Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes
Abstract
:1. Introduction
2. Results and Discussion
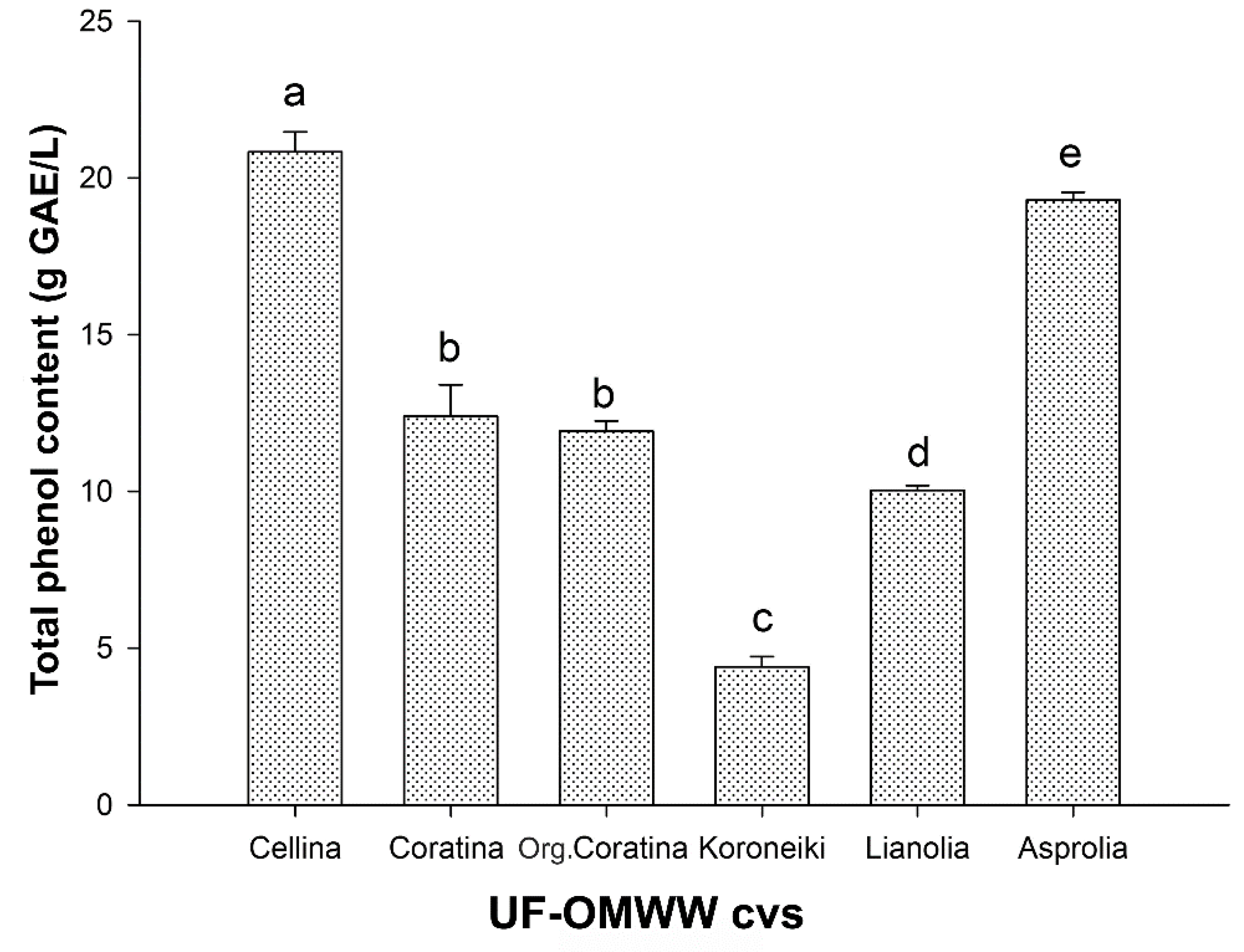
2.1. Total Phenol Content of UF Fractions and HPLC-DAD Analysis
2.2. Antioxidant Activities
2.2.1. Antioxidant Activity of UF Fractions
2.2.2. Antioxidant Activity on LDL
2.3. Possible Protective Mechanism of OMWW on Keratinocytes Cell Cultures
2.3.1. Effect of UF Fractions on HEKa Cell Viability
2.3.2. Effects of UF Fractions on UVA-Irradiated HEKa
Effect on Cell Viability of UF Fractions on UVA-Damaged HEKa
Pro-Apoptotic Effect of UF Fractions on UVA-Damaged HEKa
ROS-Quenching Ability of UF-OMWW in HEKa Cells
3. Materials and Methods
3.1. Chemicals
3.2. Olive Mill Wastewater (OMWW) Samples
3.3. Determination of Phenol Content
3.4. HPLC-DAD Analysis
3.5. Evaluation of Antioxidant Activity of UF Fractions
3.5.1. TEAC Method
3.5.2. LDL Antioxidant Assay
Headspace–Solid Phase Microextraction
Gas Chromatography–Ion Trap–Mass Spectrometry Analysis
3.6. Cell Cultures
3.6.1. Cell Viability Assay
3.6.2. UVA/UF-OMWW Combined Treatments
3.6.3. Annexin V/PI Staining
3.6.4. ROS Detection in HEKa Cell Cultures
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Michałowicz, J.; Duda, W. Phenols—Sources and toxicity. Polish J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Biotechnol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Erses Yay, A.S.; Oral, H.V.; Onay, T.T.; Yenigün, O. A study on olive oil mill wastewater management in Turkey: A questionnaire and experimental approach. Resour. Conserv. Recycl. 2012, 60, 64–71. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Elia, I.; Petalas, A.V.; Halvadakis, C.P. Removal of total phenols from olive-mill wastewater using an agricultural by-product, olive pomace. J. Hazard. Mater. 2008, 160, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Cardinali, A.; Cicco, N.; Linsalata, V.; Minervini, F.; Pati, S.; Pieralice, M.; Tursi, N.; Lattanzio, V. Biological activity of high molecular weight phenolics from olive mill wastewater. J. Agric. Food Chem. 2010, 58, 8585–8590. [Google Scholar] [CrossRef]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Bleve, G.; Gallo, A.; Altomare, C.; Vurro, M.; Maiorano, G.; Cardinali, A.; D’Antuono, I.; Marchi, G.; Mita, G. In vitro activity of antimicrobial compounds against Xylella fastidiosa, the causal agent of the olive quick decline syndrome in Apulia (Italy). FEMS Microbiol. Let. 2018, 365, fnx281. [Google Scholar] [CrossRef] [Green Version]
- Bavaro, S.L.; D’Antuono, I.; Cozzi, G.; Haidukowski, M.; Cardinali, A.; Logrieco, A.F. Inhibition of aflatoxin B1 production by verbascoside and other olive polyphenols. World Mycotoxin J. 2016, 9, 545–553. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef] [Green Version]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging Role of Phenolic Compounds as Natural Food Additives in Fish and Fish Products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Robards, K. Potent antioxidant biophenols from olive mill waste. Food Chem. 2008, 111, 171–178. