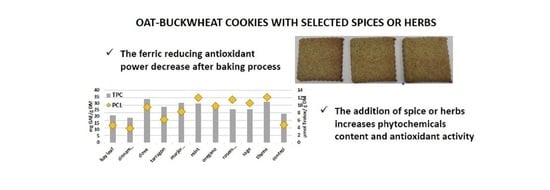
Phytochemicals and Antioxidant Activity in Oat-Buckwheat Dough and Cookies with Added Spices or Herbs
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolics Content (TPC)
2.2. Determination of Bioactive Compounds by HPLC-MS/MS
2.3. Determination of Reducing Potential and Antioxidant Activity
2.4. Correlation Study
3. Materials and Methods
3.1. Chemicals
3.2. Materials
3.3. Cookies Preparation
3.4. Extraction Procedure
3.5. Determination of Total Phenolic Content (TPC)
3.6. Determination of Contents of Phenolic Acids and Flavonoids Using High-Pressure Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
3.7. Determination of Reducing Potential by the Ferric Reducing Power (FRAP) Assay and Antioxidant Activity Using the Photochemiluminescence (PCL) Method
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Varma, P.; Bhankharia, H.; Bhatia, S. Oats: A multi-functional grain. J. Clin. Prev. Cardiol. 2016, 5, 9–17. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Bączek, N.; Jarmułowicz, A.; Wronkowska, M.; Haros, C.M. Assessment of the glycaemic index, content of bioactive compounds, and their in vitro bioaccessibility in oat-buckwheat breads. Food Chem. 2020, 330, 127199. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Szawara-Nowak, D.; Wronkowska, M. Bioaccessibility of anti-AGEs activity, antioxidant capacity and phenolics from water biscuits prepared from fermented buckwheat flours. LWT Food Sci. Technol. 2020, 123, 109051. [Google Scholar] [CrossRef]
- Wronkowska, M.; Jarmułowicz, A.; Lamparski, G.; Jeliński, T.; Haros, C.M. Oat-buckwheat breads—Technological quality, staling and sensory properties. Irish J. Agric. Food Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Olech, M. The impact of processing parameters on the content of phenolic compounds in new gluten-free precooked buckwheat pasta. Molecules 2019, 24, 1262. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 676:1995. 1995. Spices and Condiments—Botanical Nomenclature. Available online: https://www.iso.org/standard/4844.html (accessed on 15 March 2021).
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini-review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Papageorgiou, M. Aromatic plants of Lamiaceae family in a traditional bread recipe: Effects on quality and phytochemical content. J. Food Biochem. 2019, 43, e13020. [Google Scholar] [CrossRef]
- Przygodzka, M.; Zielińska, D.; Ciesarová, Z.; Kukurová, K.; Zieliński, H. Comparison of methods for evaluation of the antioxidant capacity and phenolic compounds in common spices. LWT-Food Sci. Technol. 2014, 58, 321. [Google Scholar] [CrossRef]
- Mišan, A.; Mimica-Dukić, N.; Sakač, M.; Mandić, A.; Sedej, I.; Šimurina, O.; Tumbas, V. Antioxidant activity of medicinal plant extracts in cookies. J. Food Sci. 2011, 76, C1239–C1244. [Google Scholar] [CrossRef]
