem. Comm. 2011, 2, 935–941. [Google Scholar] [CrossRef]
- Castle, R.N. (Ed.) The Chemistry of Heterocyclic Compounds Pyridazines; Wiley: Hoboken, NJ, USA, 2009; Volume 28, ISBN 978-0-470-18849-1. [Google Scholar]
- Heinisch, G.; Holzer, W.; Kunz, F.; Langer, T.; Lukavsky, P.; Pechlaner, C.; Weissenberger, H. On the Bioisosteric Potential of Diazines: Diazine Analogues of the Combined Thromboxane A2 Receptor Antagonist and Synthetase Inhibitor Ridogrel. J. Med. Chem. 1996, 39, 4058–4064. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. The Pharmacological Importance of Some Diazine Containing Drug Molecules. Sci. Online Pub. Trans. Org. Chem. 2014, 1, 1–17. [Google Scholar]
- Akhtar, W.; Shaquiquzzaman, M.; Akhter, M.; Verma, G.; Khan, M.F.; Alam, M.M. The therapeutic journey of pyridazinone. Eur. J. Med. Chem. 2016, 123, 256–281. [Google Scholar] [CrossRef]
- Seth, S.; Sharma, A.; Raj, D. Pyridazinones: A wonder nucleus with scaffold of pharmacological activities. Am. J. Biol. Pharm. Res. 2014, 1, 105–116. [Google Scholar]
- Dubey, S.; Bhosle, P.A. Pyridazinone: An important element of pharmacophore possessing broad spectrum of activity. Med. Chem. Res. 2015, 24, 3579–3598. [Google Scholar] [CrossRef]
- Bartlett, M.S.; Shaw, M.M.; Smith, J.W.; Meshnick, S.R. Efficacy of Sulfamethoxypyridazine in a Murine Model of Pneumocystiscarinii Pneumonia. Antimic. Agents Chemother. 1998, 42, 934–935. [Google Scholar] [CrossRef] [Green Version]
- Vigil-De Gracia, P.; Lasso, M.; Ruiz, E.; Vega-Malek, J.C.; De Mena, F.T.; Lopez, J.C. Severe hypertension in pregnancy: Hydralazine or labetalol: A randomized clinical trial. Eur. J. Obstet. Gynecol. Reproduc. Biol. 2006, 128, 157–162. [Google Scholar] [CrossRef]
- Papp, Z.; Édes, I.; Fruhwald, S.; De Hert, S.G.; Salmenperä, M.; Leppikangas, H.; Mebazaa, A.; Landoni, G.; Grossini, E.; Caimmi, P.; et al. Levosimendan: Molecular Mechanisms and Clinical Implications: Consensus of Experts on the Mechanisms of Action of Levosimendan. Int. J. Cardiol. 2012, 159, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan, J.P.; Mouget-Goniot, C.; Worms, P.; Biziere, K. Effect of the antidepressant minaprine on both forms of monoamine oxidase in the rat. Biochem. Pharm. 1986, 35, 973–978. [Google Scholar] [CrossRef]
- Contreras, J.M.; Rival, Y.M.; Chayer, S.; Bourguignon, J.J.; Wermuth, C.G. Aminopyridazines as acetylcholinesterase inhibitors. J. Med. Chem. 1999, 42, 730–741. [Google Scholar] [CrossRef]
