Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Nanocomposites
2.2. The Effect of pH
2.3. Contact Time and Kinetic Study
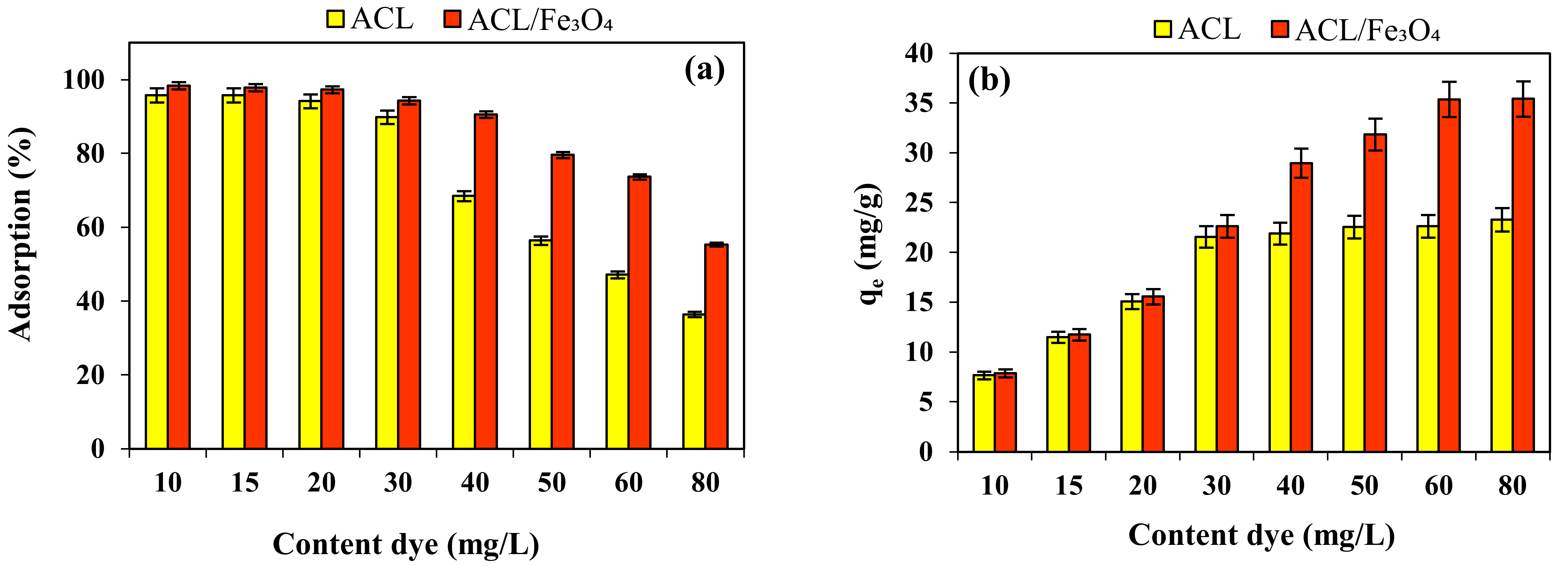
2.4. Effect of Initial CV Content and Isotherm Study
2.5. Effect of Absorbent Dose
2.6. Effect of Temperature and Thermodynamic Study
2.7. Comparison of Adsorption Capacity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Adsorbents
3.3. Characteristics of Adsorbents
3.4. Adsorption Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rai, P.; Gautam, R.K.; Banerjee, S.; Rawat, V.; Chattopadhyaya, M.C. Synthesis and characterization of a novel SnFe2O4@activated carbon magnetic nanocomposite and its effectiveness in the removal of crystal violet from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 2281–2291. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Patil, A.P.; Bhanvase, B.A.; Sonawane, S.H. Ultrasonically prepared poly(acrylamide)-kaolin composite hydrogel for removal of crystal violet dye from wastewater. J. Environ. Chem. Eng. 2015, 3, 1152–1162. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Znad, H.; Hasan, M.N. Efficient encapsulation of toxic dye from wastewater using biodegradable polymeric adsorbent. J. Mol. Liq. 2021, 329, 115541. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Esvandi, Z.; Khatooni, H.; Ramavandi, B. Evaluation of two cationic dyes removal from aqueous environments using CNT/MgO/CuFe2O4 magnetic composite powder: A comparative study. J. Environ. Chem. Eng. 2021, 9, 104752. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. African 2019, 5, e00116. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Aghdasinia, H.; Mohammadi, R.; Ramavandi, B. Modification of bio-hydroxyapatite generated from waste poultry bone with MgO for purifying methyl violet-laden liquids. Environ. Sci. Pollut. Res. 2020, 27, 44218–44229. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Aghamohammadi-Bavil, O.; Foroutan, R.; Arsalani, N. Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int. J. Biol. Macromol. 2020, 159, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Mohammadi, R.; MousaKhanloo, F.; Sahebi, S.; Ramavandi, B.; Kumar, P.S.; Vardhan, K.H. Performance of montmorillonite/graphene oxide/CoFe2O4 as a magnetic and recyclable nanocomposite for cleaning methyl violet dye-laden wastewater. Adv. Powder Technol. 2020, 31, 3993–4004. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Sohrabi, N.; Sahebi, S.; Farjadfard, S.; Esvandi, Z.; Ramavandi, B. Calcined alluvium of agricultural streams as a recyclable and cleaning tool for cationic dye removal from aqueous media. Environ. Technol. Innov. 2020, 17, 100530. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Essandoh, M.; Garcia, R.A.; Palochik, V.L.; Gayle, M.R.; Liang, C. Simultaneous adsorption of acidic and basic dyes onto magnetized polypeptidylated-Hb composites. Sep. Purif. Technol. 2021, 255, 117701. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 2021, 323, 115034. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Adsorptive decontamination of synthetic wastewater containing crystal violet dye by employing Terminalia arjuna sawdust waste. Groundw. Sustain. Dev. 2018, 7, 30–38. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Xue, K.; Ma, Q.-L.; Ma, T.; Ma, Y.-L.; Sun, Y.-G.; Ji, W.-X. Removal of hazardous crystal violet dye by low-cost P-type zeolite/carbon composite obtained from in situ conversion of coal gasification fine slag. Microporous Mesoporous Mater. 2021, 312, 110742. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Hasan, M.M.; Shenashen, M.A.; Hasan, M.N.; Znad, H.; Salman, M.S.; Awual, M.R. Natural biodegradable polymeric bioadsorbents for efficient cationic dye encapsulation from wastewater. J. Mol. Liq. 2021, 323, 114587. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A.; Jannesar, R. Comparative study on ultrasonic assisted adsorption of dyes from single system onto Fe3O4 magnetite nanoparticles loaded on activated carbon: Experimental design methodology. Ultrason. Sonochem. 2017, 34, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, H.; Xing, B. Preparation of surfactant modified magnetic expanded graphite composites and its adsorption properties for ionic dyes. Appl. Surf. Sci. 2021, 537, 147995. [Google Scholar] [CrossRef]
- Soares, S.F.; Amorim, C.O.; Amaral, J.S.; Trindade, T.; Daniel-da-Silva, A.L. On the efficient removal, regeneration and reuse of quaternary chitosan magnetite nanosorbents for glyphosate herbicide in water. J. Environ. Chem. Eng. 2021, 9, 105189. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, C.; An, L.; Zhang, X.; Wang, F.; Huang, Y.; Qu, M.; Yu, Y. Biocompatible porous boron nitride nano/microrods with ultrafast selective adsorption for dyes. J. Environ. Chem. Eng. 2021, 9, 104797. [Google Scholar] [CrossRef]
- Esvandi, Z.; Foroutan, R.; Peighambardoust, S.J.; Akbari, A.; Ramavandi, B. Uptake of anionic and cationic dyes from water using natural clay and clay/starch/MnFe2O4 magnetic nanocomposite. Surf. Interfaces 2020, 21, 100754. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.-P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Hosseini, S.V.; Regenstein, J.M. Improved mechanical and antibacterial properties of active LDPE films prepared with combination of Ag, ZnO and CuO nanoparticles. Food Packag. Shelf Life 2019, 22. [Google Scholar] [CrossRef]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M.; Ramalingam, R.J.; Al-Lohedan, H.A. Comparative investigation on the structural, morphological, optical, and magnetic properties of CoFe2O4 nanoparticles. Ceram. Int. 2017, 43, 7682–7689. [Google Scholar] [CrossRef]
- Wu, Q.; Siddique, M.S.; Yu, W. Iron-nickel bimetallic metal-organic frameworks as bifunctional Fenton-like catalysts for enhanced adsorption and degradation of organic contaminants under visible light: Kinetics and mechanistic studies. J. Hazard. Mater. 2021, 401, 123261. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Vahdati Khakhi, J. Solution combustion synthesis (SCS) of theranostic ions doped biphasic calcium phosphates; kinetic of ions release in simulated body fluid (SBF) and reactive oxygen species (ROS) generation. Mater. Sci. Eng. C 2021, 118, 111533. [Google Scholar] [CrossRef]