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Konczak, I.; Rehman, A.U.; Robards, K. Chemistry and bioactivity of olive biophenols in some antioxidant and antiproliferative in vitro bioassays. Chem. Res. Toxicol. 2009, 22, 227–234. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Nomikos, T.; Karantonis, H.C.; Apostolakis, C.; Pliakis, E.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S. Biological activity of acetylated phenolic compounds. J. Agric. Food Chem. 2007, 55, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G.; Mikhal’chik, E.V.; Suprun, M.V.; Pastore, S.; Dal Toso, R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell. Mol. Biol. 2007, 53, 84–91. [Google Scholar]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Chang, M.K.; Binder, C.J.; Shaw, P.X.; Witztum, J.L. Oxidized low density lipoprotein and innate immune receptors. Curr. Opin. Lipidol. 2003, 14, 437–445. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Montedoro, G.; Galli, C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 1995, 117, 25–32. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef]
- Seidel, V.; Verholle, M.; Malard, Y.; Tillequin, F.; Fruchart, J.C.; Duriez, P.; Bailleul, F.; Teissier, E. Phenylpropanoids from Ballota nigra L. inhibit in vitro LDL peroxidation. Phytother. Res. 2000, 14, 93–98. [Google Scholar] [CrossRef]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [PubMed] [Green Version]
- Mense, S.M.; Hei, T.K.; Ganju, R.K.; Bhat, H.K. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ. Health Perspect. 2008, 116, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, K.H.; Barve, A.; Yu, S.; Huang, M.T.; Kong, A.N.T. Cancer chemoprevention by phytochemicals: Potential molecular targets, biomarkers and animal models. Acta Pharmacol. Sin. 2007, 28, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P.M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einspahr, J.G.; Stratton, S.P.; Bowden, G.T.; Alberts, D.S. Chemoprevention of human skin cancer. Crit. Rev. Oncol. Hematol. 2002, 41, 269–285. [Google Scholar] [CrossRef]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Alcaraz, M.; Micol, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. B Biol. 2014, 136, 12–18. [Google Scholar] [CrossRef]
- Svobodová, A.; Zdařilová, A.; Mališková, J.; Mikulková, H.; Walterová, D.; Vostalová, J. Attenuation of UVA-induced damage to human keratinocytes by silymarin. J. Dermatol. Sci. 2007, 46, 21–30. [Google Scholar] [CrossRef]
- Huang, C.C.; Fang, J.Y.; Wu, W.; Chiang, H.S.; Wei, Y.J.; Hung, C.F. Protective effects of (-)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch. Dermatol. Res. 2005, 296, 473–481. [Google Scholar] [CrossRef]
- Basu-Modak, S.; Gordon, M.J.; Dobson, L.H.; Spencer, J.P.E.; Rice-Evans, C.; Tyrrell, R.M. Epicatechin and its methylated metabolite attenuate UVA-induced oxidative damage to human skin fibroblasts. Free Radic. Biol. Med. 2003, 35, 910–921. [Google Scholar] [CrossRef]
- Tobi, S.E.; Gilbert, M.; Paul, N.; McMillan, T.J. The green tea polyphenol, epigallocatechin-3-gallate, protects against the oxidative cellular and genotoxic damage of UVA radiation. Int. J. Cancer 2002, 102, 439–444. [Google Scholar] [CrossRef]
