In Vivo and In Vitro Assays Evaluating the Biological Activity of Taurine, Glucose and Energetic Beverages
Abstract
1. Introduction
2. Results
2.1. Toxicity/Antitoxicity
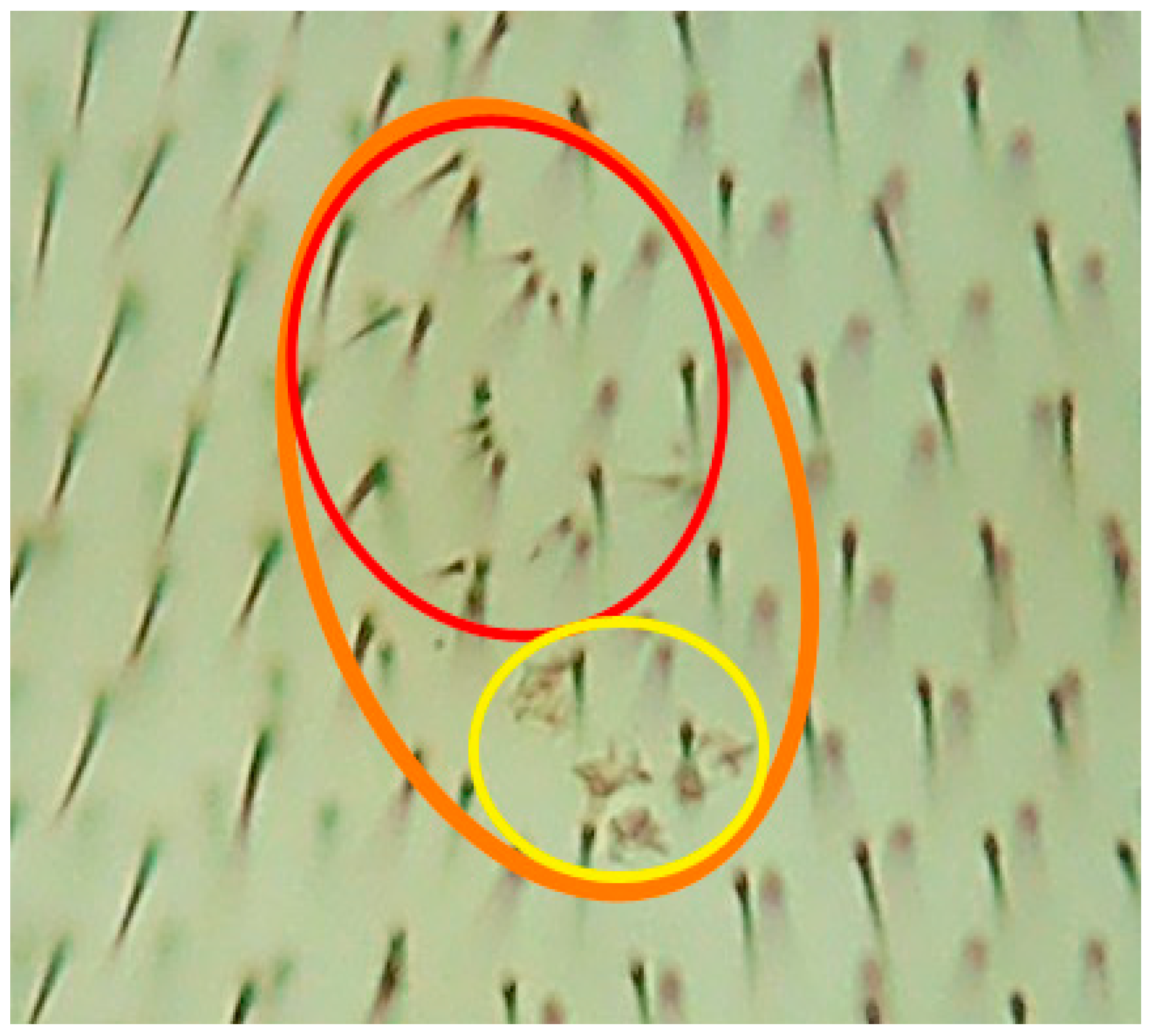
2.2. Genotoxicity/Antigenotoxicity
2.3. Lifespan
2.4. Cytotoxicity
2.5. Internucleosomal DNA Fragmentation
2.6. Comet Assay
2.7. Methylation Status
3. Discussion
4. Materials and methods
4.1. Samples
4.2. Fly Stocks
4.3. Cell Culture Conditions
4.4. In Vivo Assays
4.4.1. Toxicity and Antitoxicity Assays
4.4.2. Genotoxicity and Antigenotoxicity Assays
4.4.3. Chronic Treatments: Lifespan and Healthspan Assays
4.5. In Vitro Assays
4.5.1. Cytotoxicity Assay
4.5.2. DNA Fragmentation Status
4.5.3. Clastogenicity: SCGE (Comet Assay)
4.5.4. Methylation Status of HL-60 Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ehlers, A.; Marakis, G.; Lampen, A.; Hirsch-Ernst, I. Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe. Food Chem. Toxicol. 2019, 130, 109–121. [Google Scholar] [CrossRef]
- Vercammen, K.A.; Koma, J.W.; Bleich, S.N. Trends in energy drink consumption among U.S. adolescents and adults, 2003–2016. Am. J. Prevent. Med. 2019, 56, 827–833. [Google Scholar] [CrossRef]
- Kammerer, M.; Jaramillo, J.A.; García, A.; Calderín, J.C.; Valbuena, L.H. Effects of energy drink major bioactive compounds on the performance of young adults in fitness and cognitive tests: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alsunni, A.A. Energy drink consumption: Beneficial and adverse health effects. Int. J. Health Sci. 2015, 9, 468. [Google Scholar] [CrossRef]
- Bouckenooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Verner, A.; Craig, S.; Mc Guire, W. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1033–1041. [Google Scholar]
- Lee, S.E.; Lee, S.Y.; Jo, E.J.; Kim, M.Y.; Yang, M.S.; Chang, Y.S.; Kim, S.H. A case of taurine-containing drink induced anaphylaxis. As. Pac. Allergy 2013, 3, 70–73. [Google Scholar] [CrossRef][Green Version]
- Cavka, A.; Stupin, M.; Panduric, A.; Plazibat, A.; Cosic, A.; Rasic, L.; Debeljak, Z.; Martinovic, G.; Drenjancevic, I. Adrenergic system activation mediates changes in cardiovascular and psychomotoric reactions in young individuals after red bull © energy drink consumption. Int. J. Endocrinol. 2015, 10, 751530. [Google Scholar] [CrossRef]
- Ceriello, A.; dello Russo, P.; Amstad, P.; Cerutti, P. High glucose induces antioxidant enzymes in human endothelial cells in culture: Evidence linking hyperglycemia and oxidative stress. Diabetes 1996, 45, 471–477. [Google Scholar] [CrossRef]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Jankowski, M.; Angielski, S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. J. Cell. Biochem. 2011, 112, 1661–1672. [Google Scholar] [CrossRef]
- Karunakaran, U.; Park, K.G. A systematic review of oxidative stress and safety of antioxidants in diabetes: Focus on islets and their defense. Diabet. Metab. J. 2013, 37, 106–112. [Google Scholar] [CrossRef]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millán, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A.; de Castro, M.D. A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat. Res. 2011, 723, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Graf, U.; Würgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosoplila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Anjum, S.; Jyoti, S. Evaluation of the toxic potential of aspartame in third instar larvae of transgenic Drosoplila melanogaster (hsp70-lacZ) Bg9. All Res. J. Biol. 2017, 8, 16–24. [Google Scholar]
