Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| PON1 | paraoxonase 1 |
References
- Satapathy, S.K.; Sanyal, A.J. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin. Liver Dis. 2015, 35, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Babu, S.; Krishnan, B.; Ravindran, N.C. Non-alcoholic fatty liver disease: A clinical update. J. Clin. Transl. Hepatol. 2017, 5, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Spahis, S.; Delvin, E.; Borys, J.M.; Levy, E. Oxidativestress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef]
- Ganesh, S.; Rustgi, V.K. Current pharmacologic therapy for nonalcoholic fatty liver disease. Clin. Liver Dis. 2016, 20, 351–364. [Google Scholar] [CrossRef]
- Chen, J.T.; Kotani, K. Astaxanthin as a potential protector of liver function: A review. J. Clin. Med. Res. 2016, 8, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Ferré, N.; Camps, J.; Prats, E.; Vilella, E.; Paul, A.; Figuera, L.; Joven, J. Serum paraoxonase activity: A new additional test for the improved evaluation of chronic liver damage. Clin. Chem. 2002, 48, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sariçam, T.; Kircali, B.; Köken, T. Assessment of lipid peroxidation and antioxidant capacity in non-alcoholic fatty liver disease. Turk. J. Gastroenterol. 2005, 16, 65–70. [Google Scholar] [PubMed]
- Perlemuter, G.; Davit-Spraul, A.; Cosson, C.; Conti, M.; Bigorgne, A.; Paradis, V.; Corre, M.P.; Prat, L.; Kuoch, V.; Basdevant, A.; et al. Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int. 2005, 25, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Ravasco, P.; Jesus, L.; Marques-Vidal, P.; Oliveira, C.R.; Proença, T.; Baldeiras, I.; Camilo, M.E.; Cortez-Pinto, H. Blood oxidative stress markers in non-alcoholic steatohepatitis and how it correlates with diet. Scand. J. Gastroenterol. 2008, 43, 95–102. [Google Scholar] [CrossRef]
