Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules
Abstract
1. Introduction
2. Steroids Dimers and Non-Steroidal Analogs
3. Sugars and Nucleoside-Based Dimers
4. Dimers of Known and Synthetic Anticancer Agents
5. Polyphenol Dimers
6. Terpenoid Dimers
7. Dimers of Known and Synthetic Antibacterial Agents
8. Recently Isolated Dimeric Natural Products
9. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bérubé, G. Natural and Synthetic Biologically Active Dimeric Molecules: Anticancer Agents, Anti-HIV Agents, Steroid Derivatives and Opioid Antagonists. Curr. Med. Chem. 2006, 13, 131–154. [Google Scholar] [CrossRef]
- Portoghese, P.S. The role of concepts in structure-activity relationship studies of opioid ligands. J. Med. Chem. 1992, 35, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Tamiz, A.P.; Zhang, J.; Zhang, M.; Wang, C.Z.; Johnson, K.M.; Kozikowski, A.P. Application of the Bivalent Ligand Approach to the Design of Novel Dimeric Serotonin Reuptake Inhibitors. J. Am. Chem. Soc. 2000, 122, 5393–5394. [Google Scholar] [CrossRef]
- Voloshchuk, T.; Farina, N.S.; Wauchope, O.R.; Kiprowska, M.; Haberfield, P.; Greer, A. Molecular Bilateral Symmetry of Natural Products: Prediction of Selectivity of Dimeric Molecules by Density Functional Theory and Semiempirical Calculations. J. Nat. Prod. 2004, 67, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Hadden, M.K.; Blagg, B.S.J. Dimeric approaches to anti-cancer chemotherapeutics. Anticancer Agents Med. Chem. 2009, 8, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Naha, L.; Sarker, S.D. Steroid Dimers: Chemistry and Applications in Drug Design and Delivery, 1st ed.; Wiley & Sons: West Sussex, UK, 2012; p. 440. [Google Scholar]
- Sumoto, K. Synthetic Studies on Developments for Bioactive New Leads of Oligovalent Symmetrical Molecules. Yakugaku Zasshi 2020, 140, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Krstić, N.M.; Bjelaković, M.S.; Dabović, M.M.; Pavlović, V.D. Thionation of some α,β-unsaturated steroidal ketones. Molecules 2010, 15, 3462–3477. [Google Scholar] [CrossRef]
- Krstić, N.M.; Matić, I.Z.; Juranić, Z.D.; Novaković, I.T.; Sladić, D.M. Steroid dimers—In vitro cytotoxic and antimicrobial activities. J. Steroid Biochem. Mol. Biol. 2014, 143, 365–375. [Google Scholar] [CrossRef]
- Vesper, A.-R.; Lacroix, J.; C.-Gaudreault, R.; Tajmir-Rihai, H.-A.; Bérubé, G. Synthesis of novel C2-symmetric testosterone dimers and evaluation of antiproliferative activity on androgen-dependent and -independent prostate cancer cell lines. Steroids 2016, 115, 98–104. [Google Scholar] [CrossRef]
- Chanphai, P.; Vesper, A.; Bekale, L.; Berube, G.; Tajmir-Riahi, H. Encapsulation of testosterone and its aliphatic and aromatic dimers by milk beta-lactoglobulin. Int. J. Biol. Macromol. 2015, 76, 153–160. [Google Scholar] [CrossRef]
- Chanphai, P.; Vesper, A.; Bekale, L.; Berube, G.; Tajmir-Riahi, H. Transporting testosterone and its dimers by serum proteins. J. Photochem. Photobiol. B Biol. 2015, 153, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Vesper, A.-R.; Bariyanga, J.; Bérubé, G.; Tajmir-Riahi, H.-A. Review on the steroid delivery by carrier proteins. J. Photochem. Photobiol. B Biol. 2016, 161, 184–191. [Google Scholar] [CrossRef]