- Fernández-Bedmar, Z.; Anter, J.; de La Cruz-Ares, S.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; Pérez-Guisado, J. Role of citrus juices and distinctive components in the modulation of degenerative processes: Genotoxicity, antigenotoxicity, cytotoxicity, and longevity in Drosoplila. J. Toxicol. Environ. Health A 2011, 74, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Fernández, M.; Alves-Martínez, P.; Río-Celestino, D.; Font, R.; Merinas-Amo, T.; Alonso-Moraga, Á. Safety and nutraceutical potential of caramel colour class iv using In vivo and In vitro assays. Foods 2019, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Anazetti, M.C.; Melo, P.S.; Duran, N.; Haun, M. Comparative cytotoxicity of dimethylamide-crotonin in the promyelocytic leukemia cell line (HL60) and human peripheral blood mononuclear cells. Toxicology 2003, 188, 261–274. [Google Scholar] [CrossRef]
- Fesus, L.; Szondy, Z.; Uray, I. Probing the molecular program of apoptosis by cancer chemopreventive agents. J. Cell. Biochem. Suppl. 1995, 22, 151–161. [Google Scholar] [CrossRef]
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of cancer. Science 1997, 278, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Gaido, M.L.; Cidlowski, J.A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J. Biol. Chem. 1991, 266, 18580–18585. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Jimenez-Velasco, A.; Agirre, X.; Castillejo, J.A.; Navarro, G.; San Jose-Eneriz, E.; Garate, L.; Cordeu, L.; Cervantes, F.; Prosper, F.; et al. Repetitive DNA hypomethylation in the advanced phase of chronic myeloid leukemia. Leuk. Res. 2008, 32, 487–490. [Google Scholar] [CrossRef]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef]
- Frei, H.; Wurgler, F.E. Statistical methods to decide whether mutagenicity test data from Drosoplila assays indicate a positive, negative, or inconclusive result. Mutat. Res. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Dang, T.S.; Walker, M.; Ford, D.; Valentine, R.A. Nutrigenomics: The role of nutrients in gene expression. Periodontology 2014, 64, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Beckingham, K.M.; Armstrong, J.D.; Texada, M.J.; Munjaal, R.; Baker, D.A. Drosoplila melanogaster-the model organism of choice for the complex biology of multi-cellular organisms. Gravit. Space Res. 2007, 18, 17–30. [Google Scholar]
- El Desouky, A.A.; Abo Zaid, A.; El Saify, G.H.; Noya, D.A. Ameliorative effect of omega-3 on energy drinks-induced pancreatic toxicity in adult male albino rats. Egypt. J. Histol. 2019, 42, 324–334. [Google Scholar] [CrossRef]
- Okoro, E.; Asagba, S.; Okoro, I. Effects of energy drink and alcohol on some clinical and oxidative stress parameters in rats. Biokemistri 2020, 31, 2. [Google Scholar]
- Taiwo, O.I.; Adesokan, A.A. Toxicodynamic effects of “Red Bull” energy drink in a randomised controlled study on local strains of adult rabbits. J. Biol. Life Sci 2018, 9, 46–64. [Google Scholar]
- Bigard, A. Risks of energy drinks in youths. Arch. Pediatr. Org. Off. Soc. Franc. Pediatr. 2010, 17, 1625–1631. [Google Scholar] [CrossRef]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.S. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet β cell. Free Rad. Biol. Med. 2006, 41, 177–184. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Reg. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef]
- Deshpande, S.A.; Carvalho, G.B.; Amador, A.; Phillips, A.M.; Hoxha, S.; Lizotte, K.J.; Ja, W.W. Quantifying Drosoplila food intake: Comparative analysis of current methodology. Nat. Method 2014, 11, 535–540. [Google Scholar] [CrossRef]