- Aleeva, G.N.; Molodavkin, G.M.; Voronina, T.A. Comparison of antidepressant effects of azafan, tianeptine, and paroxetine. Bul. Exp. Biol. Med. 2009, 148, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, P.B.; Reed, A.E.; Ronfeld, R.A. Pharmacokinetics of zopolrestat, a carboxylicacid aldose reductase inhibitor in normal and diabetic rats. Pharm. Res. 1991, 8, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Infante, J.R.; Reckamp, K.L.; Blumenschein, G.R.; Leal, T.A.; Waqar, S.N.; Gitlitz, B.J.; Sanborn, R.E.; Whisenant, J.G.; Du, L.; et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human PhaseI/II, Multicenter Study. Clin. Cancer Res. 2018, 24, 2771–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barra, F.; Seca, M.; Della Corte, L.; Giampaolino, P.; Ferrero, S. Relugolix for the treatment of uterine fibroids. Drugs Today 2019, 55, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Kaye, S.B.; Yap, T.A. PARP inhibitors: The race is on. Br. J. Cancer 2016, 114, 713–715. [Google Scholar] [CrossRef] [Green Version]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Eng. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Li, D.B.; Rogers-Evans, M.; Carreira, E.M. Synthesis of novel azaspiro[3.4]octanes as multifunctional modules in drug discovery. Org. Lett. 2011, 13, 6134–6136. [Google Scholar] [CrossRef]
- Burkhard, J.A.; Guérot, C.; Knust, H.; Carreira, E.M. Expanding the Azaspiro[3.3]heptanes Family: Synthesis of Novel HighlyFunctionalized Building Blocks. Org. Lett. 2012, 14, 66–69. [Google Scholar] [CrossRef]
- Li, D.B.; Rogers-Evans, M.; Carreira, E.M. Construction of multifunctional modules for drug discovery: Synthesis of novelthia/oxa-azaspiro [3.4] octanes. Org. Lett. 2013, 15, 4766–4769. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four-membered ring-containing spirocycles: Synthetic strategies and opportunities. Chem. Rev. 2014, 144, 8257–8322. [Google Scholar] [CrossRef]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [Green Version]
- Sepsey Für, C.; Riszter, G.; Gerencsér, J.; Szigetvári, A.; Dékány, M.; Hazai, L.; Keglevich, G.; Bölcskei, H. Synthesis of Spiro[Cycloalkane-pyridazinones] with High Fsp3 Character. Lett. Drug Des. Discov. 2020, 17, 731–744. [Google Scholar]
- Sepsey Für, C.; Horvát, E.J.; Szigetvári, A.; Dékány, M.; Hazai, L.; Keglevich, G.; Bölcskei, H. Synthesis of Spiro[Cycloalkane-pyridazinones] with High Fsp3 Character Part 2. Lett. Org. Chem. 2021, 18, 373–381. [Google Scholar]
- Ozturk, T.; Ertas, E.; Mert, O. A Berzelius Reagent, Phosphorus Decasulfide (P4S10), in Organic Syntheses. Chem. Rev. 2010, 110, 3419–3478. [Google Scholar] [CrossRef]