- Chanphai, P.; Agudelo, D.; Vesper, A.; Bérubé, G.; Tajmir-Riahi, H. Effect of testosterone and its aliphatic and aromatic dimers on DNA morphology. Int. J. Biol. Macromol. 2017, 95, 850–855. [Google Scholar] [CrossRef]
- Chanphai, P.; Agudelo, D.; Vesper, A.; Bérubé, G.; Tajmir-Riahi, H. Testosterone and its dimers alter tRNA morphology. J. Pharm. Biomed. Anal. 2017, 134, 269–274. [Google Scholar] [CrossRef]
- Bastien, D.; Leblanc, V.; Asselin, É.; Berube, G. First synthesis of separable isomeric testosterone dimers showing differential activities on prostate cancer cells. Bioorg. Med. Chem. Lett. 2010, 20, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Mak, P.J.; Grinkova, Y.V.; Bastien, D.; Bérubé, G.; Sligar, S.G.; Kincaid, J.R. The use of isomeric testosterone dimers to explore allosteric effects in substrate binding to cytochrome P450 CYP3A4. J. Inorg. Biochem. 2016, 158, 77–85. [Google Scholar] [CrossRef]
- Wendlandt, A.E.; Yelton, S.M.; Lou, D.; Watt, D.S.; Noonan, D.J. Synthesis and functional analysis of novel bivalent estrogens. Steroids 2010, 75, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.H.; Crowe, D.F.; Avery, M.A.; Chong, W.K.; Tanabe, M. 17-Desoxy estrogen analogues. J. Med. Chem. 1989, 32, 1642–1652. [Google Scholar] [CrossRef]
- LaFrate, A.L.; Carlson, K.E.; Katzenellenbogen, J.A. Steroidal bivalent ligands for the estrogen receptor: Design, synthesis, characterization and binding affinities. Bioorg. Med. Chem. 2009, 17, 3528–3535. [Google Scholar] [CrossRef]
- Knox, A.; Kalchschmid, C.; Schuster, D.; Gaggia, F.; Manzl, C.; Baecker, D.; Gust, R. Development of bivalent triarylalkene- and cyclofenil-derived dual estrogen receptor antagonists and downregulators. Eur. J. Med. Chem. 2020, 192, 112191. [Google Scholar] [CrossRef]
- Willson, T.M.; Henke, B.R.; Momtahen, T.M.; Charifson, P.S.; Batchelor, K.W.; Lubahn, D.B.; Moore, L.B.; Oliver, B.B.; Sauls, H.R.; Triantafillou, J.A.; et al. 3-[4-(1,2-Diphenylbut-1-enyl)phenyl]acrylic Acid: A Non-Steroidal Estrogen with Functional Selectivity for Bone over Uterus in Rats. J. Med. Chem. 1994, 37, 1550–1552. [Google Scholar] [CrossRef]
- Bentrem, D.; Dardes, R.; Liu, H.; MacGregor-Schafer, J.; Zapf, J.; Jordan, V.C. Molecular mechanism of action at estrogen receptor alpha of a new clinically relevant antiestrogen (GW7604) related to tamoxifen. J. Endocrinol. 2001, 142, 838–846. [Google Scholar] [CrossRef]
- Kieser, K.J.; Kim, D.W.; Carlson, K.E.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Characterization of the Pharmacophore Properties of Novel Selective Estrogen Receptor Downregulators (SERDs). J. Med. Chem. 2010, 53, 3320–3329. [Google Scholar] [CrossRef]
- Krejzová, J.; Šimon, P.; Vavříková, E.; Slámová, K.; Pelantová, H.; Riva, S.; Spiwok, V.; Křen, V. Enzymatic synthesis of new C-6-acylated derivatives of NAG-thiazoline and evaluation of their inhibitor activities towards fungal β-N-acetylhexosamididase. J. Mol. Catal. B Enzym. 2013, 87, 128–134. [Google Scholar] [CrossRef]