- Massie, H.R.; Williams, T.R.; DeWolfe, L.K. Changes in taurine in aging fruit flies and mice. Exp. Gerontol. 1989, 24, 57–65. [Google Scholar] [CrossRef]
- Mateo-Fernández, M.; Merinas-Amo, T.; Moreno-Millán, M.; Alonso-Moraga, Á.; Demyda-Peyrás, S. In vivo and in vitro genotoxic and epigenetic effects of two types of cola beverages and caffeine: A multiassay approach. BioMed Res. Int. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Azuma, J.; Mozaffari, M. Role of antioxidant activity of taurine in diabetes this article is one of a selection of papers from the NATO advanced research workshop on translational knowledge for heart health. Can. J. Physiol. Pharmacol. 2009, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Guo, Z.X.; Wang, A.L. The protective effects of taurine on oxidative stress, cytoplasmic free-Ca2+ and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol. 2018, 77, 457–464. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Dessouki, A.A.; Abdel-Rahman, H.G.; Eltaysh, R.; Alkahtani, S. Hepatorenal protective effects of taurine and N-acetylcysteine against fipronil-induced injuries: The antioxidant status and apoptotic markers expression in rats. Sci. Total Environ. 2019, 650, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem. J. 1988, 256, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Fortini, M.E.; Skupski, M.P.; Boguski, M.S.; Hariharan, I.K. A survey of human disease gene counterparts in the Drosoplila genome. J. Cell Biol. 2000, 150, F23–F30. [Google Scholar] [CrossRef]
- Frei, H.; Wurgler, F.E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosoplila. Mutat. Res. 1995, 334, 247–258. [Google Scholar] [CrossRef]
- Cozzi, R.; Ricordy, R.; Bartolini, F.; Ramadori, L.; Perticone, P.; De Salvia, R. Taurine and ellagic acid: Two differently-acting natural antioxidants. Environ. Mol. Mut. 1995, 26, 248–254. [Google Scholar] [CrossRef]
- Ergun, M.A.; Soysal, Y.; Kismet, E.; Akay, C.; Dundaroz, R.; Ilhan, M.N.; Imirzalioglu, N. Investigating the in vitro effect of taurine on the infant lymphocytes by sister chromatid exchange. Pediatr. Int. 2006, 48, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Mullokandov, E.; Franklin, W.; Brownlee, M. DNA damage by the glycation products of glyceraldehyde 3-phosphate and lysine. Diabetologia 1994, 37, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Baunsgaard, D.; Autrup, H.; Vogel, U.B.; Møller, P.; Lindecrona, R.; Wallin, H.; Poulsen, H.E.; Loft, S.; Dragsted, L.O. Sucrose, glucose and fructose have similar genotoxicity in the rat colon and affect the metabolome. Food Chem. Toxicol. 2008, 46, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Geyikoğlu, F. The anti-genotoxic effect of taurine on aluminum sulphate-induced DNA damage in human peripheral lymphocytes. IUFS J. Biol. 2010, 69, 25–32. [Google Scholar]
- Messina, S.A.; Dawson, R., Jr. Attenuation of oxidative damage to DNA by tairome and taurine analogs. In Taurine 4; Springer: Boston, MA, USA, 2002; Volume 483, pp. 355–367. [Google Scholar]
- Bosquesi, P.L.; Scarim, C.B.; de Oliveira, J.R.S.; de Oliveira Vizioli, E.; dos Santos, J.L.; Chin, C.M. Protective effect of taurine in the induction of genotoxicity by mutagenic drugs. J. Pharm. Pharmacol. 2018, 6, 1–9. [Google Scholar]