- Polovka, M.; Suhaj, M. The effect of irradiation and heat treatment on the composition and antioxidant properties of culinary herbs and spices—A review. Food Rev. Int. 2010, 26, 138. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef]
- Assefa, A.D.; Keum, Y.-S.; Saini, R.K. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J. Food Meas. Charact. 2018, 12, 1548–1555. [Google Scholar] [CrossRef]
- Nagy, T.O.; Solar, S.; Sontag, G.; Koenig, J. Identification of phenolic components in dried spices and the influence of irradiation. Food Chem. 2011, 128, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Scarpelli, F.; Belsito, E.L.; Caputo, P.; Rossi, C.O.; Mincione, A.; Leggio, A.; Crispini, A.; Restuccia, D.; Spizzirri, U.G.; et al. Formulation of new baking (+)-catechin based leavening agents: Effects on rheology, sensory and antioxidant features during muffin preparation. Foods 2020, 9, 1569. [Google Scholar] [CrossRef]
- Jan, U.; Ahmad, M.; Shah, U.; Baba, W.N.; Masoodi, F.A.; Maqsood, S.; Gani, A.; Wani, I.A.; Wani, S.M. Characterization of cookies made from wheat flour blended with buckwheat flour and effect on antioxidant properties. J. Food Sci. Technol. 2015, 52, 6334–6344. [Google Scholar] [CrossRef]
- Li, W.D.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Rosales-Soto, M.U.; Powers, J.R.; Alldredge, J.R. Effect of mixing time, freeze-drying and baking on phenolics, anthocyanins and antioxidant capacity of raspberry juice during processing of muffins. J. Sci. Food Agric. 2012, 92, 1511–1518. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Wang, S.Y. Antioxidant capacity of berry crops, culinary herbs, and medicinal herbs. Acta Hortic. 2003, 620, 461–473. [Google Scholar] [CrossRef]
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef]
- Guglielmetti, A.; Fernandez-Gomez, B.; Zeppa, G.; del Castillo, M.D. Nutritional quality, potential health-promoting properties, and sensory perception of an improved gluten-free bread formulation containing inulin, rice protein, and bioactive compounds extracted from coffee. Pol. J. Food Nutr. Sci. 2019, 69, 157–166. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs, and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Antioxidant capacity of foods for scavenging reactive oxidants and inhibition of plasma lipid oxidation induced by multiple oxidants. Food Funct. 2016, 7, 2156–2168. [Google Scholar] [CrossRef]
- Arashahi-D, S.; Vishalakshi, D.; Urooj, A. Evaluation of the antioxidant activity of some plant extracts and their heat, pH, and storage stability. Food Chem. 2007, 100, 1100–1105. [Google Scholar] [CrossRef]
- Shobana, S.; Akhilender Naidu, K. Antioxidant activity of selected Indian spices. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 107–110. [Google Scholar] [CrossRef]
- Lagouri, V.; Boskou, D. Screening for antioxidant activity of essential oils obtained from spices. Dev. Food Sci. 1995, 37, 869–879. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; HernándezCarlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; Volume 1. [Google Scholar] [CrossRef]