- Camps, J.; Joven, J. Chemokine ligand 2 and paraoxonase-1 in non-alcoholic fatty liver disease: The search for alternative causative factors. World J. Gastroenterol. 2015, 21, 2875–2882. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Targeting paraoxonase-1 in atherosclerosis. Expert. Opin. Ther. Targets 2013, 17, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M. Paraoxonases and cardiovascular diseases: Pharmacological and nutritional influences. Curr. Opin. Lipidol. 2005, 16, 393–399. [Google Scholar] [CrossRef]
- Furlong, C.E.; Suzuki, S.M.; Stevens, R.C.; Marsillach, J.; Richter, R.J.; Jarvik, G.P.; Checkoway, H.; Samii, A.; Costa, L.G.; Griffith, A.; et al. Human PON1, a biomarker of risk of disease and exposure. Chem. Biol. Interact. 2010, 187, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aharoni, S.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) reduces macrophage in-flammatory responses. Atherosclerosis 2013, 228, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Draganov, D.I. Lactonases with organophosphatase activity: Structural and evolutionary perspectives. Chem. Biol. Interact. 2010, 187, 370–372. [Google Scholar] [CrossRef]
- Rogovsky, H.; Hugenmatter, A.; Tawfik, D.S. The evolutionary origins of detoxifying enzymes: The mammalian serum paraoxonases (PONs) relate to bacterial homoserine lactonases. J. Biol. Chem. 2013, 288, 23914–23927. [Google Scholar] [CrossRef] [Green Version]
- Aviram, M.; Rosenblat, M.; Billecke, S.; Erogul, J.; Sorenson, R.; Bisgaier, C.L.; Newton, R.S.; La Du, B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic. Biol. Med. 1999, 26, 892–904. [Google Scholar] [CrossRef]