- Baraniak, D.; Ruszkowski, P.; Baranowski, D.; Framski, G.; Boryski, J. Nucleoside dimers analogs containing floxuridine and thymidine with unnatural linker groups: Synthesis and cancer line studies. Part III. Nucleosides Nucleotides Nucleic Acids 2019, 38, 980–1005. [Google Scholar] [CrossRef]
- Michalska, L.; Wawrzyniak, D.; Szymańska-Michalak, A.; Barciszewski, J.; Boryski, J.; Baraniak, D. Synthesis and biological assay of new 2′-deoxyuridine dimers containing a 1,2,3-triazole linker. Part I. Nucleosides Nucleotides Nucleic Acids 2018, 38, 1–18. [Google Scholar] [CrossRef]
- Baraniak, D.; Baranowski, D.; Ruszkowski, P.; Boryski, J. Nucleoside dimers analogues with a 1,2,3-triazole linkage: Conjugation of floxuridine and thymidine provides novel tools for cancer treatment. Part II. Nucleosides Nucleotides Nucleic Acids 2019, 38, 807–835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-S.; Shi, Y.; Ma, X.-N.; Xing, D.-X.; Liu, L.-D.; Liu, Y.; Zhao, Y.-X.; Sui, Q.-C.; Tan, X.-J. Synthesis, crystal structure, spectroscopic properties and potential anti-cancerous activities of four unsaturated bis-norcantharimides. J. Mol. Struct. 2016, 1115, 228–240. [Google Scholar] [CrossRef]
- Furutachi, M.; Ota, K.; Fujisaki, F.; Ikeda, R.; Yoshikawa, N.; Yokota, T.; Takeda, Y.; Yokomizo, K.; Zhou, J.-R.; Kashige, N.; et al. Anti-proliferative Activities of Some Bivalent Symmetrical 5-Substituted Hydantoin Derivatives towards Human Brain Glioma U251 Cells (U251) and Human Carcinoma Cells (KB3-1). Biol. Pharm. Bull. 2019, 42, 1953–1956. [Google Scholar] [CrossRef]
- Fujisaki, F.; Aki, H.; Naito, A.; Fukami, E.; Kashige, N.; Miake, F.; Sumoto, K. Synthesis of New 5-Substituted Hydantoins and Symmetrical Twin-Drug Type Hydantoin Derivatives. Chem. Pharm. Bull. 2014, 62, 429–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujisaki, F.; Toyofuku, K.; Egami, M.; Ishida, S.; Nakamoto, N.; Kashige, N.; Miake, F.; Sumoto, K. Antibacterial Activity of Some 5-Dialkylaminomethylhydantoins and Related Derivatives. Chem. Pharm. Bull. 2013, 61, 1090–1093. [Google Scholar] [CrossRef]
- Sumoto, K.; Furutachi, M.; Fujisaki, F.; Fujiwara, R.; Okabe, M.; Aki, H.; Kashige, N.; Miake, F. Preparation and Antibacterial Evaluation of Some Symmetrical Twin-Drug Type Bivalent Molecules. Heterocycles 2015, 91, 1668–1677. [Google Scholar] [CrossRef]
- Sumoto, K.; Furutachi, M.; Gondo, T.; Goto, S.; Fuchigami, S.; Ako, K.; Oowada, Y.; Yokomizo, K.; Zhou, J.-R.; Ishizaki, T.; et al. Novel C2-Symmetrical Phenylboronic Acid Pinacol Esters with a Few Types of Linkers and Their Biological Activities. Heterocycles 2017, 94, 1748–1758. [Google Scholar] [CrossRef]
- Furutachi, M.; Gondo, T.; Ikeda, R.; Yoshikawa, N.; Yokota, T.; Takeda, Y.; Yokomizo, K.; Zhou, J.-R.; Kashige, N.; Miake, F.; et al. Anti-proliferative Activities towards Human Brain Glioma U251 Cells and Human Carcinoma Cells (KB3-1) of Some Twin-Drug Type Bivalent C2-Symmetrical Phenylboronic Acid Derivatives. Biol. Pharm. Bull. 2019, 42, 833–836. [Google Scholar] [CrossRef]