- Erden Inal, M.; Kahraman, A.; Koken, T. Beneficial effects of quercetin on oxidative stress induced by ultraviolet A. Clin. Exp. Dermatol. 2001, 26, 536–539. [Google Scholar] [CrossRef]
- D’Antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke polyphenols produce skin anti-age effects by improving endothelial cell integrity and functionality. Molecules 2018, 23, 2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E.; Gerothanassis, I.P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Gyurkovska, V.; Alipieva, K.; Maciuk, A.; Dimitrova, P.; Ivanovska, N.; Haas, C.; Bley, T.; Georgiev, M. Anti-inflammatory activity of Devil’s claw in vitro systems and their active constituents. Food Chem. 2011, 125, 171–178. [Google Scholar] [CrossRef]
- Mazzon, E.; Esposito, E.; Di Paola, R.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Effects of verbascoside biotechnologically produced by Syringa vulgaris plant cell cultures in a rodent model of colitis. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 79–94. [Google Scholar] [CrossRef]
- Palmieri, D.; Aliakbarian, B.; Casazza, A.A.; Ferrari, N.; Spinella, G.; Pane, B.; Cafueri, G.; Perego, P.; Palombo, D. Effects of polyphenol extract from olive pomace on anoxia-induced endothelial dysfunction. Microvasc. Res. 2012, 83, 281–289. [Google Scholar] [CrossRef]
- Lecci, R.M.; Logrieco, A.; Leone, A. Pro-oxidative action of polyphenols as action mechanism for their pro-apoptotic activity. Anticancer Agents Med. Chem. 2014, 14, 1363–1375. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cordero, C.; León-González, A.J.; Calderón-Montaño, J.M.; Burgos-Morón, E.; López-Lázaro, M. Pro-Oxidant Natural Products as Anticancer Agents. Curr. Drug Targets 2012, 13, 1006–1028. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z. Increased Oxidative Stress as a Selective Anticancer Therapy. Oxid. Med. Cell. Longev. 2015, 294303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 6475624. [Google Scholar] [CrossRef] [Green Version]
- Obied, H.K.; Karuso, P.; Prenzler, P.D.; Robards, K. Novel secoiridoids with antioxidant activity from Australian olive mill waste. J. Agric. Food Chem. 2007, 55, 2848–2853. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.L. From olive drupes to olive Oil An HPLC-orbitrap-based qualitative and quantitative exploration of olive key metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F.; Galli, C. Antioxidant and other biological activities of olive mill waste waters. J. Agric. Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage. EFSA J. 2011, 9, 1–25. [Google Scholar]
- Giovannini, C.; Straface, E.; Modesti, D.; Coni, E.; Cantafora, A.; De Vincenzi, M.; Malorni, W.; Masella, R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J. Nutr. 1999, 129, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.S.; Heinonen, M.; Frankel, E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998, 61, 71–75. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, S.; Lewis, J.; Singh, B.; Schoenlein, P.; Osaki, T.; Athar, M.; Porter, A.G.; Schuster, G. Green tea polyphenol targets the mitochondria in tumor cells inducing caspase 3-dependent apoptosis. Anticancer Res. 2003, 23, 1533–1539. [Google Scholar]