- Camps, J.; Marsillach, J.; Joven, J. The paraoxonases: Role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 2009, 46, 83–106. [Google Scholar] [CrossRef]
- Perła-Kaján, J.; Jakubowski, H. Paraoxonase 1 and homocysteine metabolism. Amino Acids 2012, 43, 1405–1417. [Google Scholar] [CrossRef]
- Gugliucci, A.; Kotani, K.; Kimura, S. Paraoxonase 1 in chronic kidney failure. J. Lipids 2012, 2012, 726048. [Google Scholar] [CrossRef] [Green Version]
- Menini, T.; Gugliucci, A. Paraoxonase 1 in neurological disorders. Redox Rep. 2014, 19, 49–58. [Google Scholar] [CrossRef]
- Gugliucci, A. Paraoxonase 1 and its clinical relevance. In The HDL Handbook, 3rd ed.; Komoda, T., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 187–208. [Google Scholar]
- Kameyama, N.; Maruyama, C.; Kotani, K.; Caccavello, R.; Gugliucci, A.; Matsui, S.; Araki, R.; Maruyama, T. Postprandial paraoxonase 1 activity following consumption of recommended amounts of mixed meals in healthy males. J. Atheroscler. Thromb. 2016, 23, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Ferré, N.; Marsillach, J.; Camps, J.; Mackness, B.; Mackness, M.; Riu, F.; Coll, B.; Tous, M.; Joven, J. Paraoxonase-1 is associated with oxidative stress, fibrosis and FAS expression in chronic liver diseases. J. Hepatol. 2006, 45, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, R.N.; Zhu, Y.R.; Xing, J.C.; Lou, X.W.; He, Y.J.; Ding, Q.L.; Zhang, M.Y.; Qiu, H. Involvement of xanthine oxidase and paraoxonase 1 in the process of oxidative stress in nonalcoholic fatty liver disease. Mol. Med. Rep. 2017, 15, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Başkol, M.; Başkol, G.; Deniz, K.; Ozbakir, O.; Yücesoy, M. A new marker for lipid peroxidation: Serum paraoxonase activity in non-alcoholic steatohepatitis. Turk. J. Gastroenterol. 2005, 16, 119–123. [Google Scholar]