- Sumoto, K.; Furutachi, M.; Ejima, A.; Tsuru, R.; Goto, S.; Gondo, T.; Ako, K.; Fuchigami, S.; Fujii, S.; Okumura, A.; et al. Preparation and Biological Activity of Novel Twin-Drug Type C2-Symmetrical Cyclic Phenylboronic Acid Derivatives. Heterocycles 2017, 95, 517–524. [Google Scholar] [CrossRef]
- Sumoto, K.; Furutachi, M.; Matsumoto, A.; Tamenaga, T.; Sugita, A.; Kuroiwa, M.; Yokomizo, K.; Zhou, J.-R.; Kashige, N.; Miake, F. Preparation of Novel Bivalent Linker Mode Phenylboronic Acid Derivatives and Their Biological Evaluation. Heterocycles 2018, 96, 1088–1100. [Google Scholar] [CrossRef]
- Tendler, M.D.; Korman, S. ‘Refuin’: A Non-cytotoxic Carcinostatic Compound proliferated by a Thermophilic Actinomycete. Nat. Cell Biol. 1963, 199, 501. [Google Scholar] [CrossRef]
- Bose, D.S.; Thompson, A.S.; Ching, J.; Hartley, J.A.; Berardini, M.D.; Jenkins, T.C.; Neidle, S.; Hurley, L.H.; Thurston, D.E. Rational design of a highly efficient irreversible DNA interstrand cross-linking agent based on the pyrrolobenzodiazepine ring system. J. Am. Chem. Soc. 1992, 114, 4939–4941. [Google Scholar] [CrossRef]
- Howard, P.W.; Chen, Z.; Gregson, S.J.; Masterson, L.A.; Tiberghien, A.C.; Cooper, N.; Fang, M.; Coffils, M.J.; Klee, S.; Hartley, J.A.; et al. Synthesis of a novel C2/C2′-aryl-substituted pyrrolo[2,1-c][1,4]benzodiazepine dimer prodrug with improved water solubility and reduced DNA reaction rate. Bioorg. Med. Chem. Lett. 2009, 19, 6463–6466. [Google Scholar] [CrossRef]
- Hartley, J.A.; Hamaguchi, A.; Coffils, M.; Martin, C.R.; Suggitt, M.; Chen, Z.; Gregson, S.J.; Masterson, L.A.; Tiberghien, A.C.; Hartley, J.M.; et al. SG2285, a Novel C2-Aryl-Substituted Pyrrolobenzodiazepine Dimer Prodrug That Cross-links DNA and Exerts Highly Potent Antitumor Activity. Cancer Res. 2010, 70, 6849–6858. [Google Scholar] [CrossRef]
- Howard, P.W.; Gregson, S.J. Pyrrolobenzodiazepines. Conjugates. Patent WO/2018/069490, 14 October 2016. [Google Scholar]
- Tiberghien, A.C.; Levy, J.-N.; Masterson, L.A.; Patel, N.V.; Adams, L.R.; Corbett, S.; Williams, D.G.; Hartley, J.A.; Howard, P.W. Design and Synthesis of Tesirine, a Clinical Antibody-Drug Conjugate Pyrrolobenzodiazepine Dimer Payload. ACS Med. Chem. Lett. 2016, 7, 983–987. [Google Scholar] [CrossRef]
- Hartley, J.A.; Flynn, M.J.; Bingham, J.P.; Corbett, S.; Reinert, H.; Tiberghien, A.; Masterson, L.A.; Antonow, D.; Adams, L.; Chowdhury, S.; et al. Pre-clinical pharmacology and mechanism of action of SG3199, the pyrrolobenzodiazepine (PBD) dimer warhead component of antibody-drug conjugate (ADC) payload tesirine. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Lashari, B.H.; Vallatharasu, Y.; Kolandra, L.; Hamid, M.; Uprety, D. Rovalpituzumab Tesirine: A Novel DLL3-Targeting Antibody–Drug Conjugate. Drugs R&D 2018, 18, 255–258. [Google Scholar] [CrossRef]
- AbbVie News Center. Available online: https://news.abbvie.com/news/press-releases/abbvie-discontinues-rovalpituzumab-tesirine-rova-t-research-and-development-program.htm (accessed on 20 June 2020).
