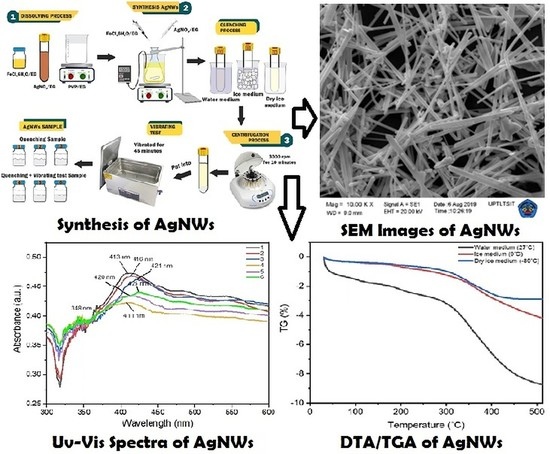
The Quenching and Sonication Effect on the Mechanical Strength of Silver Nanowires Synthesized Using the Polyol Method
Abstract
:1. Introduction
2. Results
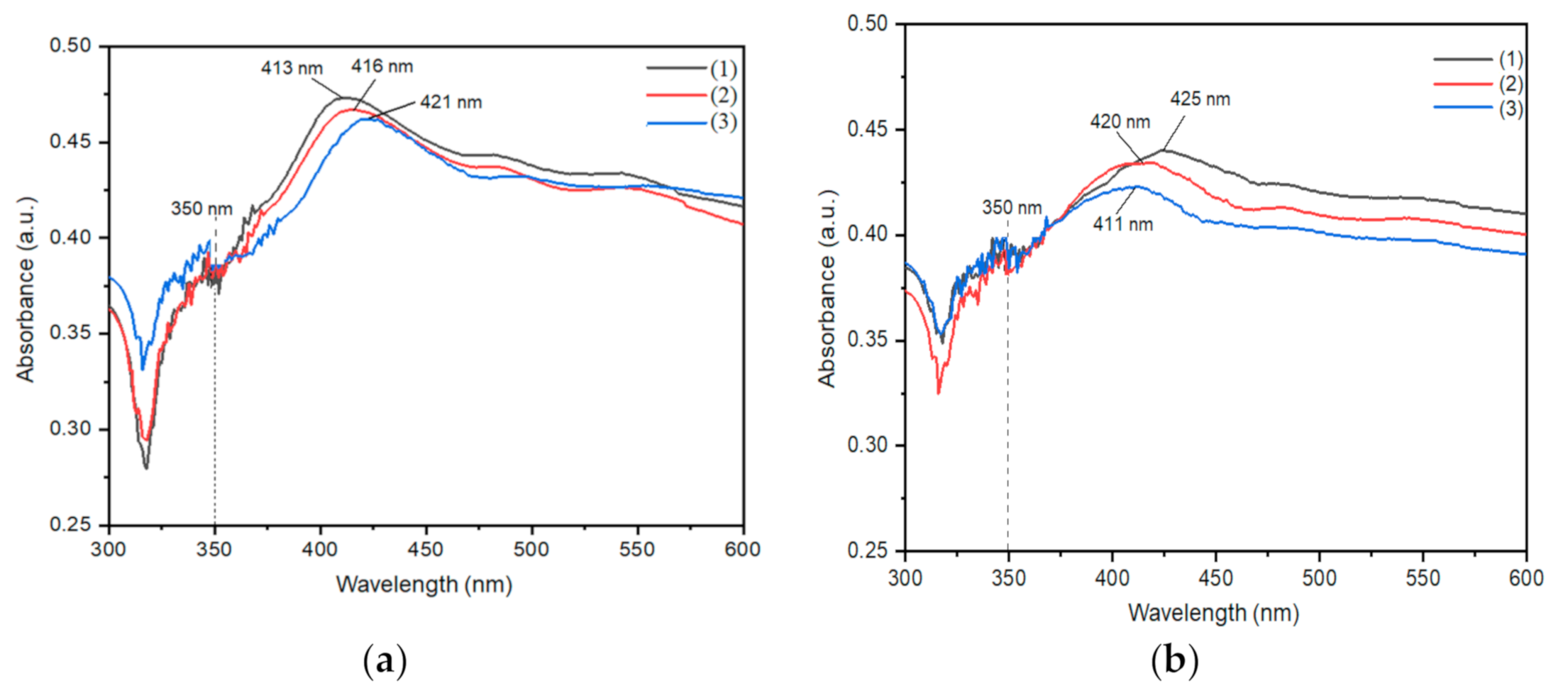
2.1. UV-Vis Analysis
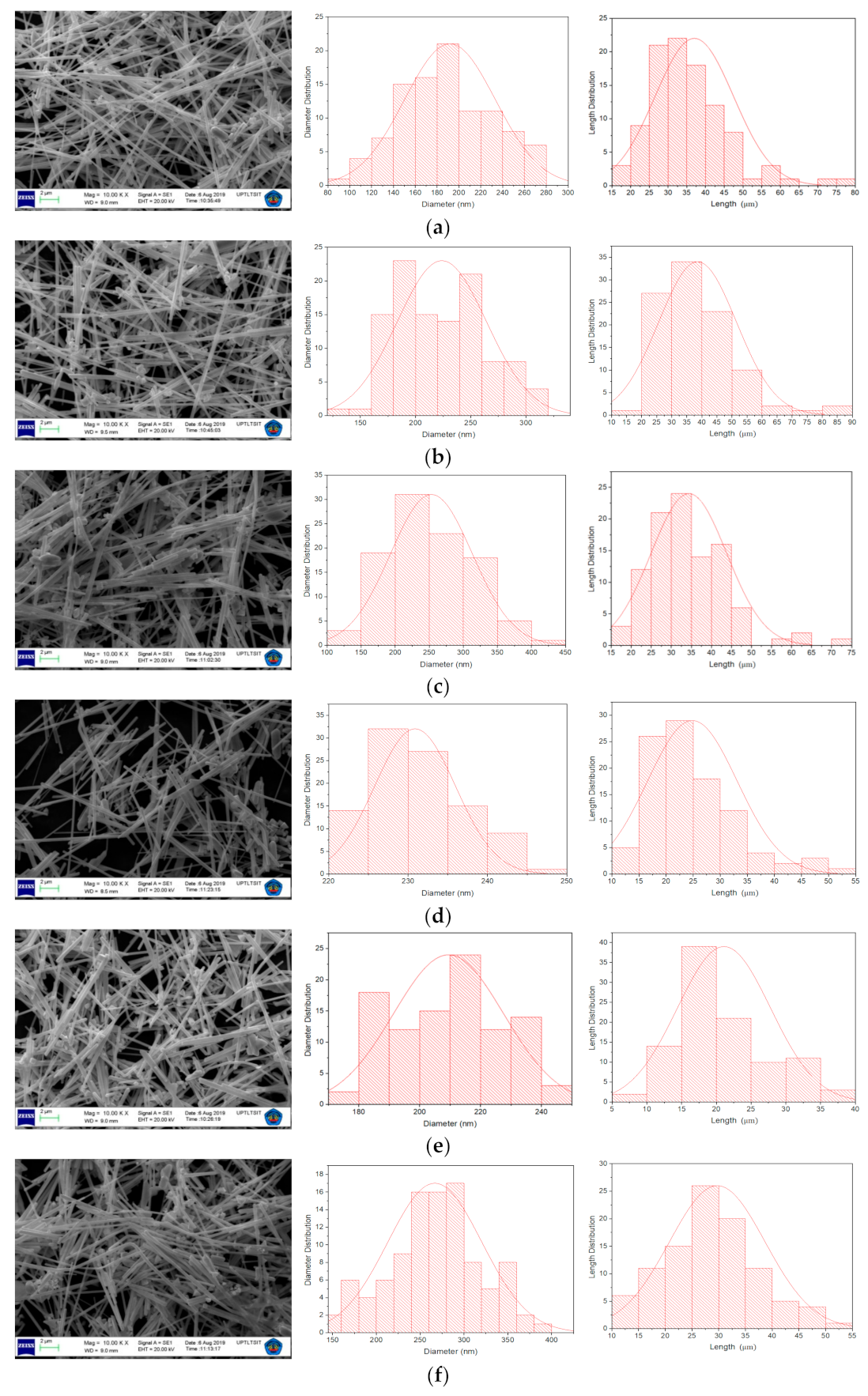
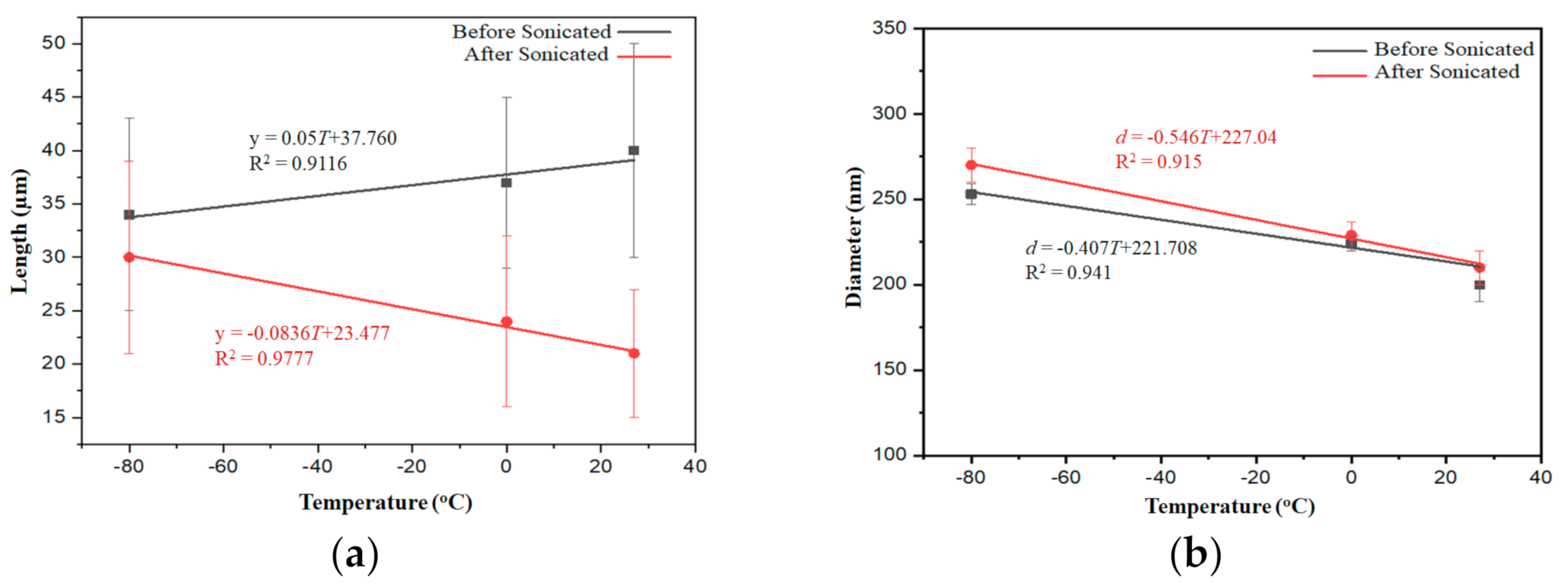
2.2. SEM and TEM Analysis
2.3. DTA/TGA Analysis
3. Discussion
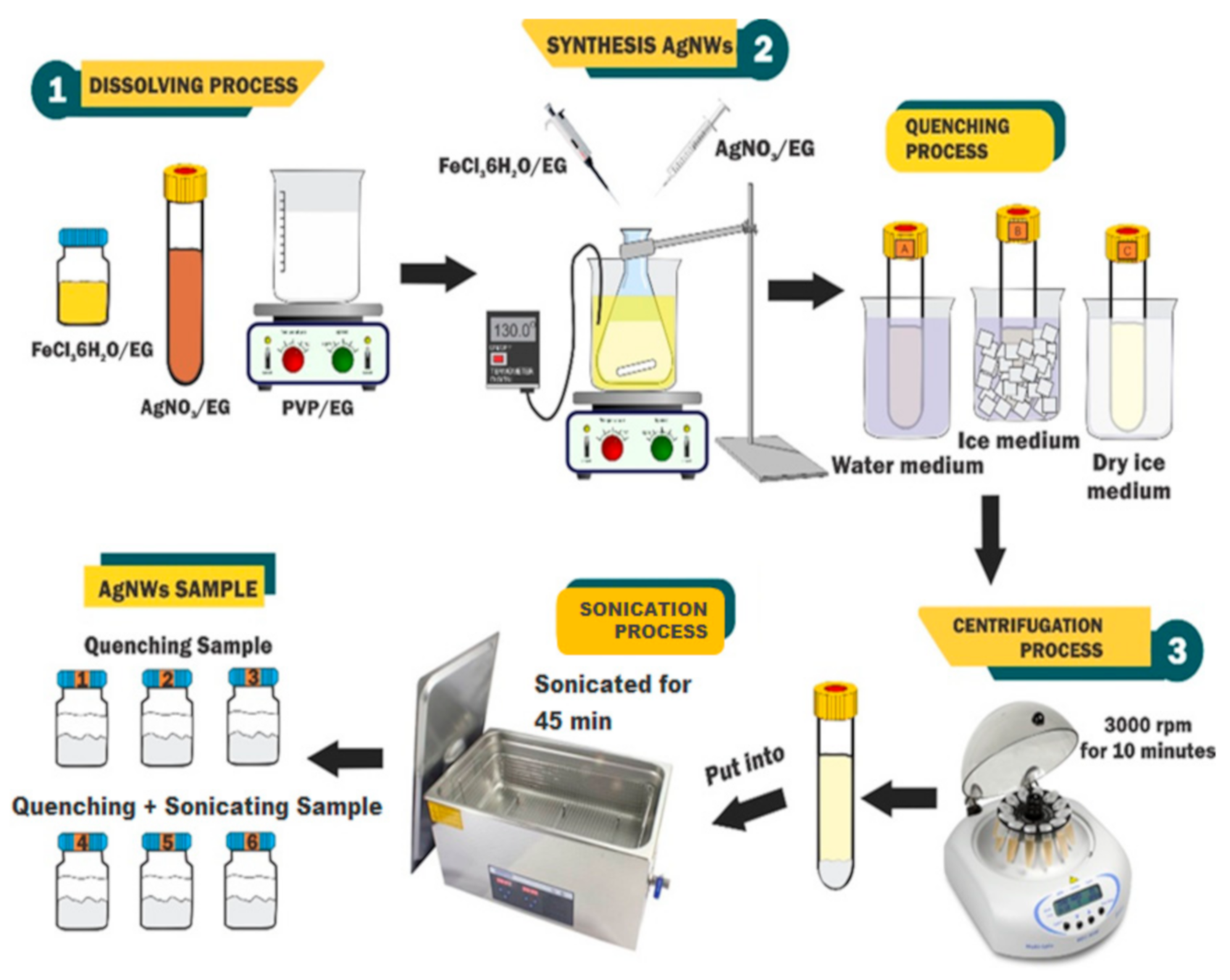
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.3. Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ganguly, S.; Mondal, S.; Das, P.; Bhawal, P.; Das, T.K.; Bose, M.; Choudhary, S.; Gangopadhyay, S.; Das, A.K.; Das, N.C. Natural saponin stabilized nano-catalyst as efficient dye-degradation catalyst. Nano-Struct. Nano-Objects 2018, 16, 86–95. [Google Scholar] [CrossRef]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.-A.; Mocan, L. Review on silver nanoparticles as a novel class of antibacterial solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, S.; Hu, J. Synthesis and characterization of size-controlled silver nanowires. Phys. Sci. Rev. 2018, 3, 20170084. [Google Scholar] [CrossRef]
- Parente, M.; Van Helvert, M.; Hamans, R.F.; Verbroekken, R.; Sinha, R.; Bieberle-Hütter, A.; Baldi, A. Simple and fast high-yield synthesis of silver nanowires. Nano Lett. 2020, 20, 5759–5764. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, Y.W.; Kim, J.E.; Yoon, S.W.; Kim, T.Y.; Noh, J.-S.; Suh, K.S. Synthesis of dimension-controlled silver nanowires for highly conductive and transparent nanowire films. Acta Mater. 2015, 83, 84–90. [Google Scholar] [CrossRef]
- Junaidi, J.; Maulidiasani, K.; Triyana, K.; Khairurrijal, K. Thin films of silver nanowires for flexible, transparent, and conductive (FTC) electrodes. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 137–144. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q. Particle size distribution of silver nanoparticles in different water conditions. J. Mater. Environ. Sci. 2013, 4, 617–620. [Google Scholar]
