Exploring Effects of Chitosan Oligosaccharides on the DSS-Induced Intestinal Barrier Impairment In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Effect of COS on the Expression of MUC2 and Occludin in HT-29 and Caco-2 Cells
2.2. Comparison of Effect of COS with Dissimilar Structures on the Expression of MUC2 and Occludin in HT-29 and Caco-2 Cells
2.3. Cell Viability after COS, HWCOS and NACOS Treatment in HT-29 and Caco-2 Cells
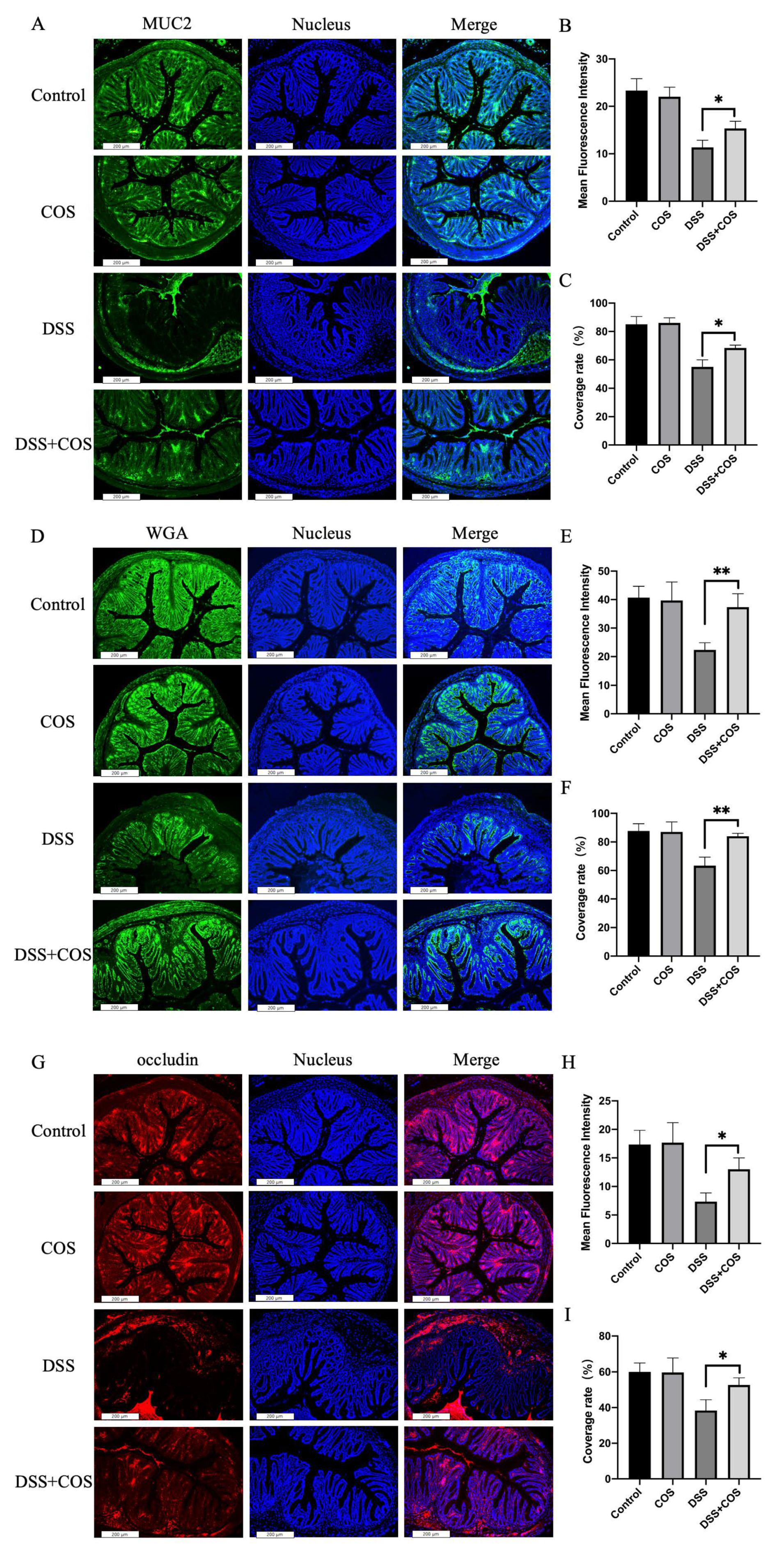
2.4. Effect of COS on Clinical Symptoms in Mice with DSS-Induced Colitis
3. Discussion
4. Materials and Methods
4.1. Preparation of COS, HWCOS and NACOS
4.2. Cell Culture
4.3. Co-Culture of COS, HWCOS, NACOS and Cells
4.4. Cell Viability Assay
4.5. Immunofluorescence Staining
4.6. RNA Extraction and Quantitative RT-PCR
4.7. Induction of Colitis and Treatment with COS
4.8. Assessment of Severity of Colitis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotze, P.G.; Underwood, F.E.; Damio, A.O.M.C.; Ferraz, J.G.P.; Kaplan, G.G. Progression of inflammatory bowel diseases throughout Latin America and the Caribbean: A Systematic Review. Clin. Gastroenterol. Hepatol. 2019, 18, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Luster, A.D.; Alon, R.; Von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Int. Congr. 2005, 1271, 135–138. [Google Scholar] [CrossRef]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; Mcdonald, D.; Gonzalez, A. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [Green Version]
- Schoultz, I.; Keita, Å. Cellular and molecular therapeutic targets in inflammatory bowel disease—Focusing on intestinal barrier function. Cells 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michielan, A.; D’Incà, R. Intestinal permeability in inflammatory bowel disease: Pathogenesis, Clinical Evaluation, and Therapy of leaky gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.; Sjvall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluis, M.V.D.; Koning, B.A.E.D.; Bruijn, A.C.J.M.D.; Velcich, A.; Meijerink, J.P.P.; Goudoever, J.B.V.; Büller, H.A.; Dekker, J.; Seuningen, I.V.; Renes, I.B. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Ma, T.; Anderson, J.M. Tight junctions and the intestinal barrier. In Physiology of the Gastrointestinal Tract, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2006; Chapter 61; pp. 1559–1594. [Google Scholar]
- Al-Sadi, R.; Nighot, P.; Nighot, M.; Haque, M.; Rawat, M.; Ma, T.Y. Lactobacillus acidophilus induces a strain-specific and toll-like receptor-2 dependent enhancement of intestinal epithelial tight junction barrier and protection against intestinal inflammation. Am. J. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mitsuro, C. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. WJG 2010, 16, 2484. [Google Scholar]
- James, S.L.; Christophersen, C.T.; Bird, A.R.; Conlon, M.A.; Rosella, O.; Gibson, P.R.; Muir, J.G. Abnormal fibre usage in UC in remission. Gut 2015, 64, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Forbes, A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, X.; Wang, Z.; Fang, J.; Yuan, T. A purified glucomannan oligosaccharide from amorphophallus konjac improves colonic mucosal barrier function via enhancing butyrate production and histone protein H3 and H4 acetylation. J. Nat. Prod. 2021, 84, 427–435. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Srlie, M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar] [CrossRef]
