The Impact of Dextran Sodium Sulfate-Induced Gastrointestinal Injury on the Pharmacokinetic Parameters of Donepezil and Its Active Metabolite 6-O-desmethyldonepezil, and Gastric Myoelectric Activity in Experimental Pigs
Abstract
:1. Introduction
2. Results
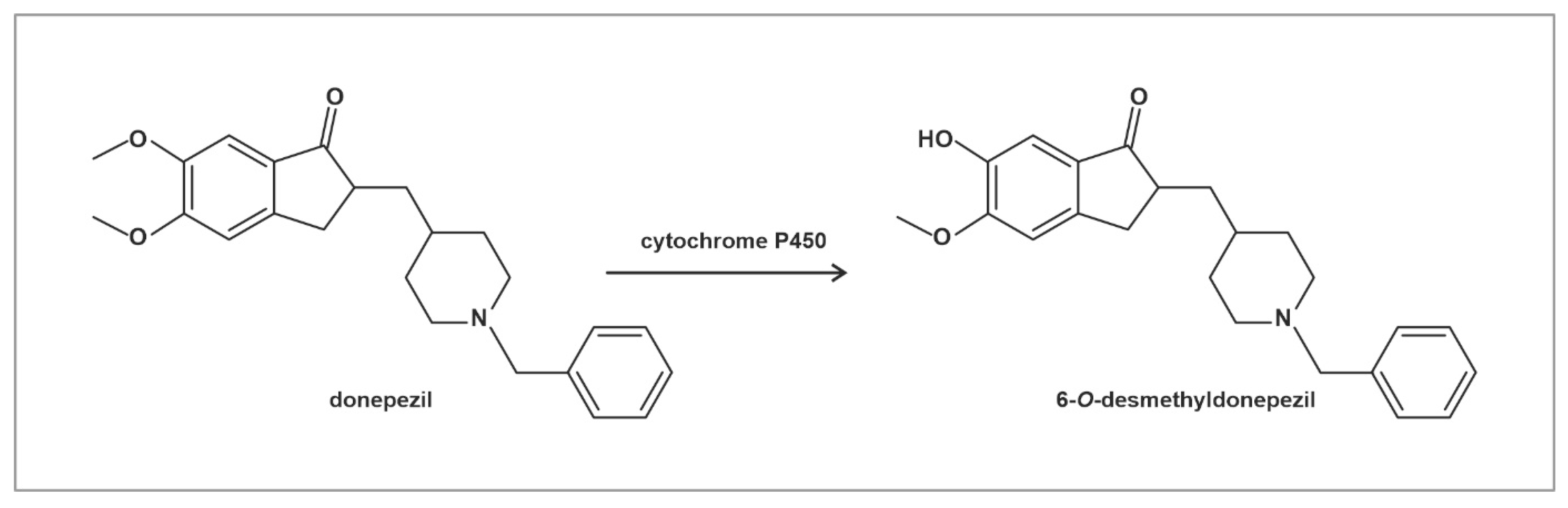
2.1. Absorption and Distribution of Donepezil and 6-O-desmethyldonepezil
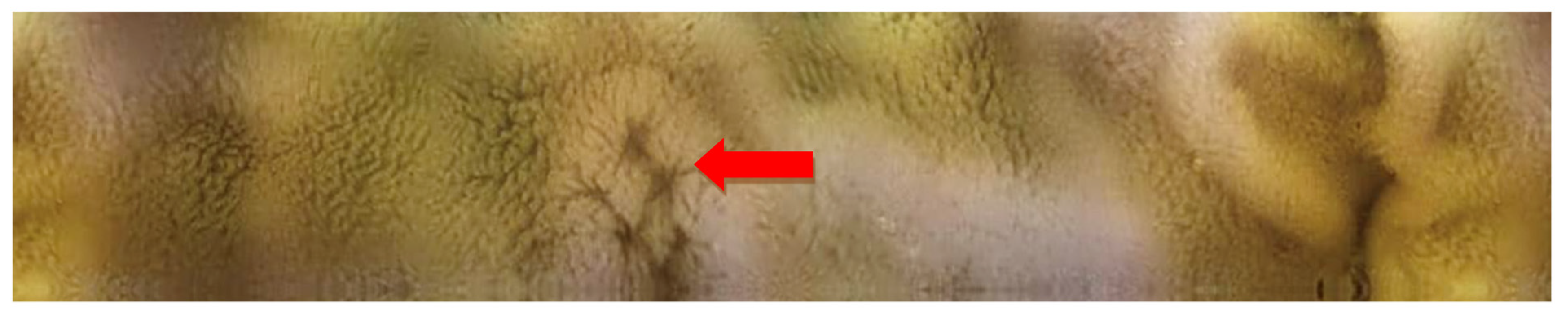
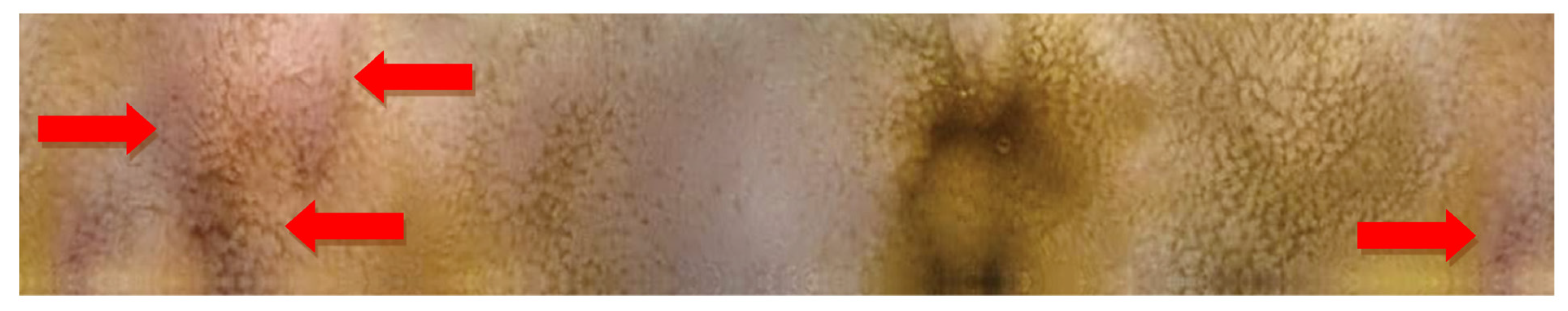
2.2. Capsule Enteroscopy
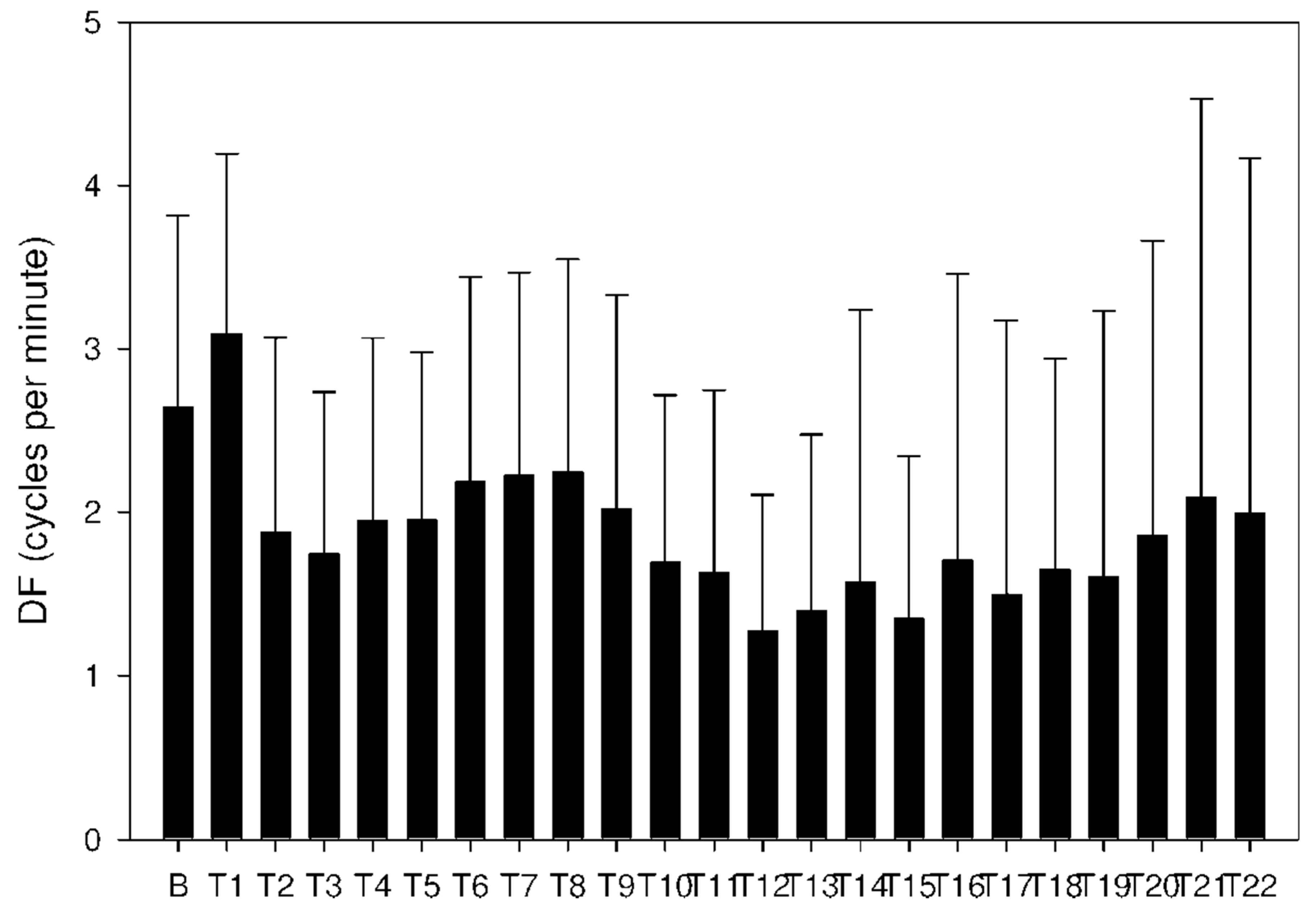
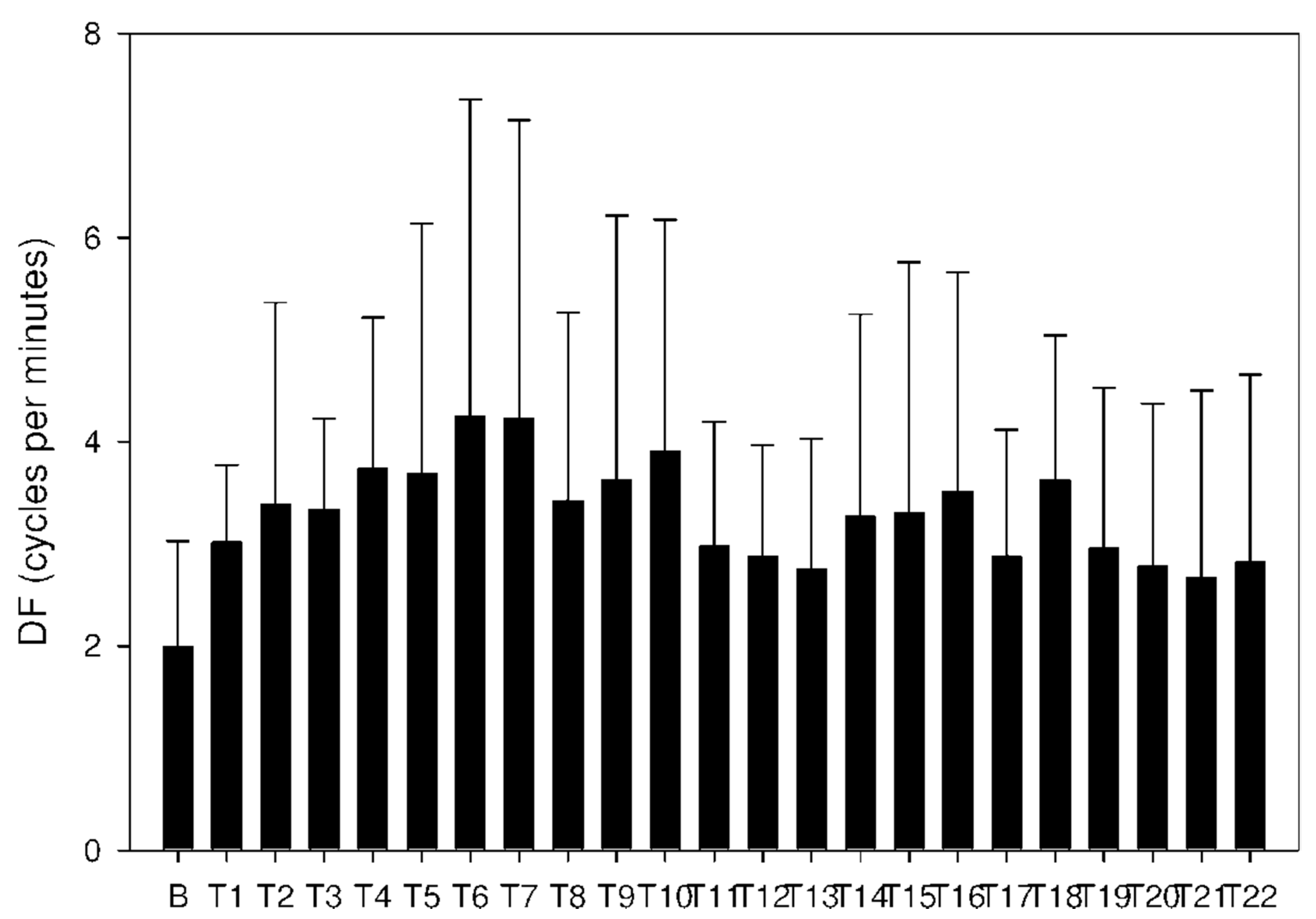
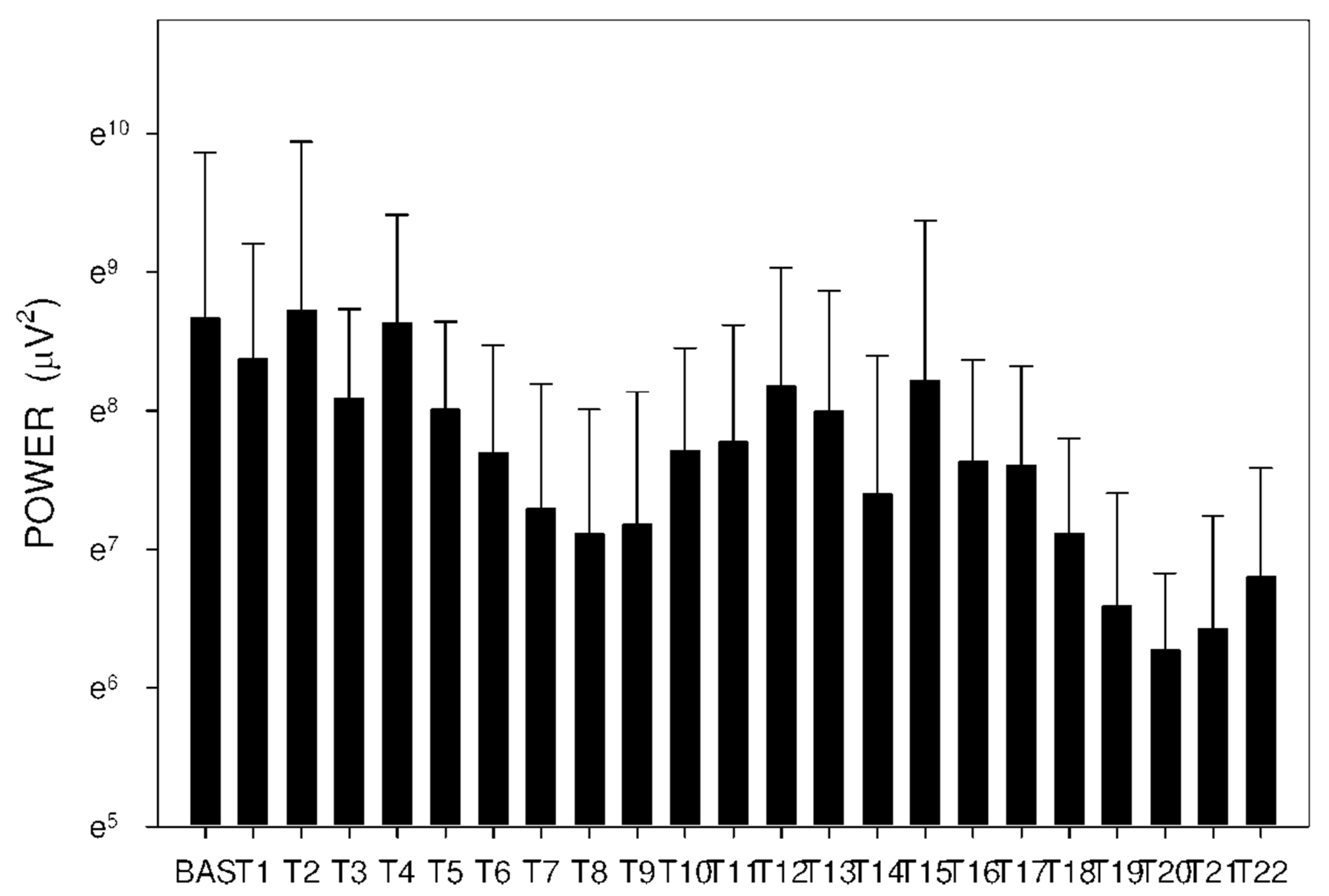
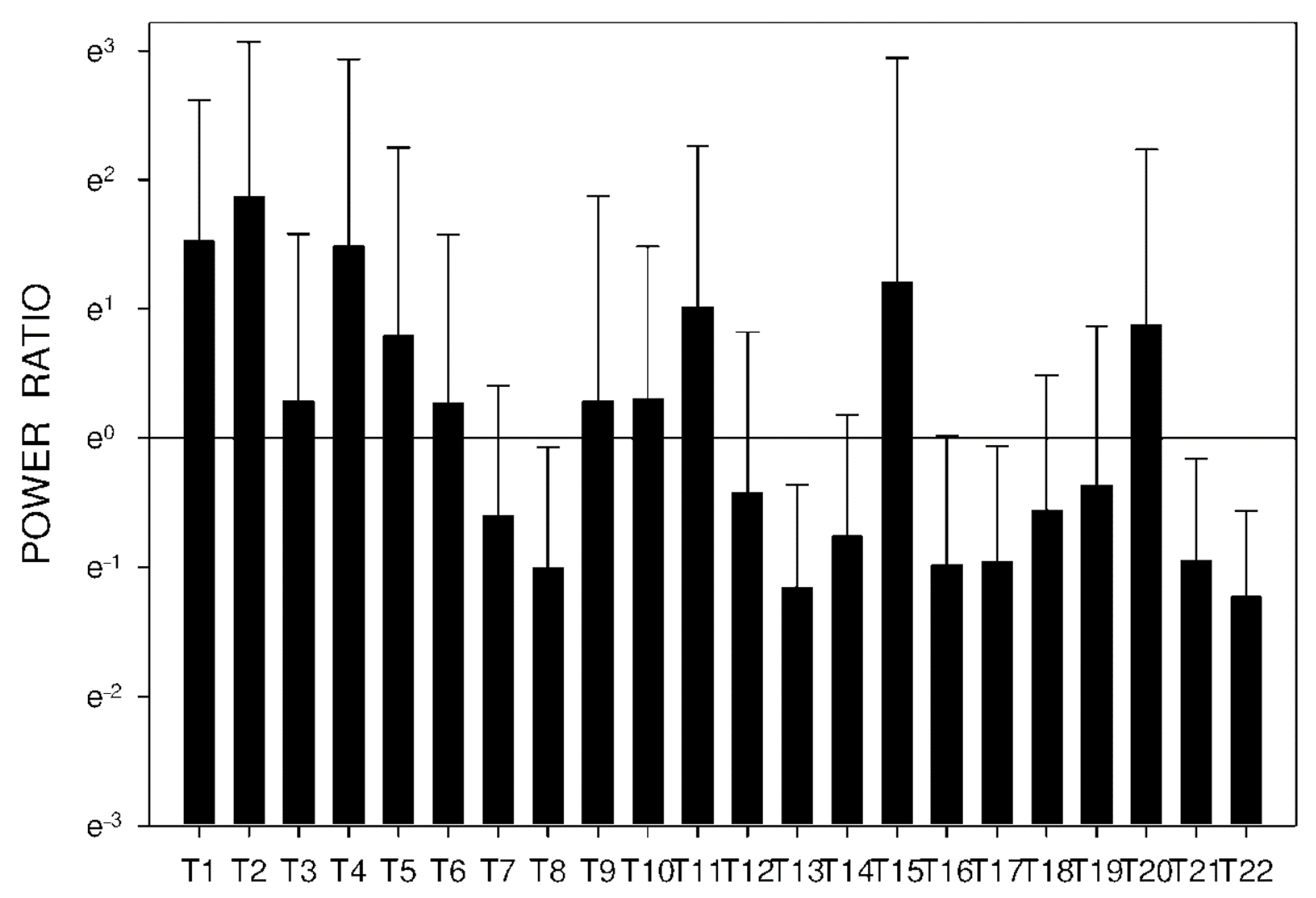
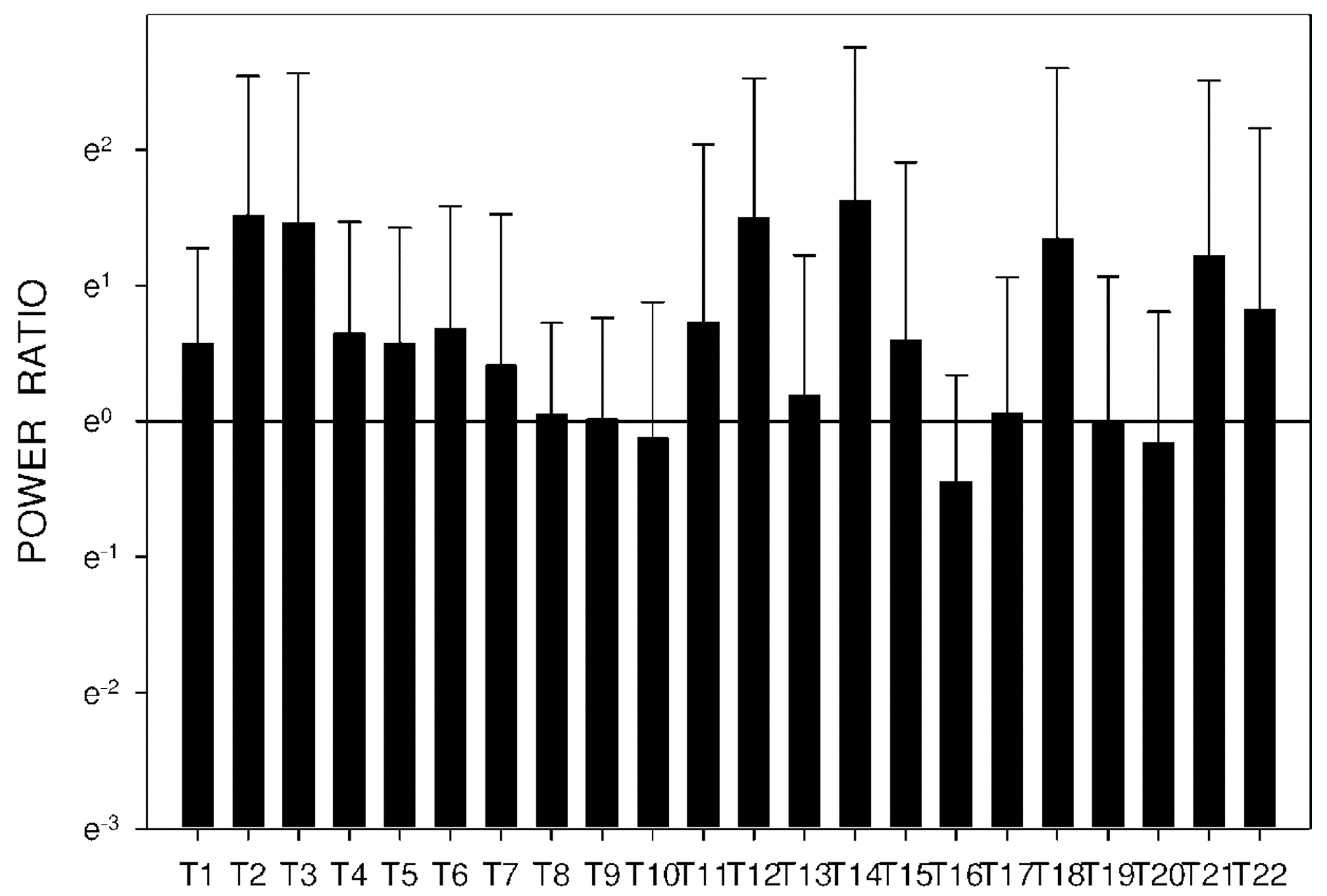
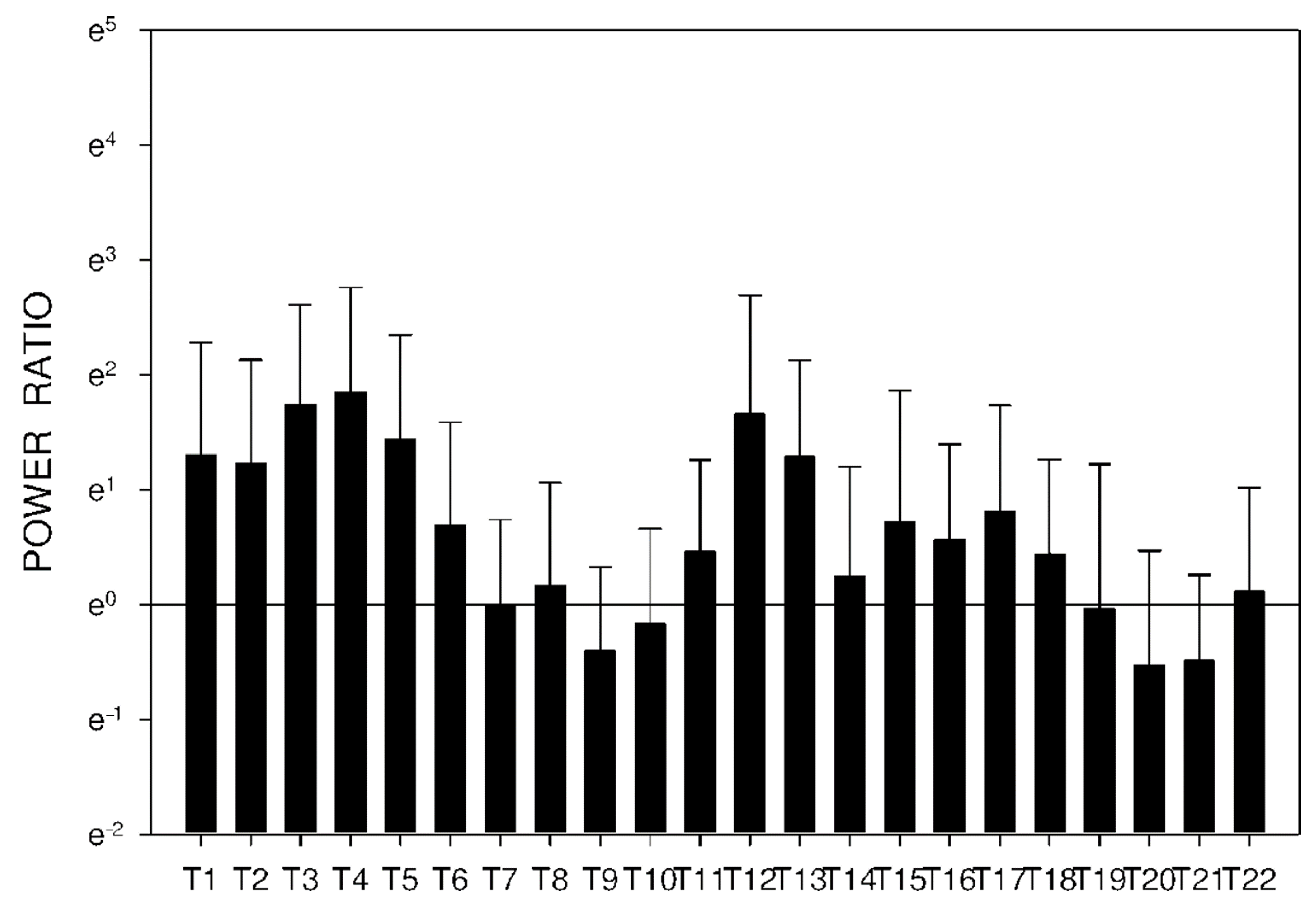
2.3. Electrogastrography
3. Discussion
4. Methods
4.1. Animals
4.2. Design of the Study
4.3. Electrogastrography
4.4. Capsule Endoscopy
4.5. LC–MS Analysis of Donepezil and 6-O-desmethyldonepezil
4.5.1. Chemicals and Reagents
4.5.2. Sample Preparation
4.5.3. LC–MS/MS Analysis
4.6. Statistical Analysis
4.7. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, CD001190. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, B. Donepezil: A review. Expert Opin. Drug. Metab. Toxicol. 2005, 1, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Taniguchi, S.; Yoshimura, T. Correlation of the intrinsic clearance of donepezil (Aricept) between in vivo and in vitro studies in rat, dog and human. Xenobiotica 1999, 29, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Atto, R.A.; Behnan, A.B.; Abachi, F.T. In vitro kinetic study of donepezil N-oxide metabolites. Irq. J. Pharm. 2011, 11, 1–9. [Google Scholar]
- Prvulovic, D.; Schneider, B. Pharmacokinetic and pharmacodynamic evaluation of donepezil for the treatment of Alzheimer’s disease. Expert Opin. Drug. Metab. Toxicol. 2014, 10, 1039–1050. [Google Scholar] [CrossRef]
- Campbell, N.L.; Perkins, A.J.; Gao, S.; Skaar, T.C.; Li, L.; Hendrie, H.C.; Fowler, N.; Callahan, C.M.; Boustani, M.A. Adherence and tolerability of Alzheimer’s disease medications: A pragmatic randomized trial. J. Am. Geriatr. Soc. 2017, 65, 1497–1504. [Google Scholar] [CrossRef]