- Fleming, J.E.; Reveillaud, I.; Niedzwiecki, A. Role of oxidative stress in Drosoplila aging. Mutat. Res. 1992, 275, 267–279. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Tollefsbol, T.O. Glucose restriction can extend normal cell lifespan and impair precancerous cell growth through epigenetic control of hTERT and p16 expression. FASEB J. 2010, 24, 1442–1453. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Feng, Y.; Ren, H.; Liu, F.; Zu, T. Effect of taurine on lifespan and antioxidant in Drosophila. In Proceedings of the Biomedical Engineering and Biotechnology (iCBEB), International Conference, Macau, Macao, 28–30 May 2012; pp. 206–209. [Google Scholar]
- Franconi, F.; Loizzo, A.; Ghirlanda, G.; Seghieri, G. Taurine supplementation and diabetes mellitus. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 32–36. [Google Scholar] [CrossRef]
- Smith, L.; Habib, I.; Shirkey, S.; Talon, B.; Milne, A.; Nadolski, J. Sexual dimorphism in the effect of a taurine supplemented diet on life span in adult Drosoplila melanogaster. Int. J. Zool. Res. 2011, 7, 34. [Google Scholar] [CrossRef]
- Suh, H.J.; Shin, B.; Han, S.H.; Woo, M.J.; Hong, K.B. Behavioral changes and survival in melanogaster: Effects of Ascorbic acid, taurine, and caffeine. Biol. Pharm. Bull. 2017, 40, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Do, C.H.; Lee, D.H. Taurine reduces ER stress in C. Elegans. J. Biomed. Sci. 2010, 17, S26. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Liu, l.; Ikeda, K.; Miura, A.; Mizushima, S.; Miki, T.; Nara, Y. Distribution of twenty-four hour urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: Results from the WHO-CARDIAC study. Hypertens. Res. 2001, 24, 453–457. [Google Scholar] [CrossRef]
- Ito, T.; Yoshikawa, N.; Inui, T.; Miyazaki, N.; Schaffer, S.W.; Azuma, J. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS ONE 2014, 9, e107409. [Google Scholar] [CrossRef]
- Troen, A.M.; French, E.E.; Roberts, J.F.; Selhub, J.; Ordovas, J.M.; Parnell, L.D.; Lai, C.-Q. Lifespan modification by glucose and methionine in Drosoplila melanogaster fed a chemically defined diet. Age 2007, 29, 29–39. [Google Scholar] [CrossRef]
- Jeon, S.H.; Lee, M.Y.; Kim, S.J.; Joe, S.G.; Kim, G.B.; Kim, I.S.; Kim, N.S.; Hong, C.U.; Kim, S.Z.; Kim, J.S.; et al. Taurine increases cell proliferation and generates an increase in [Mg2+] i accompanied by ERK 1/2 activation in human osteoblast cells. FEBS Lett. 2007, 581, 5929–5934. [Google Scholar] [CrossRef]
- Heidari, R.; Babaei, H.; Eghbal, M.A. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arch. Ind. Hygiene Toxicol. 2013, 64, 201–210. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, H.; Wang, C.; Hu, N. TUG1 knockdown enhances adriamycin cytotoxicity by inhibiting glycolysis in adriamycin-resistant acute myeloid leukemia HL60/ADR cells. RSC Adv. 2019, 9, 10897–10904. [Google Scholar] [CrossRef]
- Lee, Y.J.; Galoforo, S.S.; Berns, C.M.; Chen, J.C.; Davis, B.H.; Sim, J.E.; Corry, P.M.; Spitz, D.R. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J. Biol. Chem. 1998, 273, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Fang, L.; Gibbs, S.; Huang, Y.; Dai, Z.; Wen, P.; Zheng, X.; Sadee, W.; Sun, D. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother. Pharmacol. 2007, 59, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Ciszewicz, M.; PoLubinska, A.; Antoniewicz, A.; Suminska-Jasinska, K.; BrĘborowicz, A. Sulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicity. Trans. Res. 2009, 153, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Xian-pei, H.; Ke-ji, C.; Zheng-feng, H.; Wei-dong, H.; Ke-dan, C.; Wen-lie, C.; Xu-zheng, C.; Hai-xia, Z.; Ling, C.; Liu-qing, Y.; et al. Glucose endothelial cytotoxicity and protection by Dan Gua-Fang, a Chinese herb prescription in huVEC in hyperglycemia medium. J. Diab. Compl. 