- Ain, Q.U.; Zhang, H.; Yaseen, M.; Rasheed, U.; Liu, K.; Subhan, S.; Tong, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J. Clean. Prod. 2020, 247, 119088. [Google Scholar] [CrossRef]
- Sukla Baidya, K.; Kumar, U. Adsorption of brilliant green dye from aqueous solution onto chemically modified areca nut husk. S. Afr. J. Chem. Eng. 2021, 35, 33–43. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef]
- Sakin Omer, O.; Hussein, M.A.; Hussein, B.H.M.; Mgaidi, A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]
- Bello, M.O.; Abdus-Salam, N.; Adekola, F.A.; Pal, U. Isotherm and kinetic studies of adsorption of methylene blue using activated carbon from ackee apple pods. Chem. Data Collect. 2021, 31, 100607. [Google Scholar] [CrossRef]
- Abdus-Salam, N.; Ikudayisi-Ugbe, A.V.; Ugbe, F.A. Adsorption studies of acid dye—Eosin yellow on date palm seeds, goethite and their composite. Chem. Data Collect. 2021, 31, 100626. [Google Scholar] [CrossRef]
- De Souza, R.M.; Quesada, H.B.; Cusioli, L.F.; Fagundes-Klen, M.R.; Bergamasco, R. Adsorption of non-steroidal anti-inflammatory drug (NSAID) by agro-industrial by-product with chemical and thermal modification: Adsorption studies and mechanism. Ind. Crops Prod. 2021, 161, 113200. [Google Scholar] [CrossRef]
- Nazar de Souza, A.P.; Licea, Y.E.; Colaço, M.V.; Senra, J.D.; Carvalho, N.M.F. Green iron oxides/amino-functionalized MCM-41 composites as adsorbent for anionic azo dye: Kinetic and isotherm studies. J. Environ. Chem. Eng. 2021, 9, 105062. [Google Scholar] [CrossRef]
- Hossein Panahi, F.; Peighambardoust, S.J.; Davaran, S.; Salehi, R. Development and characterization of PLA-mPEG copolymer containing iron nanoparticle-coated carbon nanotubes for controlled delivery of Docetaxel. Polymer 2017, 117, 117–131. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Farjadfard, S.; Esmaeili, H.; Ramavandi, B.; Sorial, G.A. Eggshell nano-particle potential for methyl violet and mercury ion removal: Surface study and field application. Adv. Powder Technol. 2019, 30, 2188–2199. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Reghioua, A.; Yaseen, Z.M. Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int. J. Biol. Macromol. 2020, 163, 756–765. [Google Scholar] [CrossRef]
- Feng, M.; Yu, S.; Wu, P.; Wang, Z.; Liu, S.; Fu, J. Rapid, high-efficient and selective removal of cationic dyes from wastewater using hollow polydopamine microcapsules: Isotherm, kinetics, thermodynamics and mechanism. Appl. Surf. Sci. 2021, 542, 148633. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Zhou, L.; Xia, G.; Chen, Z.; Duan, M.; Jiang, X. The preparation of organo-bentonite by a new gemini and its monomer surfactants and the application in MO removal: A comparative study. Chem. Eng. J. 2013, 219, 469–477. [Google Scholar] [CrossRef]