- Baskol, G.; Baskol, M.; Kocer, D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin. Biochem. 2007, 40, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Samy, W.; Hassanian, M.A. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab. J. Gastroenterol. 2011, 12, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Bahari, A.; Hashemzehi, N.; Moazeni-Roodi, A.; Shafieipour, S.; Bakhshipour, A.; Ghavami, S. Serum paraoxonase and arylesterase activities in Iranian patients with nonalcoholic fatty liver disease. Pathophysiology 2012, 19, 115–119. [Google Scholar] [CrossRef]
- Torun, E.; Gökçe, S.; Ozgen, İ.T.; Aydın, S.; Cesur, Y. Serum paraoxonase activity and oxidative stress and their relationship with obesity-related metabolic syndrome and non-alcoholic fatty liver disease in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2014, 27, 667–675. [Google Scholar] [CrossRef]
- Desai, S.; Baker, S.S.; Liu, W.; Moya, D.A.; Browne, R.W.; Mastrandrea, L.; Baker, R.D.; Zhu, L. Paraoxonase 1 and oxidative stress in paediatric non-alcoholic steatohepatitis. Liver Int. 2014, 34, 110–117. [Google Scholar] [CrossRef]
- Fedelesova, M.; Kupcova, V.; Luha, J.; Turecky, L. Paraoxonase activity in sera of patients with non-alcoholic fatty liver disease. Bratisl. Lek. Listy 2017, 118, 719–720. [Google Scholar] [CrossRef] [Green Version]
- Youness, E.R.; Aly, H.F.; Nemr, M.E. Role of Apelin/monocyte chemoattractant protein-1, inflammatory, apoptotic markers in the regulation of patients with non-alcoholic fatty liver disease. Asian J. Pharm. Clin. Res. 2018, 11, 138–142. [Google Scholar] [CrossRef]