- Sahoo, B.; Dinda, S.; Kumar, B.; Panda, J.; Brahmkshatriya, P. Design, Green Synthesis, and Anti-Inflammatory Activity of Schiff Base of 1,3,4-oxadiazole Analogues. Lett. Drug Des. Discov. 2013, 11, 82–89. [Google Scholar] [CrossRef]
- Özkay, Y.; Işıkdağ, I.; Incesu, Z.; Akalın, G. Synthesis of 2-substituted-N-[4-(1-methyl-4,5-diphenyl-1H-imidazole-2-yl)phenyl]acetamide derivatives and evaluation of their anticancer activity. Eur. J. Med. Chem. 2010, 45, 3320–3328. [Google Scholar] [CrossRef]
- Harrison, T.S.; Keating, G.M. Zolpidem: A review of its use in the management of insomnia. CNS Drugs 2005, 19, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, B.; Morel, E.; Joly, D.; Perrault, G.; Sanger, D.J.; Lloyd, K.G. Pharmacological and Behavioral Profile of Alpidem as an Anxiolytic. Pharmacopsychiatry 1990, 23, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Meenakshisundaram, S.; Manickam, M.; Pillaiyar, T. Exploration of imidazole and imidazopyridine dimers as anticancer agents: Design, synthesis, and structure–activity relationship study. Archiv Pharmazie 2019, 352, e1900011. [Google Scholar] [CrossRef] [PubMed]
- Guchhait, S.K.; Chandgude, A.L.; Priyadarshani, G. CuSO4–Glucose for in Situ Generation of Controlled Cu(I)–Cu(II) Bicatalysts: Multicomponent Reaction of Heterocyclic Azine and Aldehyde with Alkyne, and Cycloisomerization toward Synthesis of N-Fused Imidazoles. J. Org. Chem. 2012, 77, 4438–4444. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Vavříková, E.; Vacek, J.; Valentova, K.; Marhol, P.; Ulrichová, J.; Kuzma, M.; Křen, V. Chemo-Enzymatic Synthesis of Silybin and 2,3-Dehydrosilybin Dimers. Molecules 2014, 19, 4115–4134. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Thongphasuk, P.; Erben, G.; Lehmann, W.-D.; Tuma, S.; Stremmel, W.; Chamulitrat, W. Significantly greater antioxidant anticancer activities of 2,3-dehydrosilybin than silybin. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 837–847. [Google Scholar] [CrossRef]
- Gažák, R.; Svobodová, A.; Psotová, J.; Sedmera, P.; Přikrylová, V.; Walterová, D.; Křen, V. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.-M.; Ghoul, M. Enzymatic acylation of flavonoids. Process. Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- Walterova, D.; Kren, V. Silybin and Silymarin—New and Emerging Applications in Medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef]
- Graf, T.; Wani, M.; Agarwal, R.; Kroll, D.; Oberlies, N. Gram-Scale Purification of Flavonolignan Diastereoisomers from Silybum marianum (Milk Thistle) Extract in Support of Preclinical in vivo Studies for Prostate Cancer Chemoprevention. Planta Med. 2007, 73, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Gavezzotti, P.; Vavrikova, E.; Valentova, K.; Fronza, G.; Kudanga, T.; Kuzma, M.; Riva, S.; Biedermann, D.; Kren, V. Enzymatic oxidative dimerization of silymarin flanovolignans. J. Mol. Catal. B Enzym. 2014, 109, 24–30. [Google Scholar] [CrossRef]
- Pertino, M.W.; Theoduloz, C.; Bastías, M.; Schmeda-Hirschmann, G. Dimeric Labdane Diterpenes: Synthesis and Antiproliferative Effects. Molecules 2013, 18, 5936–5953. [Google Scholar] [CrossRef]
- Romanucci, V.; Gravante, R.; Cimafonte, M.; Di Marino, C.; Mailhot, G.; Brigante, M.; Zarrelli, A.; Di Fabio, G. Phosphate-Linked Silibinin Dimers (PLSd): New Promising Modified Metabolites. Molecules 2017, 22, 1323. [Google Scholar] [CrossRef]