- Tan, D.; Jiang, C.; Li, Q.; Bi, S.; Song, J. Silver nanowire networks with preparations and applications: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 15669–15696. [Google Scholar] [CrossRef]
- Shi, Y.; He, L.; Deng, Q.; Liu, Q.; Li, L.; Wang, W.; Xin, Z.; Liu, R. Synthesis and applications of silver nanowires for transparent conductive films. Micromachines 2019, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, S.; Yang, H.; Chao, Y.; Jiang, S.; Wang, C. One-step synthesis of ultra-long silver nanowires of over 100 μm and their application in flexible transparent conductive films. RSC Adv. 2018, 8, 8057–8063. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Wang, J.-X.; Zeng, X.-F.; Zhang, L.-L.; Chen, J.-F. Large-scale synthesis of uniform silver nanowires by high-gravity technology for flexible transparent conductive electrodes. Ind. Eng. Chem. Res. 2019, 58, 20630–20638. [Google Scholar] [CrossRef]
- Chen, T.; Wang, H.; Yang, H.; Bai, S.; Guo, X. Mixed polyols synthesis of high aspect ratio silver nanowires for transparent conductive films. Mater. Res. Express 2018, 5, 066426. [Google Scholar] [CrossRef]
- Jones, R.S.; Draheim, R.R.; Roldo, M. Silver nanowires: Synthesis, antibacterial activity and biomedical applications. Appl. Sci. 2018, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Mirjalili, S.H.; Nateghi, M.R.; Kalantari-Fotooh, F. Preparation of silver nanowire/expanded polytetrafluoroethylene and polypropylene nanocomposites via all solution process method for antibacterial applications. J. Text. Inst. 2019, 111, 139–147. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.; Zhu, H.; Gao, B.; Garner, S.; Cimo, P.; Barcikowski, Z.; Mignerey, A.; Hu, L. Flexible, transparent, and conductive defrosting glass. Thin Solid Film. 2014, 556, 13–17. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.H.; Kim, N.-H.; Kim, J.-W. Flexible and transparent electrode based on silver nanowires and a urethane acrylate incorporating diels–Alder adducts. Mater. Des. 2015, 88, 1158–1163. [Google Scholar] [CrossRef]
- Li, W.; Meredov, A.; Shamim, A. Coat-and-print patterning of silver nanowires for flexible and transparent electronics. npj Flex. Electron. 2019, 3, 19. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, S.; Ren, J.; Khalid, B.; Peng, H.; Wu, H. A transparent, conducting tape for flexible electronics. Nano Res. 2016, 9, 917–924. [Google Scholar] [CrossRef]
- He, W.; Ye, C. Flexible transparent conductive films on the basis of ag nanowires: Design and applications: A review. J. Mater. Sci. Technol. 2015, 31, 581–588. [Google Scholar] [CrossRef]
- Ranasinghe, D.R.; Aryal, B.R.; Westover, T.R.; Jia, S.; Davis, R.C.; Harb, J.N.; Schulman, R.; Woolley, A.T. Seeding, plating and electrical characterization of gold nanowires formed on self-assembled DNA nanotubes. Molecules 2020, 25, 4817. [Google Scholar] [CrossRef]
- Selzer, F.; Floresca, C.; Kneppe, D.; Bormann, L.; Sachse, C.; Weiß, N.; Eychmüller, A.; Amassian, A.; Müller-Meskamp, L.; Leo, K. Electrical limit of silver nanowire electrodes: Direct measurement of the nanowire junction resistance. Appl. Phys. Lett. 2016, 108, 163302. [Google Scholar] [CrossRef] [Green Version]
- Nair, N.M.; Pakkathillam, J.K.; Kumar, K.; Arunachalam, K.; Ray, D.; Swaminathan, P. Printable silver nanowire and pedot:PSS nanocomposite ink for flexible transparent conducting applications. ACS Appl. Electron. Mater. 2020, 2, 1000–1010. [Google Scholar] [CrossRef]