2009, 23, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Xin, S.M.; Cheng, Z.J.; Zhang, W.B.; Zhang, R.; Qin, Y.W.; Pei, G. Activation of p38 mitogen-activated protein kinase by oxidized LDL in vascular smooth muscle cells mediation via pertussis toxin–sensitive g proteins and association with oxidized LDL-Induced cytotoxicity. Circ. Res. 1999, 84, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Zhang, X.R.; Xie, W.F.; Li, S. Effects of taurine on proliferation and apoptosis of hepatic stellate cells in vitro. Hepatobil. Pancreat. Dis. Int. 2004, 3, 106–109. [Google Scholar]
- Takatani, T.; Takahashi, K.; Uozumi, Y.; Shikata, E.; Yamamoto, Y.; Ito, T.; Matsuda, T.; Schaffer, S.; Fujio, Y.; Azuma, J. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am. J. Physiol. Cell Physiol. 2004, 287, C949–C953. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.J.; Ringold, G.M.; Heller, R.A. 1992 Cell killing and induction of manganous superoxide dismutase by tumor necrosis factor-(α is mediated by lipoxygenase metabolites of arachidonic acid. Biochem. Biophys. Res. Commun. 1995, 188, 538–546. [Google Scholar] [CrossRef]
- Salganik, R.I. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. College Nutr. 2001, 20, 464S–472S. [Google Scholar] [CrossRef]
- Ishii, N.; Ogawa, Z.; Suzuki, K.; Numakami, K.; Saruta, T.; Itoh, H. Glucose loading induces DNA fragmentation in rat proximal tubular cells. Metabolism 1996, 45, 1348–1353. [Google Scholar] [CrossRef]
- Baumgartner-Parzer, S.M.; Wagner, L.; Pettermann, M.; Grillari, J.; Gessl, A.; Waldhäusl, W. High-glucose–triggered apoptosis in cultured endothelial cells. Diabetes 1995, 44, 1323–1327. [Google Scholar] [CrossRef]
- Vaughn, A.E.; Deshmukh, M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat. Cell Biol. 2008, 10, 1477. [Google Scholar] [CrossRef]
- Mateo-Fernández, M.; Moreno-Ariza, V.; Balongo-Escobar, M.; Luque-Bravo, L.; Fernández-Bedmar, Z.N.; Martínez-Jurado, M.; Demyda-Peyrás, S.; Merinas-Amo, T. Biological effects of classic and diet soda drinks assessed in in vivo and in vitro models. Toxicol. Lett. 2015, 238, S65. [Google Scholar] [CrossRef]
- Forchhammer, L.; Ersson, C.; Loft, S.; Möller, L.; Godschalk, R.W.L.; Van Schooten, F.J.; Jones, G.D.D.; Higgins, J.A.; Coke, M.; Mistry, V.; et al. Inter-laboratory variation in DNA damage using a standard comet assay protocol. Mutagenesis 2012, 27, 665–672. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Fairbairn, D.W.; O’Neill, K.L. Necrotic DNA degradation mimics apoptotic nucleosomal fragmentation comet tail length. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 171–173. [Google Scholar] [CrossRef]
- Mochizuki, T.; Satsu, H.; Shimizu, M. Tumor necrosis factor α stimulates taurine uptake and transporter gene expression in human intestinal Caco-2 cells. FEBS Lett. 2002, 517, 92–96. [Google Scholar] [CrossRef]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Morozzi, G. Genotoxicity of alkene epoxides in human peripheral blood mononuclear cells and HL60 leukaemia cells evaluated with the comet assay. Mutat. Res. 2012, 747, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.K.; Khan, A.A.; Ali, S.N.; Mahmood, R. Chemoprotective effect of taurine on potassium bromate-induced DNA damage, DNA-protein cross-linking and oxidative stress in rat intestine. PLoS ONE 2015, 10, e0119137. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M.; Montisano, D.F.; Toledo, S.; Barrieux, A. High glucose induces DNA damage in cultured human endothelial cells. J. Clin. Investig. 1986, 77, 322. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, M.; Fujisawa, K.; Uchimuraa, I.; Numano, F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes 1996, 45, 954–959. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Campan, M.; Long, T.I.; Kim, M.; Woods, C.; Fiala, E.; Ehrlich, M.; Laird, P.W. Analysis of repetitive element DNA methylation by MethyLight. Nucl. Acids Res. 2005, 33, 6823–6836. [Google Scholar] [CrossRef] [PubMed]