- Valis, M.; Masopust, J.; Vysata, O.; Hort, J.; Dolezal, R.; Tomek, J.; Misik, J.; Kuca, K.; Karasova, J.Z. Concentration of donepezil in the cerebrospinal fluid of AD patients: Evaluation of dosage sufficiency in standard treatment strategy. Neurotox. Res. 2017, 31, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, A.B. Small intestinal disorders in the elderly. Best Pr. Res. Clin. Gastroenterol. 2009, 23, 861–874. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef]
- Hansen, K.J.; Wilson, D.B.; Craven, T.E.; Pearce, J.D.; English, W.P.; Edwards, M.S.; Ayerdi, J.; Burke, G.L. Mesenteric artery disease in the elderly. J. Vasc. Surg. 2004, 40, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bures, J.; Cyrany, J.; Kohoutova, D.; Förstl, M.; Rejchrt, S.; Kvetina, J.; Vorisek, V.; Kopacova, M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010, 16, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; McAlindon, M.E. NSAIDs and the small bowel. Curr. Opin. Gastroenterol. 2018, 34, 175–182. [Google Scholar] [CrossRef]
- Tacheci, I.; Kvetina, J.; Kunes, M.; Edakkanambeth Varayil, J.; Ali, S.M.; Pavlik, M.; Kopacova, M.; Rejchrt, S.; Bures, J.; Pleskot, M. Electrogastrography in experimental pigs: The influence of gastrointestinal injury induced by dextran sodium sulphate on porcine gastric erythromycin-stimulated myoelectric activity. Neuro. Endocrinol. Lett. 2011, 32, 131–136. [Google Scholar] [PubMed]
- Lackeyram, D.; Young, D.; Kim, C.J.; Yang, C.; Archbold, T.L.; Mine, Y.; Fan, M.Z. Interleukin-10 is differentially expressed in the small intestine and the colon experiencing chronic inflammation and ulcerative colitis induced by dextran sodium sulfate in young pigs. Physiol. Res. 2017, 66, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Kvetina, J.; Radochova, V.; Tacheci, I.; Peterova, E.; Herman, D.; Dolezal, R.; Kopacova, M.; Rejchrt, S.; Douda, T.; et al. The pharmacokinetic parameters and the effect of a single and repeated doses of memantine on gastric myoelectric activity in experimental pigs. PLoS ONE 2020, 15, e0227781. [Google Scholar] [CrossRef] [PubMed]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug. Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Suenderhauf, C.; Parrott, N. A physiologically based pharmacokinetic model of the minipig: Data compilation and model implementation. Pharm. Res. 1995, 30, 1–15. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Porcine models of digestive disease: The future of large animal translational research. Transl. Res. 2015, 166, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Tiseo, P.J.; Perdomo, C.A.; Friedhoff, L.T. Metabolism and elimination of 14C-donepezil in healthy volunteers: A single-dose study. Br. J. Clin. Pharm. 1998, 46, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Heydorn, W.E. Donepezil (E2020): A new acetylcholinesterase inhibitor. Review of its pharmacology, pharmacokinetics, and utility in the treatment of Alzheimer’s disease. Expert Opin. Investig. Drug. 1997, 10, 1527–1535. [Google Scholar] [CrossRef]
- Amjad, M.W.; Alotaibi, N.M. Formulation and in vitro characterization of donepezil-loaded chitosan nanoparticles. J. Pharm. Res. Int. 2020, 32, 57598. [Google Scholar] [CrossRef]
- Gu, F.G.; Fan, H.M.; Cong, Z.X.; Li, S.; Wang, Y.; Wu, C.Z. Preparation, characterization, and in vivo pharmacokinetics of thermosensitive in situ nasal gel of donepezil hydrochloride. Acta Pharm. 2020, 70, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, Z.P.; Wang, K.R.; Shi, J.; Liu, W.M.; Cheng, X.F.; Zhu, G.F.; Wang, Y.L.; Zhao, Y.Y.; Qiao, Z.P.; Wu, A.G.; et al. The development of advanced structural framework as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112180. [Google Scholar] [CrossRef] [PubMed]