- Przygodzka, M.; Zieliński, H.; Ciesarová, Z.; Kukurová, K.; Lamparski, G. Effect of selected spices on chemical and sensory markers in fortified rye-buckwheat cakes. Food Sci. Nutr. 2016, 4, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Horszwald, A.; Andlauer, W. Characterization of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Sawicki, T.; Mitrus, J.; Kasprzykowski, Z.; Horbowicz, M. Profile of phenolic acids and antioxidant capacity in organs of common buckwheat sprout. Acta Aliment. 2016, 45, 250–257. [Google Scholar] [CrossRef]
- Zieliński, H.; Ciesarová, Z.; Kukurovâ, K.; Zielińska, D.; Szawara-Nowak, D.; Starowicz, M.; Wronkowska, M. Effect of fermented and unfermented buckwheat flour on functional properties of gluten-free muffins. J. Food Sci. Technol. 2017, 54, 1425–1432. [Google Scholar] [CrossRef] [PubMed]


| Sample | Ferulic Acid | Sinapic Acid | p-Coumaric Acid | Rosmarinic Acid | Chlorogenic Acid | Vanillic Acid | Caffeic Acid | Total |
|---|---|---|---|---|---|---|---|---|
| Spices/herbs | ||||||||
| bay leaf | nd | nd | 6.49 ± 0.02 e | 8.18 ± 0.45 e | 8.41 ± 0.25d e | nd | nd | 23.08 |
| cinnamon | nd | nd | nd | 8.39 ± 0.58 e | nd | nd | nd | 8.39 |
| clove | 12.59 ± 1.05 b | 20.17 ± 2.24 a | 35.47 ± 0.79 b | nd | 291.20 ± 15.67 b | nd | 10.95 ± 0.65 e | 370.38 |
| tarragon | 51.67 ± 3.48 a | 4.60 ± 0.57 b | 16.71 ± 1.53 c | 1855.01 ± 121.29 d | 789.94 ± 89.61 a | nd | 101.47 ± 5.84 d,e | 2819.40 |
| marjoram | nd | nd | 16.27 ± 1.88 c | 8376.82 ± 364.39 a | 57.99 ± 4.79 d | nd | 263.49 ± 26.36 c | 8714.57 |
| mint | 7.11 ± 0.58 c | 1.04 ± 0.04 c | 11.42 ± 0.31 d | 5314.19 ± 110.39 c | 55.81 ± 1.31 d | nd | 180.57 ± 1.55 c,d | 5570.14 |
| oregano | nd | 2.01 ± 0.08 bc | 12.56 ± 0.35 d | 3454.52 ± 223.56 c | 155.22 ± 13.07 c | nd | 147.31 ± 5.77 c,d | 3771.62 |
| rosemary | 1.78 ± 0.13 d | 3.47 ± 0.16 bc | 13.34 ± 1.09 d | 6715.85 ± 604.95 b | 71.07 ± 0.14 d | 44.21 ± 2.98 | 508.66 ± 35.12 b | 7358.38 |
| sage | 2.30 ± 0.12 d | nd | 1364.00 ± 0.44 a | 7914.83 ± 74.30 a | 7.34 ± 1.33d e | nd | 757.39 ± 72.81 a | 10,045.86 |
| thyme | 1.29 ± 0.14 d | nd | 9.13 ± 0.21 d,e | 7528.76 ± 98.37 a | 41.73 ± 2.01 d | nd | 554.50 ± 39.25 b | 8135.41 |
| Cookies with | ||||||||
| bay leaf | nd | nd | nd | 5.15 ± 0.12 e | nd | nd | nd | 5.15 |
| cinnamon | nd | nd | nd | nd | nd | nd | nd | - |
| clove | 0.29 ± 0.02 b | nd | nd | nd | 6.84 ± 0.25 b | nd | nd | 7.13 |
| tarragon | 0.63 ± 0.10 a | nd | nd | 132.91 ± 14.94 c | 91.59 ± 5.62 a | nd | 5.87 ± 0.96 d | 231.00 |
| marjoram | nd | nd | nd | 174.55 ± 6.54 b | 1.25 ± 0.07 b,c | nd | 12.10 ± 0.47 c | 187.90 |
| mint | 0.21 ± 0.01 b | nd | nd | 151.82 ± 5.18 c | 1.90 ± 0.20 b,c | nd | 14.27 ± 0.31 b | 168.20 |
| oregano | nd | nd | nd | 103.18 ± 7.25 d | 2.60 ± 0.11 b,c | nd | 7.28 ± 0.28 d | 113.06 |
| rosemary | 0.25 ± 0.07 b | nd | nd | 107.24 ± 4.28 d | 1.51 ± 0.03 b,c | nd | 13.25 ± 0.26 b,c | 122.25 |
| sage | nd | nd | nd | 196.50 ± 7.17 a | 1.37 ± 0.21 b,c | nd | 19.46 ± 0.89 a | 217.33 |