- Ergun, O.; Coskun, S.; Yusufoglu, Y.; Unalan, H.E. High-performance, bare silver nanowire network transparent heaters. Nanotechnology 2016, 27, 445708. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Lee, J.-H.; Han, S.-H.; Jin, W.-Y.; Kang, J.-W.; Lee, S.-N. Silver nanowires for transparent conductive electrode to GaN-based light-emitting diodes. Appl. Phys. Lett. 2015, 106, 031118. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Bose, M.; Das, T.K.; Mondal, S.; Das, A.K.; Das, N.C. Sonochemical green reduction to prepare Ag nanoparticles decorated graphene sheets for catalytic performance and antibacterial application. Ultrason. Sonochem. 2017, 39, 577–588. [Google Scholar] [CrossRef]
- Johan, M.R.; Aznan, N.A.K.; Yee, S.T.; Ho, I.H.; Ooi, S.W.; Singho, N.D.; Aplop, F. Synthesis and growth mechanism of silver nanowires through different mediated agents (CuCl2and NaCl) polyol process. J. Nanomater. 2014, 2014, 105454. [Google Scholar] [CrossRef]
- Junaidi; Yunus, M.; Triyana, K.; Harsojo; Suharyadi, E. Chloride ion addition for controlling shapes and properties of silver nanorods capped by polyvinyl alcohol synthesized using polyol method. In Proceedings of the 3rd International Conference on Advanced Materials Science and Technology (ICAMST 2015), Semarang, Indonesia, 6–7 October 2016; AIP Publishing: Melville, NY, USA, 2016; Volume 1725, p. 20031. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-Y.; Shin, D.; Park, J. Fabrication of silver nanowire-based stretchable electrodes using spray coating. Thin Solid Film. 2016, 608, 34–43. [Google Scholar] [CrossRef]
- Feliciano, J.; Martínez-Iñesta, M.M. Synthesis and characterization of Pd, Cu, and Ag nanowires in anodic alumina membranes using solid state reduction. Mater. Lett. 2012, 82, 211–213. [Google Scholar] [CrossRef]
- Fahad, S.; Yu, H.; Wang, L.; Nazir, A.; Ullah, R.S.; Naveed, K.-U.-R.; Elshaarani, T.; Amin, B.U.; Khan, A.; Mehmood, S. Synthesis of silver nanowires with controlled diameter and their conductive thin films. J. Mater. Sci. Mater. Electron. 2019, 30, 12876–12887. [Google Scholar] [CrossRef]