- Cabré, N.; Luciano-Mateo, F.; Fernández-Arroyo, S.; Baiges-Gayà, G.; Hernández-Aguilera, A.; Fibla, M.; Fernández-Julià, R.; París, M.; Sabench, F.; Castillo, D.D.; et al. Laparoscopic sleeve gastrectomy reverses non-alcoholic fatty liver disease modulating oxidative stress and inflammation. Metabolism 2019, 99, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fadaei, R.; Meshkani, R.; Poustchi, H.; Fallah, S.; Moradi, N.; Panahi, G.; Merat, S.; Golmohammadi, T. Association of carotid intima media thickness with atherogenic index of plasma, apo B/apo A-I ratio and paraoxonase activity in patients with non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 2019, 125, 19–24. [Google Scholar] [CrossRef]
- Janac, J.; Zeljkovic, A.; Jelic-Ivanovic, Z.; Dimitrijevic-Sreckovic, V.; Miljkovic, M.; Stefanovic, A.; Munjas, J.; Vekic, J.; Kotur-Stevuljevic, J.; Spasojević-Kalimanovska, V. The association between lecithin-cholesterol acyltransferase activity and fatty liver index. Ann. Clin. Biochem. 2019, 56, 583–592. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.H.; Gruppen, E.G.; James, R.W.; Bakker, S.J.L.; Dullaart, R.P.F. Serum paraoxonase 1 activity is paradoxically maintained in nonalcoholic fatty liver disease despite low HDL cholesterol. J. Lipid Res. 2019, 60, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, G.; Neafsey, P.; Hattis, D.; Guyton, K.Z.; Johns, D.O.; Sonawane, B. Genetic polymorphism in paraoxonase 1 (PON1): Population distribution of PON1 activity. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 473–507. [Google Scholar] [CrossRef]
- Kotani, K.; Tsuzaki, K.; Sakane, N. Paraoxonase-1 gene Q192R polymorphism and reactive oxygen metabolites. J. Int. Med. Res. 2012, 40, 1513–1518. [Google Scholar] [CrossRef] [Green Version]