- López-Serra, L.; Esteller, M. Proteins that bind methylated DNA and human cancer: Reading the wrong words. Br. J. Cancer 2008, 98, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.G.; Pérez-Escuredo, J.; Castro-Santos, P.; Marcos, C.Á.; Pendás, J.L.L.; Fraga, M.F.; Hermsen, M.A. Hypomethylation of LINE-1, and not centromeric SAT-α, is associated with centromeric instability in head and neck squamous cell carcinoma. Cell. Oncol. 2012, 35, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Waye, J.; Willard, H. Structure, organization, and sequence of alpha satellite DNA from human chromosome 17: Evidence for evolution by unequal crossing-over and an ancestral pentamer repeat shared with the human X chromosome. Mol. Cell. Biol. 1986, 6, 3156–3165. [Google Scholar] [CrossRef]
- Grover, D.; Majumder, P.P.; Rao, C.B.; Brahmachari, S.K.; Mukerji, M. Nonrandom distribution of alu elements in genes of various functional categories: Insight from analysis of human chromosomes 21 and 22. Mol. Biol. Evol. 2003, 20, 1420–1424. [Google Scholar] [CrossRef]
- Lleu, P.L.; Huxtable, R. Phospholipid methylation and taurine content of synaptosomes from cerebral cortex of developing rat. Neurochem. Int. 1992, 21, 109–118. [Google Scholar] [CrossRef]
- Consortium, I.H.G.S. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860. [Google Scholar]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef]
- Yun, J.M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, K.; Li, X.; Zhang, X.; Liu, S.V. A new cultivation system for studying chemical effects on the lifespan of the fruit fly. Exp. Gerontol. 2010, 45, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.T.; Lipson, D.; Paul, S.; Brannigan, B.W.; Akhavanfard, S.; Coffman, E.J.; Contino, G.; Deshpande, V.; Lafrate, A.J.; Letovsky, S.; et al. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science 2011, 331, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Wild, L.; Flanagan, J.M. Genome-wide hypomethylation in cancer may be a passive consequence of transformation. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 50–57. [Google Scholar] [CrossRef]
- Wilson, A.S.; Power, B.E.; Molloy, P.L. DNA hypomethylation and human diseases. Biochim. Biophys. Acta Rev. Cancer 2007, 1775, 138–162. [Google Scholar] [CrossRef]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; De La Rosa, N.N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef]
- Yan, J.; Huen, D.; Morely, T.; Johnson, G.; Gubb, D.; Roote, J.; Adler, P.N. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics 2008, 180, 219–228. [Google Scholar] [CrossRef]
- Ren, N.; Charlton, J.; Adler, P.N. The flare gene, which encodes the AIP1 protein of Drosoplila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics 2007, 176, 2223–2234. [Google Scholar] [CrossRef]
- Gallagher, R.; Collins, S.; Trujillo, J.; McCredie, K.; Ahearn, M.; Tsai, S.; Metzgar, R.; Aulakh, G.; Ting, R.; Ruscetti, F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 1979, 54, 713–733. [Google Scholar] [CrossRef]
- Tasset-Cuevas, I.; Fernandez-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; de Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, A. Protective effect of borage seed oil and gamma linolenic acid on DNA: In vivo and in vitro studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef]