| thyme | nd | nd | nd | 129.19 ± 5.86 c | 1.92 ± 0.04 b,c | nd | 12.48 ± 0.88 c | 143.59 |
| control | nd | nd | nd | 6.63 ± 0.37 e | nd | nd | 2.98 ± 0.32 e | 9.61 |
| Sample | Apigenin | Rutin | Catechin | Naringenin | Luteolin | Vitexin | Kaempferol | Total |
|---|---|---|---|---|---|---|---|---|
| Spices/herbs | ||||||||
| bay leaf | nd | 0.28 ± 0.04 c | 315.85 ± 18.81 a | 1.19 ± 0.04 f | nd | 444.55 ± 27.47 a | 4.59 ± 0.20 d | 766.46 |
| cinnamon | nd | nd | 39.15 ± 1.98 b | 0.71 ± 0.03 f | nd | nd | nd | 39.86 |
| clove | nd | 0.03 ± 0.00 e | 37.69 ± 1.17 b | 14.47 ± 0.34 e | 137.26 ± 5.62 c | nd | 30.54 ± 2.18 a | 219.99 |
| tarragon | 5.48 ± 0.09 c | 0.42 ± 0.04 b | nd | 93.82 ± 6.52 b | 20.76 ± 0.20 e | nd | 17.75 ± 2.47 b | 138.23 |
| marjoram | 13.54 ± 0.92 b | 0.05 ± 0.00 e | nd | 24.98 ± 3.37 d | 236.57 ± 8.61 b | 277.90 ± 14.48 b | 6.43 ± 0.40 c | 559.47 |
| mint | 15.19 ± 0.91 a,b | 0.64 ± 0.04 a | 23.88 ± 1.21 b | 20.18 ± 2.32 d,e | 903.24 ± 35.13 a | nd | 7.73 ± 0.12 c | 970.86 |
| oregano | 15.82 ± 0.78 a | 0.10 ± 0.00 d,e | nd | 73.71 ± 6.62 c | 123.18 ± 4.31 c | nd | 9.31 ± 0.26 c | 222.12 |
| rosemary | 7.09 ± 0.10 c | 0.15 ± 0.02 d | nd | 0.93 ± 0.03 f | 197.46 ± 20.96 b | nd | nd | 205.63 |
| sage | 3.03 ± 0.25 d | 0.03 ± 0.00 e | nd | 0.77 ± 0.05 f | 107.77 ± 18.44 c | nd | nd | 111.6 |
| thyme | 4.75 ± 0.30 c | 0.02 ± 0.00 e | nd | 109.22 ± 4.12 a | 84.96 ± 1.88 d | 67.66 ± 3.51 c | 3.56 ± 0.36 d | 270.17 |
| Cookies with | ||||||||
| bay leaf | nd | 0.01 ± 0.00 | 11.69 ± 1.40 a | 0.55 ± 0.03 cd | nd | nd | nd | 12.25 |
| cinnamon | nd | 0.02 ± 0.00 | 9.70 ± 0.28 b | 0.29 ± 0.02 d | nd | nd | nd | 10.01 |
| clove | nd | 0.02 ± 0.00 | 15.19 ± 0.93 a | 0.27 ± 0.04 d | 1.53 ± 0.17 d | nd | nd | 17.01 |
| tarragon | nd | 0.02 ± 0.00 | 2.67 ± 0.14 b,c | 2.54 ± 0.45 a | nd | nd | nd | 11.09 |
| marjoram | nd | 0.02 ± 0.00 | 1.10 ± 0.56 c | 0.55 ± 0.05 c,d | 5.32 ± 0.35 b | nd | nd | 16.88 |
| mint | nd | 0.05 ± 0.00 | 12.96 ± 0.47 a | 0.78 ± 0.09 c | 36.60 ± 0.90 a | nd | nd | 50.39 |
| oregano | nd | 0.02 ± 0.00 | 1.41 ± 0.43 c | 1.41 ± 0.03 b | 3.42 ± 0.05 c | nd | nd | 6.26 |
| rosemary | nd | 0.02 ± 0.00 | 1.38 ± 0.24 c | nd | 3.37 ± 0.04 c | nd | nd | 4.77 |
| sage | nd | 0.03 ± 0.00 | 1.10 ± 0.07 c | nd | 2.21 ± 0.05 d | nd | nd | 3.35 |
| thyme | nd | 0.02 ± 0.00 | nd | 1.44 ± 0.02 b | 1.60 ± 0.06 d | nd | nd | 3.06 |
| control | nd | 0.02 ± 0.00 | 9.19 ± 0.10 b | nd | nd | nd | nd | 9.21 |
| Sample | FRAP | ||
|---|---|---|---|
| Spices/Herbs | Doughs | Cookies | |
| bay leaf | 244.64 ± 2.85 d,e | 92.63 ± 3.84 c | 7.30 ± 0.39 c,d |
| cinnamon | 220.76 ± 11.14 e | 92.19 ± 2.33 c | 6.70 ± 0.28 d |
| clove | 508.77 ± 8.57 a | 137.01 ± 2.38 a | 11.68 ± 1.15 a |
| tarragon | 374.44 ± 0.53 b | 92.93 ± 3.26 c | 9.64 ± 0.63 b |
| marjoram | 293.27 ± 5.87 c | 100.91 ± 2.80 c,b | 10.61 ± 0.67 a,b |
| mint | 378.76 ± 9.31 b | 108.79 ± 5.72 b | 10.51 ± 0.53 a,b |
| oregano | 331.68 ± 7.14 c | 93.55 ± 0.99 c | 9.59 ± 0.47 b |