- Chimirri, A.; Zappala, M.; Gitto, R.; Quartarone, S.; Bevacqua, F. Synthesis and structural features of 11H-Tetrazolo[1,5-c][2,3]benzodiazepines. Heterocycles 1999, 51, 1303–1309. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of five revolution. Drug Discov. Today Tech. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the OralBioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. Med. Chem. Commun. 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Chimirri, A.; Bevacqua, F.; Gitto, R.; Quartarone, S.; Zappala, M.; De Sarro, A.; Maciocco, L.; Biggio, G.; De Sarro, G. Synthesis and anticonvulsant activity of new 11H-triazolo[4,5-c][2,3]benzodiazepines. Med. Chem. Res. 1999, 9, 203–212. [Google Scholar]
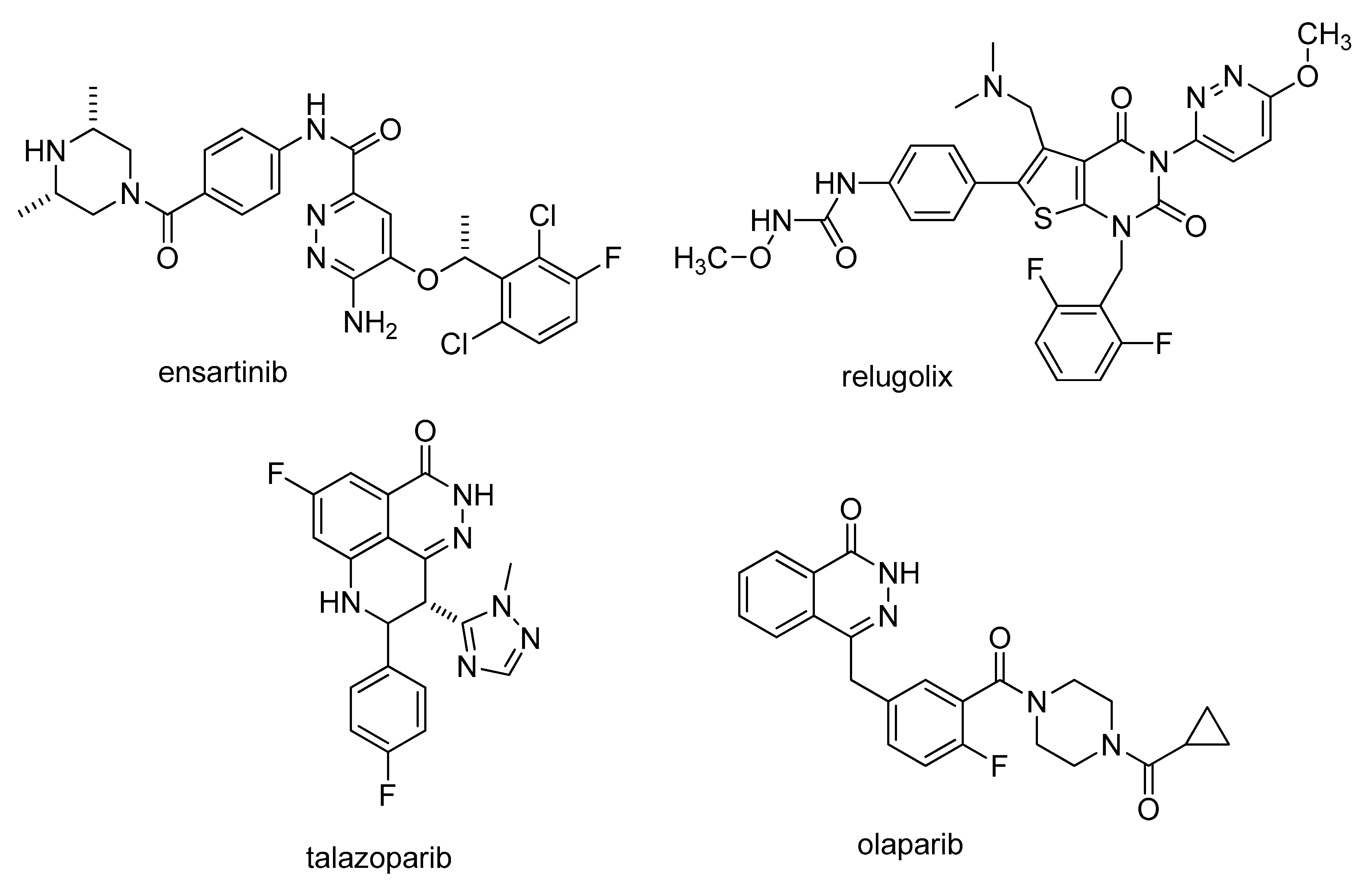

| Starting Material | R | Product | Fsp3 | LogP | ClogP | TPSA |
|---|---|---|---|---|---|---|
| 8a | OMe | 9a | 0.50 | 3.08 * | 3.55 | 61.91 |
| 8b | Me | 9b | 0.50 | 3.47 * | 4.13 | 52.68 |
| 8c | Cl | 9c | 0.47 | 3.70 * | 4.34 | 52.68 |
| Drug | Fsp3 | LogP | ClogP | TPSA |
|---|---|---|---|---|
| Hydralazine | 0 | 0.73 | 1.17 | 62.77 |
| Zopolrestate | 0.16 | 4.37 | 2.55 | 82.33 |
| Levosimendan | 0.21 | 1.23 | 1.91 | 113.43 |
| Pipofezin | 0.38 | 1.98 | 3.33 | 43.67 |
| Minaprine | 0.41 | 2.70 | 3.19 | 49.22 |
| Talazoparib a | 0.16 | 2.58 | 0.35 | 81.45 |
| Relugolix a | 0.21 | 4.98 | 4.58 | 128.17 |
| Ensartinib a | 0.27 | 4.04 | 5.22 | 121.41 |
| Olaparib a | 0.34 | 2.02 | 1.24 | 82.08 |
| Starting Material | R | Product | Fsp3 | logP | ClogP | TPSA |
|---|---|---|---|---|---|---|
| 6a | OMe | 10a * | 0.35 | 5.33 | 4.36 | 49.55 |
| 6b | Me | 10b * | 0.35 | 5.72 | 4.94 | 40.32 |
| 6c | Cl | 10c * | 0.32 | 5.95 | 5.16 | 40.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepsey Für, C.; Riszter, G.; SzigetvárI, Á.; Dékány, M.; Keglevich, G.; HazaI, L.; BölcskeI, H. Novel Ring Systems: Spiro[Cycloalkane] Derivatives of Triazolo- and Tetrazolo-Pyridazines. Molecules 2021, 26, 2140. https://doi.org/10.3390/molecules26082140
Sepsey Für C, Riszter G, SzigetvárI Á, Dékány M, Keglevich G, HazaI L, BölcskeI H. Novel Ring Systems: Spiro[Cycloalkane] Derivatives of Triazolo- and Tetrazolo-Pyridazines. Molecules. 2021; 26(8):2140. https://doi.org/10.3390/molecules26082140
Chicago/Turabian StyleSepsey Für, Csilla, Gergő Riszter, Áron SzigetvárI, Miklós Dékány, György Keglevich, László HazaI, and Hedvig BölcskeI. 2021. "Novel Ring Systems: Spiro[Cycloalkane] Derivatives of Triazolo- and Tetrazolo-Pyridazines" Molecules 26, no. 8: 2140. https://doi.org/10.3390/molecules26082140
APA StyleSepsey Für, C., Riszter, G., SzigetvárI, Á., Dékány, M., Keglevich, G., HazaI, L., & BölcskeI, H. (2021). Novel Ring Systems: Spiro[Cycloalkane] Derivatives of Triazolo- and Tetrazolo-Pyridazines. Molecules, 26(8), 2140. https://doi.org/10.3390/molecules26082140