- Sachse, C.; Müller-Meskamp, L.; Bormann, L.; Kim, Y.H.; Lehnert, F.; Philipp, A.; Beyer, B.; Leo, K. Transparent, dip-coated silver nanowire electrodes for small molecule organic solar cells. Org. Electron. 2013, 14, 143–148. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Li, Z.; Turng, L.-S.; Sun, Y.; Gong, S. Silver nanowire/thermoplastic polyurethane elastomer nanocomposites: Thermal, mechanical, and dielectric properties. Mater. Des. 2014, 56, 398–404. [Google Scholar] [CrossRef]
- Margulis, G.Y.; Christoforo, M.G.; Lam, D.; Beiley, Z.M.; Bowring, A.R.; Bailie, C.D.; Salleo, A.; McGehee, M.D. Spray deposition of silver nanowire electrodes for semitransparent solid-state dye-sensitized solar cells. Adv. Energy Mater. 2013, 3, 1657–1663. [Google Scholar] [CrossRef]
- Moreno, I.; Navascues, N.; Irusta, S.; Santamaria, J. Silver nanowires/polycarbonate composites for conductive films. IOP Conf. Ser. Mater. Sci. Eng. 2012, 40, 012001. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Jiang, H.; Tu, S.; Zhang, S.; Ren, E.; Yan, B.; Yang, Q.; Chen, S. High-performance and ultraflexible PEDOT/silver nanowires/graphene films for electrochromic applications. Opt. Lett. 2020, 45, 2443–2446. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Hsueh, Y.-L.; Huang, J.-J.; Wu, J.-R. Effect of silver nitrate concentration of silver nanowires synthesized using a polyol method and their application as transparent conductive films. Thin Solid Film. 2015, 584, 243–247. [Google Scholar] [CrossRef]
- Kim, T.; Canlier, A.; Kim, G.H.; Choi, J.; Park, M.; Han, S.M. Electrostatic spray deposition of highly transparent silver nanowire electrode on flexible substrate. ACS Appl. Mater. Interfaces 2013, 5, 788–794. [Google Scholar] [CrossRef]