- Yang, C.M.; Ferket, P.R.; Hong, Q.H.; Zhou, J.; Cao, G.T.; Zhou, L.; Chen, A.G. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J. Anim. Sci. 2012, 90, 2671–2676. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. Sci. Technol. Asp. Ind. Important Polysacch. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Shin, W.; Kim, H.J. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. USA 2018, 115, E10539–E10547. [Google Scholar] [CrossRef] [Green Version]
- Han, F.F.; Zhang, H.W.; Xia, X.; Xiong, H.T.; Song, D.G.; Zong, X.; Wang, Y.Z. Porcine beta-Defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, T.; Kurozumi, S.; Kiyose, M.; Tsuka, T.; Murahata, Y.; Itoh, N.; Minami, S.; Sato, K.; Okamoto], Y. Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr. Polym. 2015, 115, 448–456. [Google Scholar]
- Wu, H.; Aam, B.B.; Wang, W.; Norberg, A.L.; Sorlie, M.; Eijsink, V.G.H.; Du, Y. Inhibition of angiogenesis by chitooligosaccharides with specific degrees of acetylation and polymerization. Carbohydr. Polym. 2012, 89, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Mengíbar, M.; Mateos-Aparicio, I.; Miralles, B.; Heras, Á. Influence of the physico-chemical characteristics of chito-oligosaccharides (COS) on antioxidant activity. Carbohydr. Polym. 2013, 97, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Yarullina, L.G.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Maksimov, I.V. Influence of chitooligosaccharides with different acetylation degrees on the H2O2 content and the activity of oathogenesis-related proteins in potato plants infected with phytophthora infestans. Appl. Biochem. Microbiol. 2018, 54, 528–534. [Google Scholar] [CrossRef]
- Mankertz, J.; Schulzke, J.D. Altered permeability in inflammatory bowel disease: Pathophysiology and clinical implications. Curr. Opin. Gastroenterol. 2007, 23, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Zhou, Q.G. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [CrossRef]
- Van der Post, S.; Thomsson, K.A.; Hansson, G.C. Multiple enzyme approach for the characterization of glycan modifications on the C-Terminus of the intestinal MUC2 mucin. J. Proteome Res. 2014, 13, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Maeda, K.; Nakamura, M.; Yamamura, T.; Sawada, T.; Mizutani, Y.; Ito, T.; Ishikawa, T.; Furukawa, K.; Ohno, E.; et al. Filtrated adipose tissue-derived mesenchymal stem cell lysate ameliorates experimental acute colitis in mice. Dig. Dis. Sci. 2021, 6, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, Y.; Song, Y.; Gao, Y.; Zhao, F.; Luo, Y.; Qian, F.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020, 11, 5205–5222. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Lin, C.-H.; Nagarajan, B.; Sankaranarayanan, N.V.; Desai, U.R.; Liu, M.-Y. Tamarind xyloglucan attenuates dextran sodium sulfate induced ulcerative colitis: Role of antioxidation. J. Funct. Foods 2018, 42, 327–338. [Google Scholar] [CrossRef]
- Huang, T.-C.; Tsai, S.-S.; Liu, L.-F.; Liu, Y.L.; Liu, H.-J.; Chuang, K.P. Effect of Arctium lappa L. in the dextran sulfate sodium colitis mouse model. World J. Gastroenterol. 2010, 16, 4193–4199. [Google Scholar] [CrossRef] [PubMed]
- Kidibule, P.E. Tailored enzymatic synthesis of chitooligosaccharides with different deacetylation degrees and their anti-inflammatory activity. Catalysts 2019, 9, 405. [Google Scholar]