- Darreh-Shori, T.; Meurling, L.; Pettersson, T.; Hugosson, K.; Hellström-Lindahl, E.; Andreasen, N.; Minthon, L.; Nordberg, A. Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer’s disease following chronic donepezil treatment. J. Neural Transm. (Vienna) 2006, 113, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, P.J.; Rogers, S.L.; Friedhoff, L.T. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following evening administration. Br. J. Clin. Pharm. 1998, 46, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darreh-Shori, T.; Soininen, H. Effects of cholinesterase inhibitors on the activities and protein levels of cholinesterases in the cerebrospinal fluid of patients with Alzheimer’s disease: A review of recent clinical studies. Curr. Alzheimer Res. 2010, 7, 67–73. [Google Scholar] [CrossRef]
- Geerts, H.; Guillaumat, P.O.; Grantham, C.; Bode, W.; Anciaux, K.; Sachak, S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005, 1033, 186–193. [Google Scholar] [CrossRef]
- Groppa, F.; Coin, A.; De Rosa, G.; Granziera, S.; Alexopoulos, C.; Pamio, M.V.; Padrini, R. Monitoring plasma levels of donepezil, 5-O-desmethyl-donepezil, 6-O-desmethyl-donepezil, and donepezil-N-oxide by a novel HPLC method in patients with Alzheimer disease. Drug Monit. 2016, 38, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C.; Rogers, S.J. Donepezil: A clinical review of current and emerging indications. Expert Opin. Pharm. 2004, 5, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Jun, D.; Hrabinová, M.; Tacheci, I.; Kvetina, J.; Pavlik, M.; Rejchrt, S.; Douda, T.; Kunes, M.; Kuca, K.; et al. Impact of tacrine and 7-methoxytacrine on gastric myoelectrical activity assessed using electrogastrography in experimental pigs. Neuro. Endocrinol. Lett. 2015, 36, 150–155. [Google Scholar]
- Bures, J.; Kvetina, J.; Pavlik, M.; Kunes, M.; Kopacova, M.; Rejchrt, S.; Jun, D.; Hrabinova, M.; Kuca, K.; Tacheci, I. Impact of paraoxon followed by acetylcholinesterase reactivator HI-6 on gastric myoelectric activity in experimental pigs. Neuro. Endocrinol. Lett. 2013, 34, 79–83. [Google Scholar] [PubMed]
- Kopacova, M.; Tacheci, I.; Kvetina, J.; Bures, J.; Kunes, M.; Spelda, S.; Tycova, V.; Svoboda, Z.; Rejchrt, S. Wireless video capsule enteroscopy in preclinical studies: Methodical design of its applicability in experimental pigs. Dig. Dis. Sci. 2010, 55, 626–630. [Google Scholar] [CrossRef] [Green Version]