- Balasubramanian, S.; Eckert, R.L. Keratinocyte proliferation, differentiation, and apoptosis—Differential mechanisms of regulation by curcumin, EGCG and apigenin. Toxicol. Appl. Pharmacol. 2007, 224, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.Z.; Jandova, J.; Janda, J.; Vleugels, F.R.; Elliott, D.A.; Sligh, J.E. Resveratrol-sensitized UVA induced apoptosis in human keratinocytes through mitochondrial oxidative stress and pore opening. J. Photochem. Photobiol. B 2012, 113, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Dell’Aquila, M.E.; Bogliolo, L.; Russo, R.; Martino, N.A.; Filioli Uranio, M.; Ariu, F.; Amati, F.; Sardanelli, A.M.; Linsalata, V.; Ferruzzi, M.G.; et al. Prooxidant effects of verbascoside, a bioactive compound from olive oil mill wastewater, on in vitro developmental potential of ovine prepubertal oocytes and bioenergetic/oxidative stress parameters of fresh and vitrified oocytes. BioMed Res. Int. 2014, 878062. [Google Scholar] [CrossRef] [Green Version]
- Lundvig, D.M.; Pennings, S.W.; Brouwer, K.M.; Mtaya-Mlangwa, M.; Mugonzibwa, E.; Kuijpers-Jagtman, A.M.; Wagener, F.A.; Von den Hoff, J.W. Cytoprotective responses in HaCaT keratinocytes exposed to high doses of curcumin. Exp. Cell Res. 2015, 336, 298–307. [Google Scholar] [CrossRef]
- Venza, M.; Visalli, M.; Beninati, C.; De Gaetano, G.V.; Teti, D.; Venza, I. Cellular mechanisms of oxidative stress and action in melanoma. Oxid. Med. Cell. Longev. 2015, 481782. [Google Scholar] [CrossRef] [Green Version]
- Kelfkens, G.; de Gruijl, F.R.; van der Leun, J.C. Tumorigenesis by short-wave ultraviolet A: Papillomas versus squamous cell carcinomas. Carcinogenesis 1991, 12, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Ragione, F.D.; Cucciolla, V.; Borriello, A.; Pietra, V.D.; Pontoni, G.; Racioppi, L.; Manna, C.; Galletti, P.; Zappia, V. Hydroxytyrosol, a natural molecule occurring in olive oil, induces cytochrome c-dependent apoptosis. Biochem. Biophys. Res. Commun. 2000, 278, 733–739. [Google Scholar] [CrossRef]
- Villiotou, V.; Deliconstantinos, G. Nitric-Oxide, Peroxynitrite and Nitroso-Compounds Formation by Ultraviolet-a (Uva) Irradiated Human Squamous-Cell Carcinoma Potential Role of Nitric-Oxide in Cancer Prognosis. Anticancer Res. 1995, 15, 931–942. [Google Scholar] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Rodrigo, R.; Bosco, C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 142, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Mumtaz Hadi, S. A prooxidant mechanism for the anticancer and chemopreventive properties of plant polyphenols. Curr. Drug Targets 2012, 13, 1738–1749. [Google Scholar] [CrossRef]