- Lee, S.H.; Senevirathne, M.; Ahn, C.B.; Kim, S.K.; Je, J.Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef]
- Pangestuti, R.; Bak, S.S.; Kim, S.K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef]
- Fernandes, J.O.C.; Spindola, H.; De Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.O.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Chen, Y.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals 2019, 9, 493. [Google Scholar] [CrossRef] [Green Version]
- Junping, Z.; Gong, C.; Qiongyu, L.; Siming, J.; Cui, F.; Xiaoming, Z.; Heng, Y.; Yuguang, D.; Hongtao, L. Chitin oligosaccharide modulates gut microbiota and attenuates High-Fat-Diet-induced metabolic syndrome in mice. Mar. Drugs 2018, 16, 66. [Google Scholar]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Cani, P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Li, R.; Jiao, S.; Wei, J.; Yan, Y.; Wang, Z.A.; Li, J.; Du, Y. Blood-brain barrier permeable chitosan oligosaccharides interfere with beta-amyloid aggregation and alleviate beta-amyloid protein mediated neurotoxicity and neuroinflammation in a dose- and degree of polymerization-dependent manner. Mar. Drugs 2020, 18, 488. [Google Scholar] [CrossRef]
- Jing, B.; Wang, Z.A.; Zhang, C.; Deng, Q.; Wei, J.; Luo, Y.; Zhang, X.; Li, J.; Du, Y. Establishment and application of peristaltic human Gut-Vessel microsystem for studying Host-Microbial interaction. Front. Bioeng. Biotechnol. 2020, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.B.; Zheng, J.P.; Ren, L.S.; Jiao, S.M.; Feng, C.; Du, Y.G.; Liu, H.T. Enteromorpha prolifera oligomers relieve pancreatic injury in streptozotocin (STZ)-induced diabetic mice. Carbohydr. Polym. 2019, 206, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.C.-H.; Ibrahim, A.E.K.; Dawson, S.; Parashar, D.; Howat, W.J.; Guttula, K.; Miller, R.; Fearnhead, N.S.; Winton, D.J.; Neves, A.A.; et al. Detection of colorectal dysplasia using fluorescently labelled lectins. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yomogida, S.; Kojima, Y.; Tsutsumi-Ishii, Y.; Hua, J.; Nagaoka, I. Glucosamine, a naturally occurring amino monosaccharide, suppresses dextran sulfate sodium-induced colitis in rats. Int. J. Mol. Med. 2008, 22, 317–323. [Google Scholar] [CrossRef] [PubMed]






| Name | Molecular Weight Range | Deacetylation Degree |
|---|---|---|
| COS | 363–1329 Da | >95% |
| HWCOS | 4000–6000 Da | >90% |
| NACOS | 300–1700 Da | <10% |
| Gene | Sequences | |
|---|---|---|
| MUC2 | F: GACCCGCACTATGTCACCTT | R: GGACAGGACACCTTGTCGTT |
| OCLN | F: ACAAGCGGTTTTATCCAGAGTC | R: GTCATCCACAGGCGAAGTTAA |
| GAPDH | F: GTGAAGGTCGGAGTCAACG | R: TGAGGTCAATGAAGGGGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wen, R.; Liu, D.; Zhang, C.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on the DSS-Induced Intestinal Barrier Impairment In Vitro and In Vivo. Molecules 2021, 26, 2199. https://doi.org/10.3390/molecules26082199
Wang Y, Wen R, Liu D, Zhang C, Wang ZA, Du Y. Exploring Effects of Chitosan Oligosaccharides on the DSS-Induced Intestinal Barrier Impairment In Vitro and In Vivo. Molecules. 2021; 26(8):2199. https://doi.org/10.3390/molecules26082199
Chicago/Turabian StyleWang, Yujie, Rong Wen, Dongdong Liu, Chen Zhang, Zhuo A. Wang, and Yuguang Du. 2021. "Exploring Effects of Chitosan Oligosaccharides on the DSS-Induced Intestinal Barrier Impairment In Vitro and In Vivo" Molecules 26, no. 8: 2199. https://doi.org/10.3390/molecules26082199