- Tacheci, I.; Kvetina, J.; Bures, J.; Osterreicher, J.; Kunes, M.; Pejchal, J.; Rejchrt, S.; Spelda, S.; Kopacova, M. Wireless capsule endoscopy in enteropathy induced by nonsteroidal anti-inflammatory drugs in pigs. Dig. Dis. Sci. 2010, 55, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Zdarova Karasova, J.; Sestak, V.; Korabecny, J.; Mezeiova, E.; Palicka, V.; Kuca, K.; Mzik, M. 1-Benzyl-4-methylpiperidinyl moiety in donepezil: The priority ticket across the blood-brain-barrier in rats. J. Chrom. B 2018, 1092, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Tveden-Nyborg, P.; Bergmann, T.K.; Lykkesfeldt, J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin. Pharm. Toxicol. 2018, 123, 233–235. [Google Scholar]
- Explanatory Report on the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS 123); Council of Europe: Strasbourg, France, 2009.
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
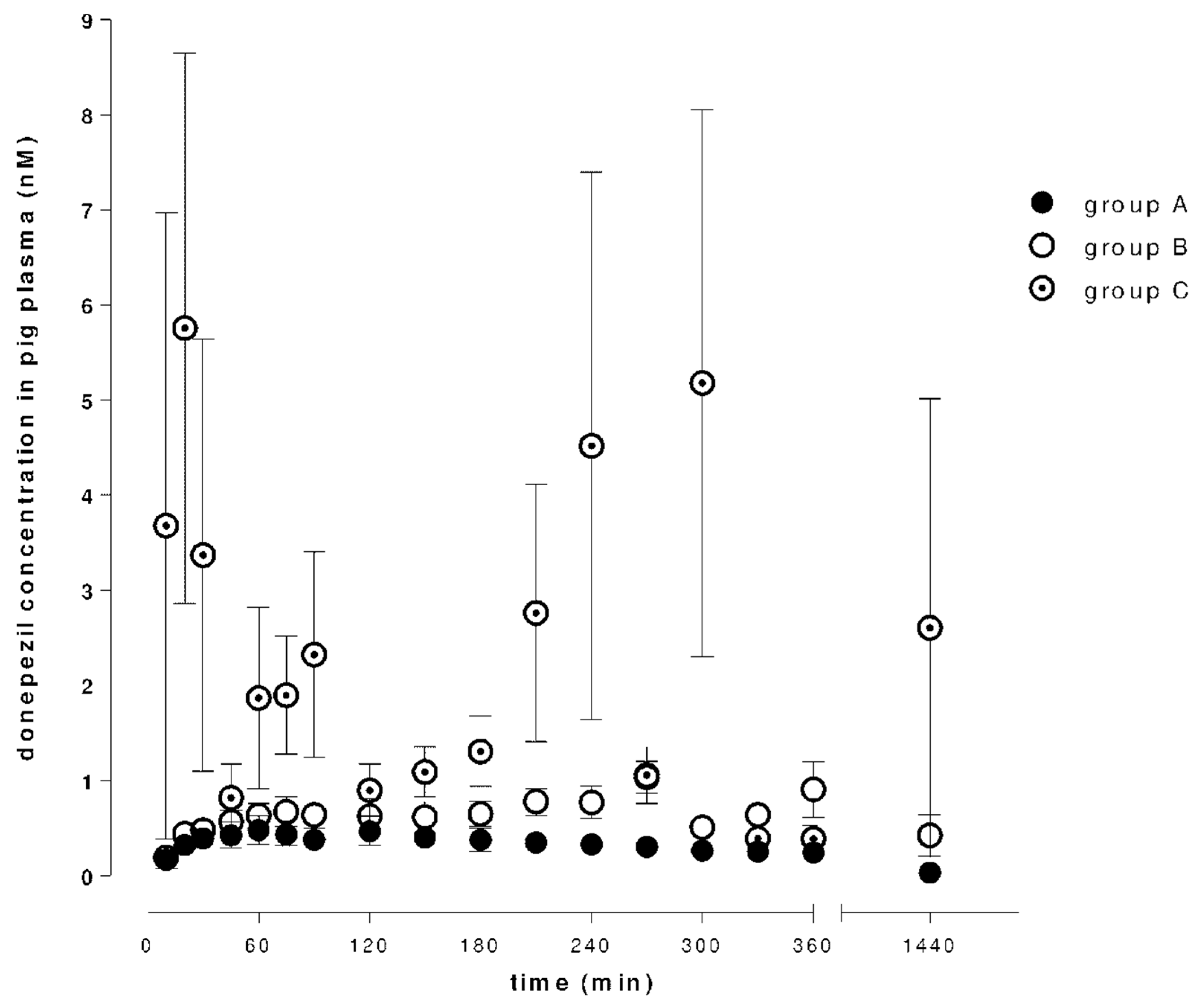





| Groups | Donepezil | 6-O-desmethyldonepezil | ||||
|---|---|---|---|---|---|---|
| Cmax ng/mL | Tmax min | AUClast min.ng/mL | Cmax ng/mL | Tmax min | AUClast min.ng/mL | |
| A | 0.55 ± 0.13 | 87.50 ± 14.32 | 232.5 ± 41.72 | 0.21 ± 0.007 | 125.0 ± 13.07 | 22.1 ± 8.50 |
| B | 1.25 ± 0.19 | 267.5 ± 38.79 | 877.67 ± 234.8 | 0.21 ± 0.06 | 275.0 ± 24.91 | 121.52 ± 69.58 |
| C | 12.08 ± 2.28 | 306.67 ± 209.24 | 1913.67 ± 872.77 | 0.52 ± 0.15 | 157.5 ± 35.66 | 208.3 ± 83.1 |
| Tissue | Donepezil (nM) | Ratio | O-DNP (nM) | Ratio |
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | |||
| plasma | 1.77 ± 0.35 | 1.00 | 0.43 ± 0.10 | 1.00 |
| stomach | 72.16 ± 15.89 | 40.76 | 1.45 ± 0.22 | 3.37 |
| proximal jejunum | 370.53 ± 112.94 | 209.34 | 17.25 ± 4.02 | 40.12 |
| mid jejunum | 104.61 ± 68.27 | 59.10 | 4.33 ± 0.88 | 10.07 |
| ileum | 14.42 ± 1.30 | 8.15 | 3.96 ± 1.19 | 9.21 |
| colon | 14.61 ± 1.54 | 8.25 | 6.60 ± 4.69 | 15.35 |