- Abraham, S.K. Antigenotoxicity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis 1994, 9, 383–386. [Google Scholar] [CrossRef]
- Anter, J.; Tasset, I.; Demyda-Peyras, S.; Ranchal, I.; Moreno-Millan, M.; Romero-Jimenez, M.; Muntané, J.; Luque de Castro, M.D.; Serran-Muñoz, A.; Alonso-Moraga, A. Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product alperujo, hydroxytyrosol, tyrosol and verbascoside. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 772, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.V. OpenComet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, T.; Tasset-Cuevas, I.; Díaz-Carretero, A.M.; Alonso-Moraga, A.; Calahorro, F. Role of choline in the modulation of degenerative processes: In vivo and In vitro studies. J. Med. Food 2017, 20, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, G.; Raji, O.Y.; Markopoulou, S.; Gosney, J.R.; Bryan, J.; Warburton, C.; Walshaw, M.; Sheard, J.; Field, J.K.; Liloglou, T. DNA methylation biomarkers offer improved diagnostic efficiency in lung cancer. Cancer Res. 2012, 72, 5692–5701. [Google Scholar] [CrossRef]
- Liloglou, T.; Bediaga, N.G.; Brown, B.R.; Field, J.K.; Davies, M.P. Epigenetic biomarkers in lung cancer. Cancer Lett. 2014, 342, 200–212. [Google Scholar] [CrossRef]







| CRB (mg/mL) | Survival (%) | SFRB (mg/mL) | Survival (%) | TAU (mM) | Survival (%) | GLU (mM) | Survival (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Simple Treatment (1) | Combined Treatment (2) | Simple Treatment | Combined Treatment | Simple Treatment | Combined Treatment | Simple Treatment | Combined Treatment | ||||
| 0 | 100 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 0 | 100 | 100 |
| H2O2 | - | 38.46 | H2O2 | - | 58 | H2O2 | - | 38.46 | H2O2 | - | 60 |
| 1.02 | 97 | 49.23 * (4) | 0.16 | 77.65 * | 65 | 0.25 | 94 | 40.76 | 2.7 | 98 | 60 |
| 4.06 | 93 | 47.69 * | 0.625 | 80.3 * | 87 * | 1 | 78 * | 57.69 * | 11 | 92.65 | 84.35 * |
| 8.13 | 88 | 51.53 * | 1.25 | 74 * | 106.68 * | 2 | 77 * | 56.9 * | 21.86 | 90.32 | 88.65 * |
| 32.5 | 75 * (3) | 40 | 5 | 93 | 69 * | 8 | 76 * | 40 | 87.5 | 70.3 * | 79 * |
| 130 | 59 * | 39 | 20 | 95.32 | 53.65 | 32 | 78 * | 42.3 | 350 | 65 * | 72 * |
| Clones Per Wings (Number of Spots) (1) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Wings | Small Single Spots | Large Simple Spots | Twin Spots | Total Spots | Mann-Whitney Test (2) | IP (%) (3) |
| Number | (1–2 Cells) | (>2 Cells) | m = 5 | m = 2 | |||
| m = 2 | m = 5 | ||||||
| H2O | 41 | 0.147 (6) | 0.048 (2) | 0 | 0.195 (8) | ||
| H2O2 (0.15 M) | 40 | 0.375 (15) | 0.05 (2) | 0 | 0.425 (17)+ | ||
| SIMPLE TREATMENT | |||||||
| CRB (mg/mL) | |||||||
| [4.06] | 40 | 0.2 (8) | 0 | 0 | 0.2 (8)i | λ | |
| [130] | 40 | 0.3 (12) | 0.025 (1) | 0 | 0.325 (13)i | λ | |
| SFRB (mg/mL) | |||||||
| [0.625] | 36 | 0.055 (2) | 0.027 (1) | 0 | 0.083 (3)- | ||
| [20] | 40 | 0.125 (5) | 0 | 0 | 0.125 (5)- | ||
| TAU (mM) | |||||||
| [1] | 37 | 0.027 (1) | 0.08 (3) | 0 | 0.108 (4)- | ||
| [32] | 40 | 0.15 (6) | 0.05 (2) | 0 | 0.2 (8)i | λ | |
| GLU (mM) | |||||||
| [11] | 40 | 0.125 (5) | 0.05 (2) | 0 | 0.175 (7)i | λ | |
| [350] | 40 | 0.1 (4) | 0.025 (1) | 0 | 0.125 (5)- | ||
| COMBINED TREATMENT (mwh/flr3) | |||||||
| CRB (mg/mL) | |||||||
| [4.06] | 40 | 0.275 (11) | 0.1 (4) | 0 | 0.375 (15) β | λ | |
| [130] | 44 | 0.11 (5) | 0.023 (1) | 0 | 0.13 (6) * | 69.4 | |
| SFRB (mg/mL) | |||||||
| [0.625] | 38 | 0.079 (3) | 0.053 (2) | 0.026 (1) | 0.158 (6) * | 62.8 | |