- Stern, C.; Castera, L. Non-invasive diagnosis of hepatic steatosis. Hepatol. Int. 2017, 11, 70–78. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef]
- Abid, A.; Taha, O.; Nseir, W.; Farah, R.; Grosovski, M.; Assy, N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J. Hepatol. 2009, 51, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Al-Jiffri, O.H. Oxidative stress biomarkers among Saudi patients with non-alcoholic steatohepatitis versus chronic hepatitis C. Eur. J. Gen. Med. 2016, 13, 81–85. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1. 2020. Available online: https://training.cochrane.org/handbook/current (accessed on 22 March 2021).
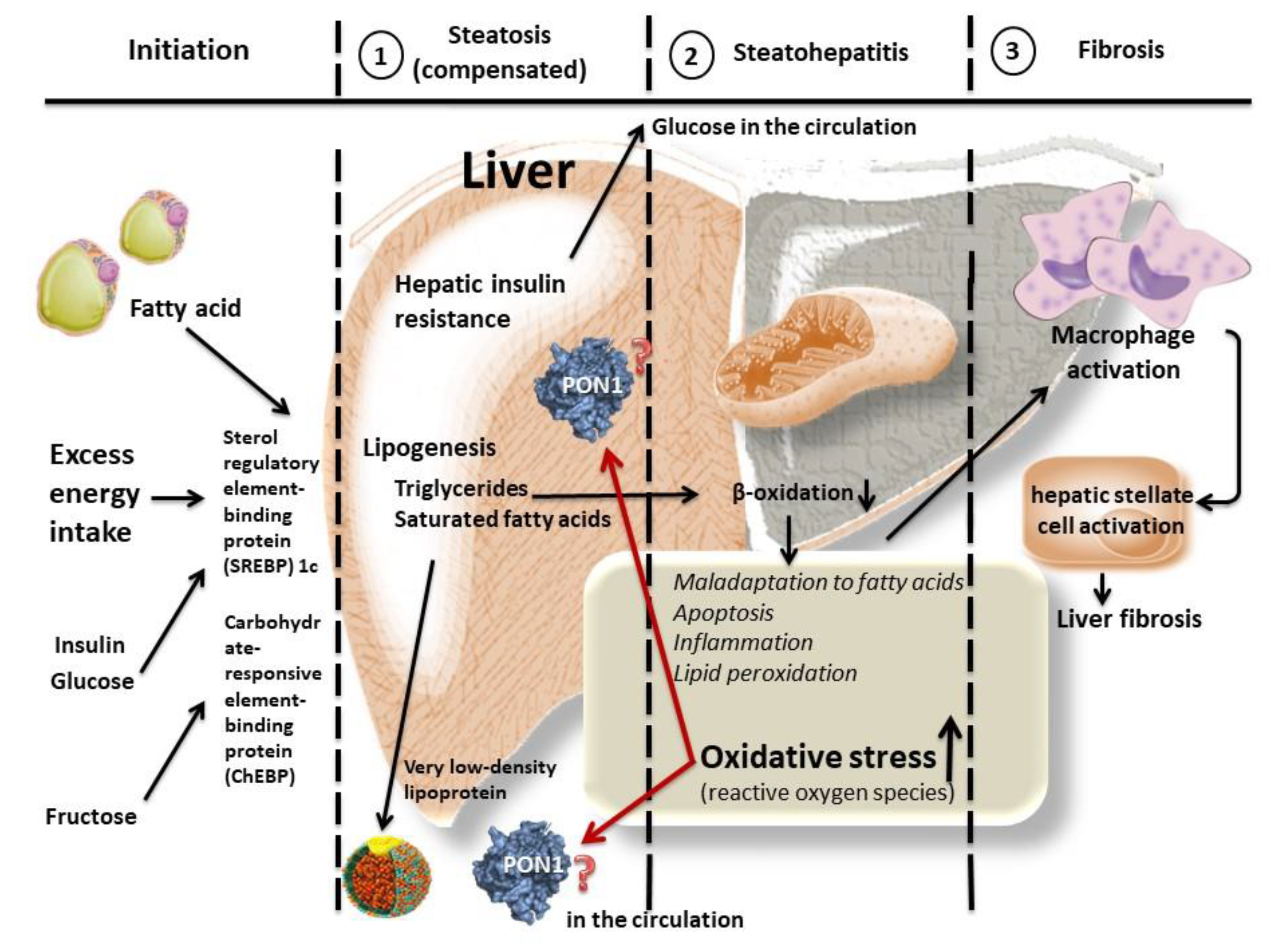
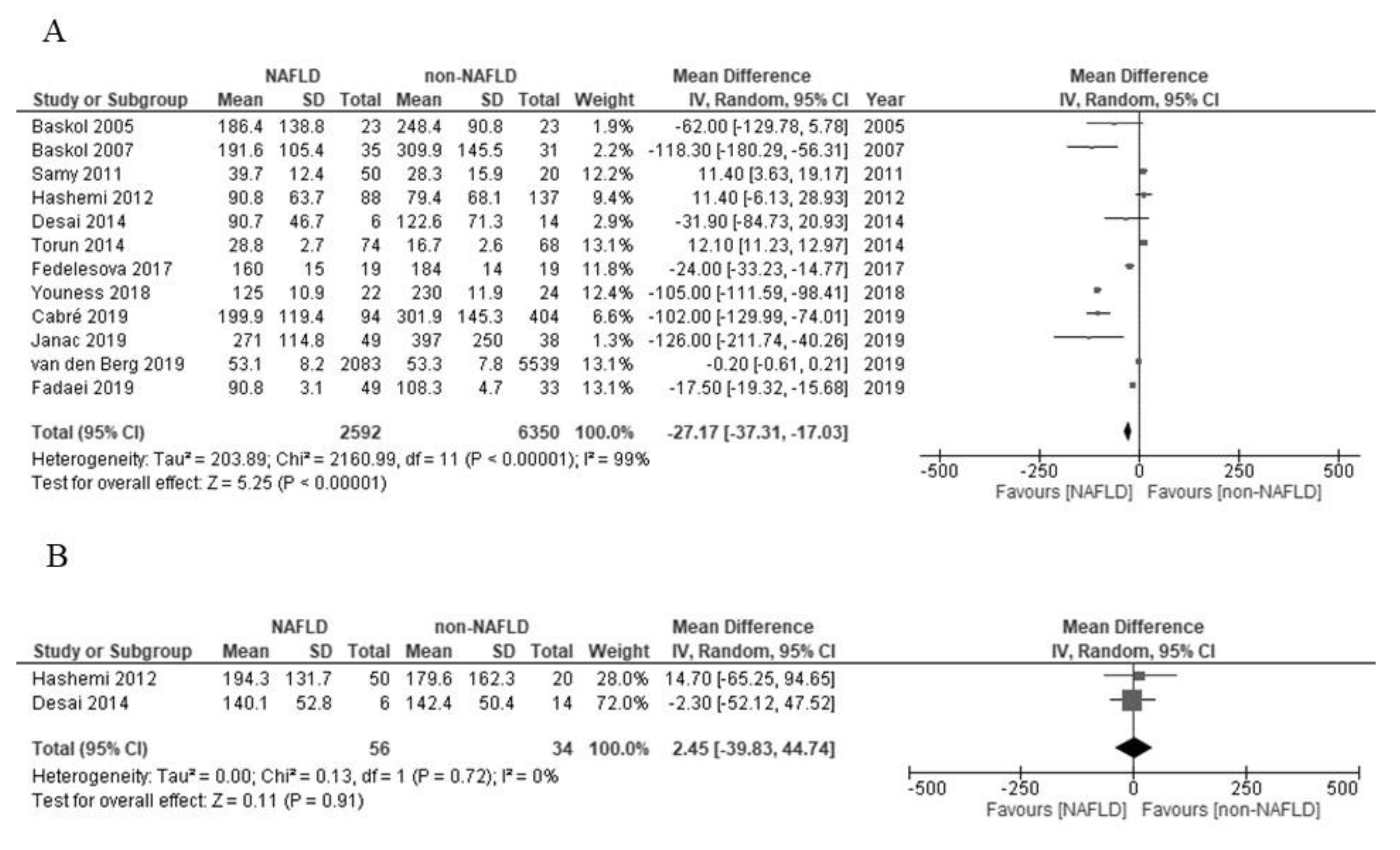
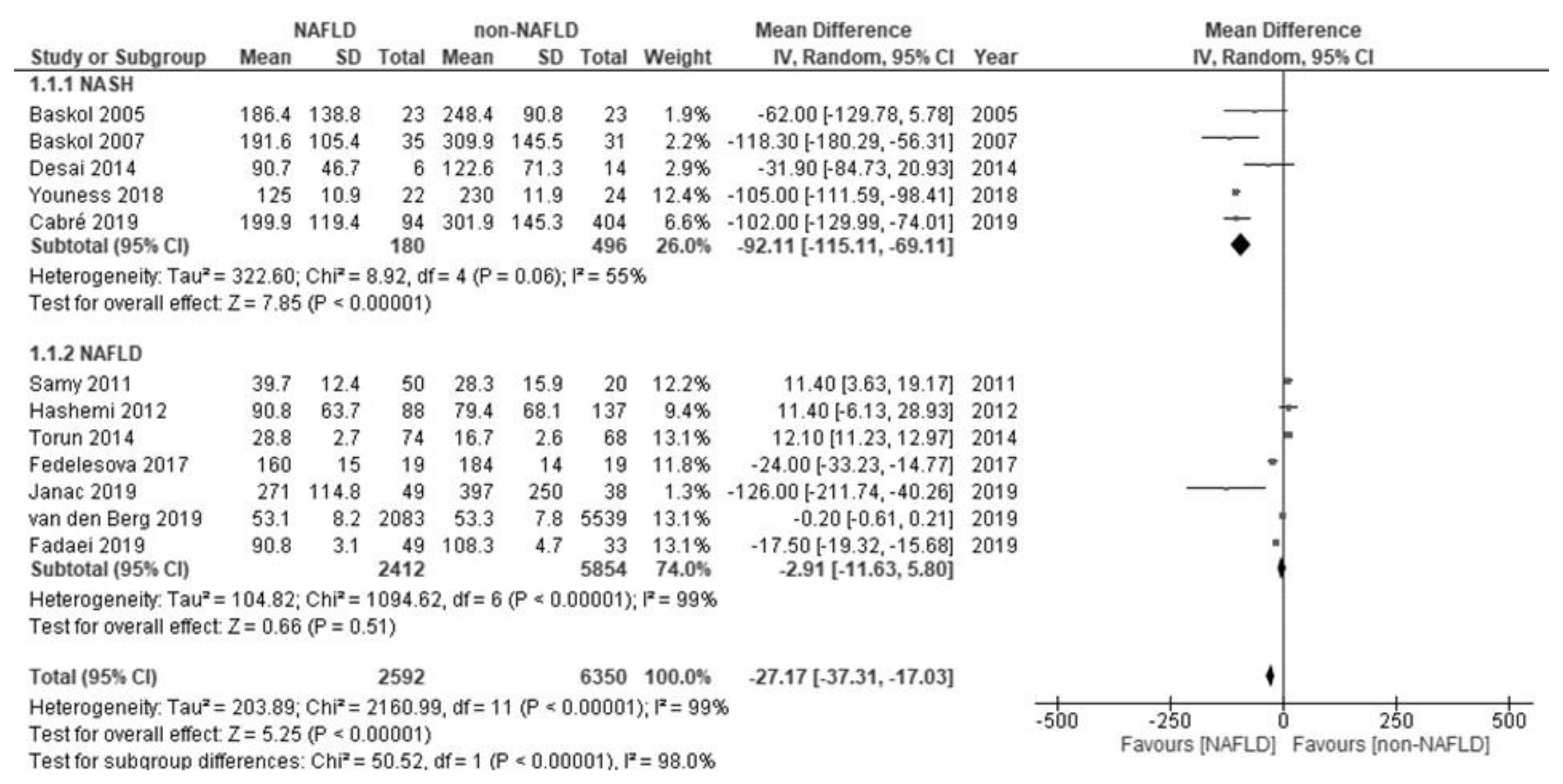
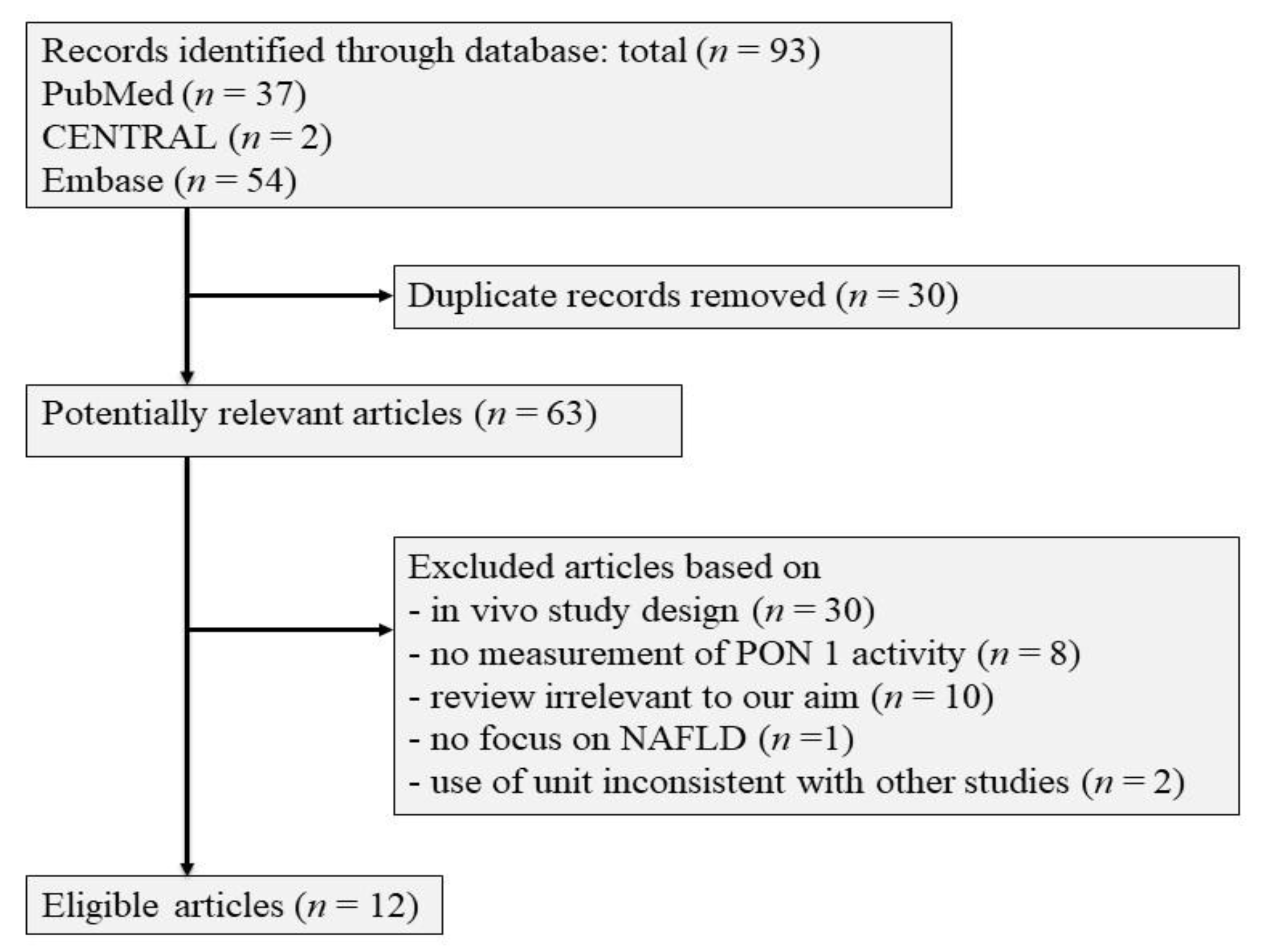
| Authors (Reference) | Age | Gender | Diagnosis | Activity in NAFLD (U/L) | Activity in Non-NAFLD (U/L) | Additional Notes |
|---|---|---|---|---|---|---|
| Paraoxonase | ||||||
| Baskol et al. [26] | 40 years (mean) | Men/women: 9/14 | NASH; biopsy (histology) | 186.4 ± 138.8 | 248.4 ± 90.8 | Serum PON1 does not always correspond to the grade of NASH. |
| Baskol et al. [27] | 39 years (mean) | Men/women: 22/13 | NASH; biopsy (histology) | 191.6 ± 105.4 | 309.9 ± 145.5 | |
| Samy et al. [28] | 47 years (mean) | Men/women: 22/28 | NAFLD; ultrasonography | 39.7 ± 12.4 | 28.3 ± 15.9 | Statin treatment increases serum PON1. |
| Hashemi et al. [29] | 40 years (mean) | Men/women: 50/33 | NAFLD; ultrasonography | 90.8 ± 63.7 | 79.4 ± 68.1 | |
| Torun et al. [30] | About 13 years | Men/women: 26/83 | NAFLD; ultrasonography | 28.8 ± 2.7 | 16.7 ± 2.6 | |