- Ahmadi, A.; Foroutan, R.; Esmaeili, H.; Tamjidi, S. The role of bentonite clay and bentonite clay@MnFe2O4 composite and their physico-chemical properties on the removal of Cr(III) and Cr(VI) from aqueous media. Environ. Sci. Pollut. Res. 2020, 27, 14044–14057. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Wang, D.; Ma, X.; Fan, L.; Ding, T.; Ye, X.; Liu, D. Enhanced adsorption of Congo red using chitin suspension after sonoenzymolysis. Ultrason. Sonochem. 2021, 70, 105327. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hosseini, S.S.; Akbari, A.; Ramavandi, B. Hydroxyapatite biomaterial production from chicken (femur and beak) and fishbone waste through a chemical less method for Cd2+ removal from shipbuilding wastewater. J. Hazard. Mater. 2021, 413, 125428. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, Z.; Kumar, P.S.; Foroutan, R.; Kafaei, R.; Arfaeinia, H.; Farjadfard, S.; Ramavandi, B. Ultrasonic-assisted synthesis of Populus alba activated carbon for water defluorination: Application for real wastewater. Korean J. Chem. Eng. 2019, 36, 1595–1603. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Mirzaee, S.A.; Martinez, S.S.; Alavi, S.; Ahmadi, M.; Jaafarzadeh, N. Adsorption of textile dye in activated carbons prepared from DVD and CD wastes modified with multi-wall carbon nanotubes: Equilibrium isotherms, kinetics and thermodynamic study. Chem. Eng. Res. Des. 2019, 141, 290–301. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of methylene blue on chemically modified lychee seed biochar: Dynamic, equilibrium, and thermodynamic study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S. The kinetic and thermodynamic study of the adsorption Lissamine Green B dye by micro-particle of wild plants from aqueous solutions. Egypt. J. Aquat. Res. 2019, 45, 231–238. [Google Scholar] [CrossRef]
- Dehghani, Z.; Sedghi-Asl, M.; Ghaedi, M.; Sabzehmeidani, M.M.; Adhami, E. Ultrasound-assisted adsorption of paraquat herbicide from aqueous solution by graphene oxide/ mesoporous silica. J. Environ. Chem. Eng. 2021, 9, 105043. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; El-Sonbati, A.Z.; Hawas, A.R. Magnetic alginate beads with high basic dye removal potential and excellent regeneration ability. Can. J. Chem. 2017, 95, 807–815. [Google Scholar] [CrossRef]
- Aref, L.; Navarchian, A.H.; Dadkhah, D. Adsorption of Crystal Violet Dye from Aqueous Solution by Poly(Acrylamide-co-Maleic Acid)/Montmorillonite Nanocomposite. J. Polym. Environ. 2017, 25, 628–639. [Google Scholar] [CrossRef]
- Abdolahi, G.; Dargahi, M.; Ghasemzadeh, H. Synthesis of starch-g-poly (acrylic acid)/ZnSe quantum dot nanocomposite hydrogel, for effective dye adsorption and photocatalytic degradation: Thermodynamic and kinetic studies. Cellulose 2020, 27, 6467–6483. [Google Scholar] [CrossRef]
- Hoang, B.N.; Nguyen, T.T.; Bui, Q.P.T.; Bach, L.G.; Vo, D.-V.N.; Trinh, C.D.; Bui, X.-T.; Nguyen, T.D. Enhanced selective adsorption of cation organic dyes on polyvinyl alcohol/agar/maltodextrin water-resistance biomembrane. J. Appl. Polym. Sci. 2020, 137, 48904. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Synthesis of Guar gum/bentonite a novel bionanocomposite: Isotherms, kinetics and thermodynamic studies for the removal of Pb (II) and crystal violet dye. J. Mol. Liq. 2018, 249, 805–814. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Das, P. Optimization of crystal violet dye removal using novel soil-silver nanocomposite as nanoadsorbent using response surface methodology. J. Environ. Chem. Eng. 2014, 2, 708–714. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A. The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: Application of ultrasound wave, optimization and modeling. Ultrason. Sonochem. 2017, 34, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Ali, H.; Muhammad, S.K. Biosorption of crystal violet from water on leaf biomass of Calotropis procera. J. Environ. Sci. Technol. 2008, 1, 143–150. [Google Scholar] [CrossRef]
- Jayasantha Kumari, H.; Krishnamoorthy, P.; Arumugam, T.K.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Druzian, S.P.; Zanatta, N.P.; Côrtes, L.N.; Streit, A.F.M.; Dotto, G.L. Preparation of chitin nanowhiskers and its application for crystal violet dye removal from wastewaters. Environ. Sci. Pollut. Res. 2019, 26, 28548–28557. [Google Scholar] [CrossRef]
- Hamidzadeh, S.; Torabbeigi, M.; Shahtaheri, S.J. Removal of crystal violet from water by magnetically modified activated carbon and nanomagnetic iron oxide. J. Environ. Heal. Sci. Eng. 2015, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Druzian, S.P.; Zanatta, N.P.; Borchardt, R.K.; Côrtes, L.N.; Streit, A.F.M.; Severo, E.C.; Gonçalves, J.O.; Foletto, E.L.; Lima, E.C.; Dotto, G.L. Chitin-psyllium based aerogel for the efficient removal of crystal violet from aqueous solutions. Int. J. Biol. Macromol. 2021, 179, 366–376. [Google Scholar] [CrossRef]
- Ganea, I.-V.; Nan, A.; Baciu, C.; Turcu, R. Effective Removal of Crystal Violet Dye Using Neoteric Magnetic Nanostructures Based on Functionalized Poly(Benzofuran-co-Arylacetic Acid): Investigation of the Adsorption Behaviour and Reusability. Nanomaterials 2021, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Shariati, S.; Besharati, N. Adsorption of Crystal Violet and Methylene Blue on Azolla and Fig Leaves Modified with Magnetite Iron Oxide Nanoparticles. Int. J. Environ. Res. 2017, 11, 197–206. [Google Scholar] [CrossRef]
- Falaki, Z.; Bashiri, H. Preparing an adsorbent from the unused solid waste of Rosewater extraction for high efficient removal of Crystal Violet. J. Iran. Chem. Soc. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Successful Application of Eucalyptus Camdulensis Biochar in the Batch Adsorption of Crystal Violet and Methylene Blue Dyes from Aqueous Solution. Sustainability 2021, 13, 3600. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J. Nanocomposite films containing organoclay nanoparticles as an antimicrobial (active) packaging for potential food application. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]










| Kinetic Model | Adsorbent | |
|---|---|---|
| ACL | ACL/Fe3O4 | |
| Pseudo-first order | ||
| qe cal | 7.51 | 7.808 |
| KP1st | 0.0608 | 0.0819 |
| R2 | 0.9151 | 0.9484 |
| RMSE | 0.5142 | 0.3687 |
| Pseudo-second order | ||
| qe.cal | 8.545 | 8.648 |
| KP2st | 0.009 | 0.0138 |
| R2 | 0.9664 | 0.9730 |
| RMSE | 0.3235 | 0.2666 |
| Elovich equation | ||
| α (mg/g min) | 2.049 | 4.34 |
| β (g/mg) | 0.6328 | 0.7057 |
| R2 | 0.9642 | 0.9156 |
| RMSE | 0.3337 | 0.4719 |
| Intraparticle diffusion | ||
| Ki,1 (mg/g min1/2) | 1.0021 | 1.0836 |