- Preston, C.; Xu, Y.; Han, X.; Munday, J.N.; Hu, L. Optical haze of transparent and conductive silver nanowire films. Nano Res. 2013, 6, 461–468. [Google Scholar] [CrossRef]
- Selzer, F.; Weiß, N.; Bormann, L.; Sachse, C.; Gaponik, N.; Müller-Meskamp, L.; Eychmüller, A.; Leo, K. Highly conductive silver nanowire networks by organic matrix assisted low-temperature fusing. Org. Electron. 2014, 15, 3818–3824. [Google Scholar] [CrossRef]
- Mao, H.; Feng, J.; Ma, X.; Wu, C.; Zhao, X. One-dimensional silver nanowires synthesized by self-seeding polyol process. J. Nanoparticle Res. 2012, 14, 1–15. [Google Scholar] [CrossRef]
- Rajesh, D.; Sunandana, C.; Desapogu, R. Controlled formation of porous silver nanowires. Chem. Phys. Lett. 2014, 610-611, 363–368. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.; Lai, W.-Y.; Chen, Y.; Huang, W. A rapid synthesis of high aspect ratio silver nanowires for high-performance transparent electrodes. Chin. J. Chem. 2014, 33, 147–151. [Google Scholar] [CrossRef]
- Junaidi; Triyana, K.; Hui, H.; Wu, L.Y.L.; Suharyadi, E.; Harsojo. The silver nanowires synthesized using different molecule weight of polyvinyl pyrrolidone for controlling diameter and length by one-pot polyol method. In Proceedings of the 2016 Conference on Fundamental and Applied Science for Advanced Technology (CONFAST 2016), Yogyakarta, Indonesia, 25–26 January 2016; AIP Publishing: Melville, NY, USA, 2016; Volume 1746, p. 20014. [Google Scholar] [CrossRef]
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.-P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 452001. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, H.-S.; Noh, Y.-J.; Na, S.-I.; Kim, H.-K. Brush painting of transparent PEDOT/Ag nanowire/PEDOT multilayer electrodes for flexible organic solar cells. Sol. Energy Mater. Sol. Cells 2013, 114, 15–23. [Google Scholar] [CrossRef]