| Desai et al. [31] | 12–18 years | Men/women: 4/2 | NASH; biopsy (histology) | 90.7 ± 46.7 | 122.6 ± 71.3 | PON1 mRNA and protein levels in liver increase in NASH. |
| Fedelesova et al. [32] | Not detailed | Total 19 (gender: not detailed) | NAFLD; not detailed | 160 ± 15 | 184 ± 14 | |
| Youness et al. [33] | 46 years (mean) | Men/women: 12/10 | NASH; biopsy (histology) | 125.0 ± 10.9 | 230.0 ± 11.9 | |
| Cabré et al. [34] | 46 years (mean) | Men/women: 25/69 | NASH; biopsy (histology) | 199.9 ± 119.4 | 301.9 ± 145.3 | |
| Fadaei et al. [35] | 51 years (median) | Total 49 (gender: not detailed) | NAFLD; ultrasonography | 90.8 ± 3.1 | 108.3 ± 4.7 | |
| Janac et al. [36] | 48 years (mean) | Men/women: 16/33 | NAFLD; the fatty liver index | 271 ± 114.8 | 397 ± 250.0 | |
| van den Berg et al. [37] | 54 years (mean) | Men/women: 1422/661 | NAFLD; the fatty liver index | 53.1 ± 8.15 | 53.3 ± 7.78 | |
| Arylesterase | ||||||
| Hashemi et al. [29] | 40 years (mean) | Men/women: 50/33 | NAFLD; ultrasonography | 194.3 ± 131.7 | 179.6 ± 162.3 | |
| Desai et al. [31] | 12–18 years | Men/women: 4/2 | NASH; biopsy (histology) | 140.1 ± 52.8 | 142.4 ± 50.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotani, K.; Watanabe, J.; Miura, K.; Gugliucci, A. Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Molecules 2021, 26, 2323. https://doi.org/10.3390/molecules26082323
Kotani K, Watanabe J, Miura K, Gugliucci A. Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Molecules. 2021; 26(8):2323. https://doi.org/10.3390/molecules26082323
Chicago/Turabian StyleKotani, Kazuhiko, Jun Watanabe, Kouichi Miura, and Alejandro Gugliucci. 2021. "Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis" Molecules 26, no. 8: 2323. https://doi.org/10.3390/molecules26082323