| muscle | 6.26 ± 0.29 | 3.54 | 9.91 ± 0.50 | 23.05 |
| fat | 5.68 ± 0.74 | 3.20 | 6.46 ± 2.25 | 15.02 |
| skin | 5.22 ± 0.68 | 2.95 | 7.88 ± 0.94 | 18.33 |
| spleen | 15.34 ± 1.74 | 8.66 | 3.26 ± 0.27 | 7.58 |
| liver | 127.14 ± 8.57 | 71.83 | 44.33 ± 5.31 | 103.09 |
| kidney | 25.56 ± 3.03 | 14.44 | 21.67 ± 3.44 | 50.40 |
| heart | 4.38 ± 0.21 | 2.48 | 1.00 ± 0.17 | 2.33 |
| lung | 20.73 ± 1.13 | 11.71 | 3.73 ± 0.43 | 8.67 |
| medulla | 7.99 ± 1.22 | 4.51 | 8.36 ± 1.68 | 19.44 |
| medulla oblongata | 7.02 ± 0.64 | 3.97 | 7.03 ± 0.97 | 16.35 |
| front lobe | 5.21 ± 0.32 | 2.94 | 3.95 ± 0.25 | 9.19 |
| cerebellum | 4.73 ± 0.20 | 2.67 | 3.60 ± 0.15 | 8.37 |
| pituitary gland | 13.12 ± 0.99 | 7.41 | 8.40 ± 0.48 | 19.54 |
| hippocampus | 5.22 ± 0.44 | 2.95 | 4.31 ± 0.56 | 10.02 |
| diencephalon | 4.58 ± 0.37 | 2.58 | 3.82 ± 0.20 | 8.88 |
| urine | 45.88 ± 9.68 | 25.92 | 62.90 ± 12.48 | 146.28 |
| bile | 601.63 ± 112.12 | 339.90 | 444.35 ± 132.62 | 1033.37 |
| Period | A: Donepezil 5 mg Mean ± Std Dev Median IQR | B: Donepezil 10 mg Mean ± Std Dev Median IQR | C: Donepezil + DSS Mean ± Std Dev Median IQR | Significance p Value |
|---|---|---|---|---|
| Basal | 2.2 ± 1.1 | 2.6 ± 1.2 | 2.0 ± 1.0 | A-B: NS |
| 3.1 | 3.1 | 2.2 | A-C: NS | |
| 1.1–3.1 | 1.2–3.5 | 0.9–3.1 | B-C: p < 0.001 | |
| T1 | 2.3 ± 1.1 | 3.1 ± 1.1 | 3.0 ± 0.8 | A-B: p < 0.001 |
| 2.8 | 3.3 | 3.3 | A-C: p < 0.001 | |
| 0.9–3.3 | 2.6–3.5 | 3.1–3.5 | B-C: NS | |
| T4 | 1.0 ± 0.5 | 1.9 ± 1.1 | 3.7 ± 1.5 | A-B: p < 0.001 |
| 0.9 | 1.4 | 3.3 | A-C: p < 0.001 | |
| 0.7–1.2 | 0.9–2.8 | 3.3–4.0 | B-C: p < 0.001 | |
| T7 | 1.0 ± 0.6 | 2.2 ± 1.2 | 4.2 ± 2.9 | A-B: p < 0.001 |
| 0.9 | 2.6 | 3.1 | A-C: p < 0.001 | |
| 0.7–1.2 | 1.2–3.1 | 2.8–7.0 | B-C: p < 0.001 | |
| T9 | 2.5 ± 3.6 | 2.0 ± 1.3 | 3.6 ± 2.6 | A-B: p = 0.011 |
| 0.9 | 1.4 | 3.1 | A-C: p < 0.001 | |
| 0.7–1.4 | 0.9–3.1 | 1.4–3.5 | B-C: p < 0.001 | |
| T11 | 1.3 ± 1.4 | 1.6 ± 1.1 | 3.0 ± 1.2 | A-B: p = 0.017 |
| 0.9 | 1.3 | 3.3 | A-C: p < 0.001 | |
| 0.7–1.4 | 0.7–2.6 | 2.6–3.5 | B-C: p < 0.001 | |