- Wang, J.; Jiu, J.; Araki, T.; Nogi, M.; Sugahara, T.; Nagao, S.; Koga, H.; He, P.; Suganuma, K. Silver nanowire electrodes: Conductivity improvement without post-treatment and application in capacitive pressure sensors. Nano-Micro Lett. 2015, 7, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Huang, H. Fabrication of three-dimensional sandwich-like silver nanowire network coated bilayer graphene nanostructures for flexible display. Appl. Opt. 2020, 59, 9878–9883. [Google Scholar] [CrossRef]
- Junaidi, J.; Triyana, K.; Suharyadi, E.; Harsojo; Wu, L.Y. The roles of polyvinyl alcohol (PVA) as the capping agent on the polyol method for synthesizing silver nanowires. J. Nano Res. 2017, 49, 174–180. [Google Scholar] [CrossRef]


| Medium | Temperature (°C) | Heat Process | Loss Mass (%) |
|---|---|---|---|
| Water | 30–330 | Endothermic | 3.94 |
| 330–500 | Exothermic | 4.71 | |
| Ice | 30–330 | Endothermic | 1.54 |
| 330–500 | Exothermic | 2.53 | |
| Dry Ice | 30–330 | Endothermic | 1.35 |
| 330–500 | Exothermic | 1.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junaidi, J.; Saputra, M.W.; Marjunus, R.; Sembiring, S.; Hadi, S. The Quenching and Sonication Effect on the Mechanical Strength of Silver Nanowires Synthesized Using the Polyol Method. Molecules 2021, 26, 2167. https://doi.org/10.3390/molecules26082167
Junaidi J, Saputra MW, Marjunus R, Sembiring S, Hadi S. The Quenching and Sonication Effect on the Mechanical Strength of Silver Nanowires Synthesized Using the Polyol Method. Molecules. 2021; 26(8):2167. https://doi.org/10.3390/molecules26082167
Chicago/Turabian StyleJunaidi, Junaidi, Muhamad Wahyudi Saputra, Roniyus Marjunus, Simon Sembiring, and Sutopo Hadi. 2021. "The Quenching and Sonication Effect on the Mechanical Strength of Silver Nanowires Synthesized Using the Polyol Method" Molecules 26, no. 8: 2167. https://doi.org/10.3390/molecules26082167