| [20] | 40 | 0.2 (8) | 0.025 (1) | 0.075 (3) | 0.3 (12) * | 29.4 | |
| TAU (mM) | |||||||
| [1] | 40 | 0.125 (5) | 0 | 0 | 0.125 (5) * | 70.6 | |
| [32] | 38 | 0.236 (9) | 0.026 (1) | 0 | 0.263 (10) * | 38.1 | |
| GLU (mM) | |||||||
| [11] | 40 | 0.075 (3) | 0.025 (1) | 0 | 0.1 (4) * | 76.5 | |
| [350] | 40 | 0.175 (7) | 0.025 (1) | 0 | 0.2 (8) * | 52.9 | |
| Mean Lifespan | Mean Lifespan Difference | Healthspan (80th Percentile) | Healthspan Difference | |
|---|---|---|---|---|
| (days) | (%) a | (days) | (%) a | |
| CRB (mg/mL) | ||||
| Control | 58.797 ± 2.27 | 0 | 37.553 ± 1.96 | 0 |
| 1.02 | 56.191 ± 2.22 | −4.4 | 34 ± 3.327 | −9.3 |
| 4.06 | 57.671 ± 2.548 | −2 | 26.625 ± 2.23 * | −29 |
| 8.13 | 61.59 ± 2.233 | 4.76 | 35.4 ± 3.637 | −5.6 |
| 32.5 | 59.742 ± 2.637 | 1.6 | 32.4 ± 3.04 | −13.6 |
| 130 | 56.425 ± 3.06 | −4 | 27.5 ± 2.1 ** | −26.6 |
| SFRB (mg/mL) | ||||
| Control | 67.08 ± 3.6 | 0 | 32.4 ± 1.78 | 0 |
| 0.16 | 62.75 ± 2.5 | −6.4 | 33.75 ± 0.975 | 4.16 |
| 0.625 | 68.46 ± 3.4 | 2.2 | 36.7 ± 3.3 | 13.27 |
| 1.27 | 68.22 ± 2.7 | 1.8 | 36.8 ± 2.21 | 13.58 |
| 5 | 64.37 ± 3.24 | −4 | 25.75 ± 0.94 ** | −20.5 |
| 20 | 54.7 ± 2.7 *** | −18.35 | 30.25 ± 1.5 | −6.6 |
| TAU (mM) | ||||
| Control | 64.97 ± 2.426 | 0 | 37.78 ± 1.94 | 0 |
| 0.25 | 67.94 ± 2.603 | 4.57 | 34.98 ± 3.954 | −7.3 |
| 1 | 66.81 ± 3.215 | 2.81 | 30.22 ± 1.878 | −20 |
| 2 | 61.83 ± 2.25 | −4.8 | 35.357 ± 0.9 | −6.4 |
| 8 | 70.142 ± 2.385 | 7.95 | 36.8 ± 2.832 | −2.6 |
| 32 | 66.04 ± 2.055 | 1.6 | 36.57 ± 3.55 | −3.2 |
| GLU (mM) | ||||
| Control | 59.67 ± 2.92 | 0 | 32.63 ± 1.49 | 0 |
| 2.7 | 61.25 ± 2.12 | 2.65 | 31.14 ± 1.53 | −4.5 |
| 11 | 64.9 ± 2.05 | 8.76 | 41 ± 3.4 | 25.65 |
| 21.86 | 63.21 ± 2.08 | 5.9 | 30.66 ± 3.62 | −5.8 |
| 87.5 | 60.5 ± 2.5 | 1.39 | 29.9 ± 1.96 | −8.4 |
| 350 | 59 ± 3.1 | −1.2 | 21.5 ± 0.76 *** | −34 |
| Primer | Forward Primer Sequence 5′ to 3′ (N) | Reverse Primer Sequence 5′ to 3′ (N) |
|---|---|---|
| ALU-C4 | GGTTAGGTATAGTGGTTTATATTTGTAATTTTAGTA (−36) | ATTAACTAAACTAATCTTAAACTCCTAACCTCA (−33) |
| ALU-M1 | ATTATGTTAGTTAGGATGGTTTCGATTTT (−29) | CAATCGACCGAACGCGA (−17) |
| LINE-1-M1 | GGACGTATTTGGAAAATCGGG (−21) | AATCTCGCGATACGCCGTT (−19) |
| SAT-α-M1 | TGATGGAGTATTTTTAAAATATACGTTTTGTAGT (−34) | AATTCTAAAAATATTCCTCTTCAATTACGTAAA (−33) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo-Fernández, M.; Valenzuela-Gómez, F.; Font, R.; Del Río-Celestino, M.; Merinas-Amo, T.; Alonso-Moraga, Á. In Vivo and In Vitro Assays Evaluating the Biological Activity of Taurine, Glucose and Energetic Beverages. Molecules 2021, 26, 2198. https://doi.org/10.3390/molecules26082198
Mateo-Fernández M, Valenzuela-Gómez F, Font R, Del Río-Celestino M, Merinas-Amo T, Alonso-Moraga Á. In Vivo and In Vitro Assays Evaluating the Biological Activity of Taurine, Glucose and Energetic Beverages. Molecules. 2021; 26(8):2198. https://doi.org/10.3390/molecules26082198
Chicago/Turabian StyleMateo-Fernández, Marcos, Fernando Valenzuela-Gómez, Rafael Font, Mercedes Del Río-Celestino, Tania Merinas-Amo, and Ángeles Alonso-Moraga. 2021. "In Vivo and In Vitro Assays Evaluating the Biological Activity of Taurine, Glucose and Energetic Beverages" Molecules 26, no. 8: 2198. https://doi.org/10.3390/molecules26082198
APA StyleMateo-Fernández, M., Valenzuela-Gómez, F., Font, R., Del Río-Celestino, M., Merinas-Amo, T., & Alonso-Moraga, Á. (2021). In Vivo and In Vitro Assays Evaluating the Biological Activity of Taurine, Glucose and Energetic Beverages. Molecules, 26(8), 2198. https://doi.org/10.3390/molecules26082198