| T15 | 1.6 ± 1.8 | 1.3 ± 1.0 | 3.3 ± 2.5 | A-B: NS |
| 0.9 | 0.9 | 3.1 | A-C: p < 0.001 | |
| 0.7–1.4 | 0.7–1.4 | 1.6–3.8 | B-C: p < 0.001 | |
| T18 | 1.4 ± 0.9 | 1.6 ± 1.3 | 3.6 ± 1.4 | A-B: NS |
| 1.2 | 1.2 | 3.3 | A-C: p < 0.001 | |
| 0.9–1.4 | 0.7–2.1 | 3.3–4.0 | B-C: p < 0.001 | |
| T22 | 1.5 ± 1.8 | 2.0 ± 2.2 | 2.8 ± 1.8 | A-B: NS |
| 1.2 | 1.2 | 3.1 | A-C: p < 0.001 | |
| 0.7–1.4 | 0.9–2.6 | 1.2–3.8 | B-C: p < 0.001 |
| Period | A: Donepezil 5 mg Mean ± Std Dev Median IQR | B: Donepezil 10 mg Mean ± Std Dev Median IQR | C: Donepezil + DSS Mean ± Std Dev Median IQR | Significance p Value |
|---|---|---|---|---|
| Basal | 2632 ± 1855 | 1938 ± 1658 | 5812 ± 13433 | A-B: NS |
| 2153 | 1441 | 1438 | A-C: NS | |
| 1226–3616 | 825–2550 | 668–4139 | B-C: NS | |
| T1 | 7459 ± 14605 | 3068 ± 3963 | 4330 ± 5648 | A-B: p = 0.001 |
| 2772 | 1957 | 2262 | A-C: NS | |
| 1555–5703 | 592–3426 | 1392–5031 | B-C: NS | |
| T3 | 4337 ± 11106 | 4891 ± 12753 | 3254 ± 2955 | A-B: NS |
| 570 | 579 | 2205 | A-C: p < 0.001 | |
| 296–1748 | 200–1555 | 1370–3932 | B-C: p < 0.001 | |
| T8 | 769 ± 1347 | 1015 ± 820 | 1227 ± 1780 | A-B: p < 0.001 |
| 408 | 803 | 745 | A-C: p < 0.001 | |
| 205–712 | 414–1414 | 461–1064 | B-C: NS | |
| T10 | 2123 ± 7376 | 838 ± 1007 | 2234 ± 2457 | A-B: NS |
| 342 | 587 | 739 | A-C: p < 0.001 | |
| 209–817 | 168–1180 | 304–4497 | B-C: p < 0.001 | |
| T12 | 762 ± 1623 | 5176 ± 11805 | 3569 ± 4773 | A-B: p = 0.002 |
| 346 | 703 | 1114 | A-C: p < 0.001 | |
| 191–637 | 193–3181 | 378–5583 | B-C: NS | |
| T16 | 1475 ± 4554 | 652 ± 834 | 2068 ± 2235 | A-B: NS |
| 319 | 269 | 1201 | A-C: p < 0.001 | |
| 184–603 | 113–946 | 473–2740 | B-C: p < 0.001 | |
| T20 | 3023 ± 9753 | 779 ± 1630 | 531 ± 393 | A-B: p < 0.001 |
| 423 | 215 | 425 | A-C: NS | |
| 208–1129 | 77–987 | 184–925 | B-C: p = 0.007 | |
| T22 | 527 ± 426 | 3061 ± 11286 | 900 ± 1074 | A-B: NS |
| 422 | 253 | 559 | A-C: NS | |
| 202–681 | 124–993 | 239–1264 | B-C: NS |
| Compound | Parent Ion | Normalized Collision Energy | Selected Product Ion | tR (min) |