| rosemary | 469.55 ± 37.06 a | 99.56 ± 2.50 c | 8.92 ± 0.56 bc |
| sage | 392.18 ± 13.95 b | 95.39 ± 1.32 c | 8.94 ± 0.49 b,c |
| thyme | 284.77 ± 17.89 d | 98.34 ± 2.44 c | 10.96 ± 0.64 a |
| control | - | 6.50 ± 0.73 d | 7.77 ± 0.25 c,d |
| Sample | ACW | ACL | PCL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spices/Herbs | Doughs | Cookies | Spices/Herbs | Doughs | Cookies | Spices/Herbs | Doughs | Cookies | |
| bay leaf | 1741.09 ± 132.78 a | 7.91 ± 0.33 d | 4.02 ± 0.17 c | 385.61 ± 7.80 g | 14.34 ± 0.64 c | 9.35 ± 0.40 g | 2126.7 | 22.25 | 13.37 |
| cinnamon | 732.08 ± 24.00 c | 7.15 ± 0.39 d | 1.86 ± 0.08 c | 259.26 ± 3.03 h | 13.20 ± 0.38 c | 9.12 ± 0.38 g | 991.34 | 20.35 | 10.98 |
| clove | 873.00 ± 35.98 c | 21.98 ± 0.59 b | 15.60 ± 3.38 a | 2268.13 ± 62.96 a | 20.62 ± 0.72 a | 11.69 ± 0.41 f | 3141.13 | 42.60 | 27.29 |
| tarragon | 993.16 ± 60.82 b | 14.28 ± 3.84 c | 4.89 ± 0.12 c | 454.01 ± 21.93 g | 13.78 ± 0.69 c | 12.70 ± 0.45 e | 1447.17 | 28.06 | 17.59 |
| marjoram | 865.51 ± 55.60 bc | 17.98 ± 1.93 bc | 8.55 ± 0.32 b | 784.96 ± 24.45 f | 17.48 ± 0.64 b | 15.40 ± 0.43 d | 1650.47 | 35.46 | 23.95 |
| mint | 821.90 ± 38.18 c | 27.81 ± 1.86 a | 14.59 ± 0.26 a | 1018.38 ± 37.20 e | 18.25 ± 1.19 b | 19.86 ± 0.19 a,b | 1840.28 | 46.06 | 34.45 |
| oregano | 1097.30 ± 70.56 b | 14.09 ± 1.08 c | 10.50 ± 0.15 b | 1307.33 ± 16.96 c | 15.60 ± 0.76 c | 17.46 ± 0.93 c | 2490.26 | 29.69 | 27.96 |
| rosemary | 889.28 ± 44.23 b,c | 18.47 ± 1.09 b | 11.53 ± 0.32 b | 1393.26 ± 48.06 b,c | 19.09 ± 0.74 a | 21.28 ± 0.54 a | 2282.54 | 37.56 | 32.81 |
| sage | 6.96 ± 0.29 e | 12.93 ± 2.39 c,d | 11.84 ± 0.51 a,b | 1457.30 ± 21.57 b | 16.15 ± 0.59 b | 18.43 ± 0.72 b | 1464.26 | 29.08 | 30.27 |
| thyme | 3.58 ± 0.24 e | 15.12 ± 1.97 b,c | 16.47 ± 1.40 a | 1113.25 ± 27.86 d | 14.76 ± 1.00 c | 18.23 ± 0.41 b | 1116.83 | 29.88 | 34.70 |
| control | - | 6.83 ± 0.06 d | 3.05 ± 0.04 c | - | 11.09 ± 0.45 d | 10.34 ± 0.22 f | - | 17.92 | 13.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starowicz, M.; Arpaci, S.; Topolska, J.; Wronkowska, M. Phytochemicals and Antioxidant Activity in Oat-Buckwheat Dough and Cookies with Added Spices or Herbs. Molecules 2021, 26, 2267. https://doi.org/10.3390/molecules26082267
Starowicz M, Arpaci S, Topolska J, Wronkowska M. Phytochemicals and Antioxidant Activity in Oat-Buckwheat Dough and Cookies with Added Spices or Herbs. Molecules. 2021; 26(8):2267. https://doi.org/10.3390/molecules26082267
Chicago/Turabian StyleStarowicz, Małgorzata, Saruhan Arpaci, Joanna Topolska, and Małgorzata Wronkowska. 2021. "Phytochemicals and Antioxidant Activity in Oat-Buckwheat Dough and Cookies with Added Spices or Herbs" Molecules 26, no. 8: 2267. https://doi.org/10.3390/molecules26082267
APA StyleStarowicz, M., Arpaci, S., Topolska, J., & Wronkowska, M. (2021). Phytochemicals and Antioxidant Activity in Oat-Buckwheat Dough and Cookies with Added Spices or Herbs. Molecules, 26(8), 2267. https://doi.org/10.3390/molecules26082267