- Korkina, L.; De Luca, C.; Pastore, S. Plant polyphenols and human skin: Friends or foes. Ann. N. Y. Acad. Sci. 2012, 1259, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Leo, L.; Leone, A.; Longo, C.; Lombardi, D.A.; Raimo, F.; Zacheo, G. Antioxidant compounds and antioxidant activity in “early potatoes”. J. Agric. Food Chem. 2008, 56, 4154–4163. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Bruno, A.; Linsalata, V.; Minervini, F.; Garbetta, A.; Tufariello, M.; Mita, G.; Logrieco, A.F.; Bleve, G.; Cardinali, A. Fermented Apulian table olives: Effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. 2018, 248, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Teissedre, P.L.; Frankel, E.N.; Waterhouse, A.L.; Peleg, H.; German, J.B. Inhibition of In Vitro Human LDL Oxidation by Phenolic Antioxidants from Grapes and Wines. J. Sci. Food Agric. 1996, 70, 55–61. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Piraino, S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar. Drugs 2013, 11, 1728–1762. [Google Scholar] [CrossRef] [Green Version]
- Potapovich, A.I.; Kostyuk, V.A.; Kostyuk, T.V.; De Luca, C.; Korkina, L.G. Effects of pre- and post-treatment with plant polyphenols on human keratinocyte responses to solar UV. Inflamm. Res. 2013, 62, 773–780. [Google Scholar] [CrossRef]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Polyphenols (µg/mL) | Cellina | Coratina | Organic Coratina | Asprolia | Koroneiki | Lianolia |
|---|---|---|---|---|---|---|
| Hydroxytyrosol | 563.1 | 407.2 | 697.3 | 690.4 | 201.5 | 791.8 |
| Tyrosol | 105.6 | 122.0 | 132.0 | 174.3 | 58.0 | 163.8 |
| Caffeic Acid | 22.7 | 4.5 | 4.0 | 18.7 | 6.7 | 7.5 |
| Cumaric Acid | 3.7 | 2.8 | 3.3 | 37.7 | 6.0 | 4.1 |
| Verbascoside | 3.0 | 159.7 | 120.3 | 11.1 | nd * | 65.7 |
| Isoverbascoside | nd * | 19.5 | 15.4 | nd * | nd * | nd * |
| Caffeoyl-6-secologanoside | nd * | 5.3 | nd * | 18.5 | 4.4 | 4.6 |
| Comselogoside | nd * | 11.2 | 15.7 | 41.5 | 6.1 | 10.2 |
| Total | 698.1 | 732.2 | 988.0 | 992.2 | 282.7 | 1047.7 |
| Polyphenol Concentrations (µg/mL) | Cellina | Coratina | Organic Coratina | Koroneiki | Lianolia | Asprolia |
|---|---|---|---|---|---|---|
| 0.01 | 20.6 ± 0.8 a,A | 14.0 ±1.1 a,A | 14.3 ± 1.2 a,A | 18.3 ± 2.1 a,A | 28.4 ± 2.7 a,B | 14.6 ± 0.1 a,A |
| 0.03 | 28.3 ± 1,0 b,A | 50.9 ±6.2 b,B | 50.4 ± 7.6 b,B | 84.2 ± 6.8 b,C | 43.7 ± 4.2 b,B | 29.3 ± 0.2 b,A |
| 0.1 | 78.3 ± 0.9 c,A | 64.2 ±8.1 b,A | 64.1 ± 9.7 b,A | 90.5 ± 3.2 b,B | 81.5 ± 7.9 b,B | 83.4 ± 0.6 c,A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecci, R.M.; D’Antuono, I.; Cardinali, A.; Garbetta, A.; Linsalata, V.; Logrieco, A.F.; Leone, A. Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes. Molecules 2021, 26, 2153. https://doi.org/10.3390/molecules26082153
Lecci RM, D’Antuono I, Cardinali A, Garbetta A, Linsalata V, Logrieco AF, Leone A. Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes. Molecules. 2021; 26(8):2153. https://doi.org/10.3390/molecules26082153
Chicago/Turabian StyleLecci, Raffaella Marina, Isabella D’Antuono, Angela Cardinali, Antonella Garbetta, Vito Linsalata, Antonio F. Logrieco, and Antonella Leone. 2021. "Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes" Molecules 26, no. 8: 2153. https://doi.org/10.3390/molecules26082153
APA StyleLecci, R. M., D’Antuono, I., Cardinali, A., Garbetta, A., Linsalata, V., Logrieco, A. F., & Leone, A. (2021). Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes. Molecules, 26(8), 2153. https://doi.org/10.3390/molecules26082153