|---|---|---|---|---|
| Donepezil | 380.22 | 35 | 362.21 | 3.08 |
| 6-O-desmethyldonepezil | 366.21 | 35 | 348.20 | 2.95 |
| D7-donepezil | 387.26 | 35 | 369.25 | 3.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bures, J.; Tacheci, I.; Kvetina, J.; Radochova, V.; Prchal, L.; Kohoutova, D.; Valis, M.; Novak, M.; Dolezal, R.; Kopacova, M.; et al. The Impact of Dextran Sodium Sulfate-Induced Gastrointestinal Injury on the Pharmacokinetic Parameters of Donepezil and Its Active Metabolite 6-O-desmethyldonepezil, and Gastric Myoelectric Activity in Experimental Pigs. Molecules 2021, 26, 2160. https://doi.org/10.3390/molecules26082160
Bures J, Tacheci I, Kvetina J, Radochova V, Prchal L, Kohoutova D, Valis M, Novak M, Dolezal R, Kopacova M, et al. The Impact of Dextran Sodium Sulfate-Induced Gastrointestinal Injury on the Pharmacokinetic Parameters of Donepezil and Its Active Metabolite 6-O-desmethyldonepezil, and Gastric Myoelectric Activity in Experimental Pigs. Molecules. 2021; 26(8):2160. https://doi.org/10.3390/molecules26082160
Chicago/Turabian StyleBures, Jan, Ilja Tacheci, Jaroslav Kvetina, Vera Radochova, Lukas Prchal, Darina Kohoutova, Martin Valis, Martin Novak, Rafael Dolezal, Marcela Kopacova, and et al. 2021. "The Impact of Dextran Sodium Sulfate-Induced Gastrointestinal Injury on the Pharmacokinetic Parameters of Donepezil and Its Active Metabolite 6-O-desmethyldonepezil, and Gastric Myoelectric Activity in Experimental Pigs" Molecules 26, no. 8: 2160. https://doi.org/10.3390/molecules26082160
APA StyleBures, J., Tacheci, I., Kvetina, J., Radochova, V., Prchal, L., Kohoutova, D., Valis, M., Novak, M., Dolezal, R., Kopacova, M., Rejchrt, S., Sestak, V., Knoblochova, V., Peterova, E., & Zdarova Karasova, J. (2021). The Impact of Dextran Sodium Sulfate-Induced Gastrointestinal Injury on the Pharmacokinetic Parameters of Donepezil and Its Active Metabolite 6-O-desmethyldonepezil, and Gastric Myoelectric Activity in Experimental Pigs. Molecules, 26(8), 2160. https://doi.org/10.3390/molecules26082160