| I1 (mg/g) | 0.7179 | 1.0916 |
| R2 | 0.9887 | 0.9965 |
| Ki,2 (mg/g min1/2) | 0.8855 | 0.7777 |
| I2 (mg/g) | 0.6332 | 2.4091 |
| R2 | 0.9807 | 0.954 |
| Ki,3 (mg/g min1/2) | 0.0247 | 0.0081 |
| I3 (mg/g) | 7.4444 | 7.8024 |
| R2 | 0.9495 | 0.9696 |
| Models | Adsorbent | ||
|---|---|---|---|
| Parameters | ACL | ACL/Fe3O4 | |
| Langmuir | qm (mg/g) | 23.64 | 35.31 |
| KL (L/mg) | 1.469 | 1.366 | |
| RL | 0.008–0.063 | 0.009–0.68 | |
| R2 | 0.9704 | 0.9826 | |
| RMSE | 1.122 | 1.435 | |
| Freundlich | n | 6.5 | 4.595 |
| Kf (mg/g (L/mg)1/n) | 13.78 | 18.25 | |
| R2 | 0.78 | 0.891 | |
| Dubinin–Radushkevich (D–R) | RMSE | 3.06 | 3.591 |
| E (kJ/mol) | 1.972 | 2.364 | |
| qm (mg/g) | 22.53 | 31.91 | |
| β (mol2/J2) | 1.285 × 10−7 | 8.969 × 10−8 | |
| R2 | 0.9838 | 0.8828 | |
| Temkin | RMSE | 0.8293 | 3.723 |
| bT (kJ/mol) | 0.837 | 0.45 | |
| AT (L/g) | 94.77 | 30.79 | |
| R2 | 0.8415 | 0.9737 | |
| RMSE | 2.597 | 1.904 | |
| Adsorbent | T (°C) | ΔG° (KJ/mol) | ΔH° (KJ/mol) | ΔS° (J/mol·K) |
|---|---|---|---|---|
| ACL | 25 | −9.011 | −45.382 | −120.594 |
| 30 | −8.958 | |||
| 35 | −8.551 | |||
| 40 | −8.071 | |||
| 45 | −6.793 | |||
| 50 | -6.128 | |||
| ACL/Fe3O4 | 25 | −10.117 | −56.901 | −154.915 |
| 30 | −10.241 | |||
| 35 | −9.793 | |||
| 40 | −8.666 | |||
| 45 | −7.105 | |||
| 50 | −6.742 |
| Adsorbent | qe (mg/g) CV Dye | Reference |
|---|---|---|
| Magnetite alginate | 37.5 | [50] |
| P(AAm-MA)/MMT | 20.36 | [51] |
| Starch-g-poly (acrylic acid)/ZnSe | 10 | [52] |
| Poly (acrylamide)-kaolin composite hydrogel | 23.8 | [2] |
| Polyvinyl alcohol/agar/maltodextrin | 19.17 | [53] |
| Guar gum/bentonite bionanocomposite | 167.929 | [54] |
| Soil-silver nanocomposite | 1.918 | [55] |
| Activated carbon | 35.64 | [56] |
| NaOH-modified rice husk | 44.876 | [57] |
| Leaf biomass of Calotropis procera | 4.14 | [58] |
| TLAC/Chitosan composite | 0.269–2.375 | [59] |
| Chitin nanowhiskers | 59.52 | [60] |
| AC-Fe2O3·NPLs | 16.5 | [61] |
| Chitin-psyllium based aerogel | 227.11 | [62] |
| Poly(benzofuran-co-arylacetic acid)-FA | 25.10 | [63] |
| Azolla and fig leaves modified with magnetite iron oxide nanoparticles | 25 | [64] |
| Solid waste of rosewater extraction | 78.24 | [65] |
| Eucalyptus camdulensis sawdust-derived biochar (Ec-bio) | 54.7 | [66] |
| ACL | 23.64 | This study |
| ACL/Fe3O4 magnetic nanocomposite | 35.31 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. https://doi.org/10.3390/molecules26082241
Foroutan R, Peighambardoust SJ, Peighambardoust SH, Pateiro M, Lorenzo JM. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules. 2021; 26(8):2241. https://doi.org/10.3390/molecules26082241
Chicago/Turabian StyleForoutan, Rauf, Seyed Jamaleddin Peighambardoust, Seyed Hadi Peighambardoust, Mirian Pateiro, and Jose M. Lorenzo. 2021. "Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study" Molecules 26, no. 8: 2241. https://doi.org/10.3390/molecules26082241
APA StyleForoutan, R., Peighambardoust, S. J., Peighambardoust, S. H., Pateiro, M., & Lorenzo, J. M. (2021). Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules, 26(8), 2241. https://doi.org/10.3390/molecules26082241