- Romanucci, V.; Zarrelli, A.; Guaragna, A.; Di Marino, C.; Di Fabio, G. New phosphorylating reagents for deoxyribonucleosides and oligonucleotides. Tetrahedron Lett. 2017, 58, 1227–1229. [Google Scholar] [CrossRef]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Marchiani, A.; Mammi, S.; Siligardi, G.; Hussain, R.; Tessari, I.; Bubacco, L.; Delogu, G.; Fabbri, D.; Dettori, M.A.; Sanna, D.; et al. Small molecules interacting with α-synuclein: Antiaggregating and cytoprotective properties. Amino Acids 2013, 45, 327–338. [Google Scholar] [CrossRef]
- Sultana, R. Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Slavova-Kazakova, A.K.; Angelova, S.E.; Veprintsev, T.L.; Denev, P.; Fabbri, D.; Dettori, M.A.; Kratchanova, M.; Naumov, V.V.; Trofimov, A.V.; Vasil’ev, R.F.; et al. Antioxidant potential of curcumin-related compounds studied by chemiluminescence kinetics, chain-breaking efficiencies, scavenging activity (ORAC) and DFT calculations. Beilstein J. Org. Chem. 2015, 11, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Hoenke, S.; Wiengarn, I.; Serbian, I.; Al-Harrasi, A.; Csuk, R. Synthesis of amide-spacered dimers of ursolic and oleanolic acid. Mediterr. J. Chem. 2019, 9, 24–36. [Google Scholar] [CrossRef]
- Kahnt, M.; Fisher, L.; Al-Harrasi, A.; Csuk, R. Ethylenediamine Derived Carboxamides of Betulinic and Ursolic Acid as Potential Cytotoxic Agents. Molecules 2018, 23, 2558. [Google Scholar] [CrossRef] [PubMed]
- Heller, L.; Knorrscheidt, A.; Flemming, F.; Wiemann, J.; Sommerwerk, S.; Pavel, I.Z.; Al-Harrasi, A.; Csuk, R. Synthesis and proapoptotic activity of oleanolic acid derived amides. Bioorg. Chem. 2016, 68, 137–151. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kuhfs, J.; Csuk, R. Selective killing of cancer cells with triterpenoic acid amides—The substantial role of an aromatic moiety alignment. Eur. J. Med. Chem. 2016, 122, 452–464. [Google Scholar] [CrossRef]
- Tan, Q.-G.; Luo, X.-D. Meliaceous Limonoids: Chemistry and Biological Activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Roy, A.; Saraf, S. Limonoids: Overview of Significant Bioactive Triterpenes Distributed in Plants Kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Wu, J.; Li, J.; Satyanandamurty, T.; Shen, L.; Bringmann, G. Krishnadimer A, an Axially Chiral Non-biaryl Natural Product: Discovery and Biomimetic Synthesis. Org. Lett. 2016, 19, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Yang, Y.; Liu, J.J.; Shen, L.; Shi, Z.; Wu, J. Scaffold diversity-oriented synthesis of limonoid dimers: Discovery of an axially chiral agent with in vivo anti-breast cancer activity. Org. Chem. Front. 2018, 5, 1079–1091. [Google Scholar] [CrossRef]
- Li, M.-Y.; Yang, S.-X.; Pan, J.-Y.; Xiao, Q.; Satyanandamurty, T.; Wu, J. Moluccensins A−G, Phragmalins with a Conjugated C-30 Carbonyl Group from a Krishna Mangrove, Xylocarpus moluccensis. J. Nat. Prod. 2009, 72, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Rutkowski, J.; Huczyński, A. Structure and Biological Activity of Polyether Ionophores and Their Semisynthetic Derivatives. In Bioactive Natural Products; Wiley: Hoboken, NJ, USA, 2015; pp. 107–170. [Google Scholar]
- Zardavas, D.; Baselga, J.; Piccart-Gebhart, M. Emerging targeted agents in metastatic breast cancer. Nat. Rev. Clin. Oncol. 2013, 10, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Huczyński, A. Anticancer Activity of Polyether Ionophore-Salinomycin. Anticancer Agents Med. Chem. 2015, 15, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Deng, Z.; Tian, J.; Liu, T. Synthesis and biological evaluation of salinomycin triazole analogues as anticancer agents. Eur. J. Med. Chem. 2017, 127, 900–908. [Google Scholar] [CrossRef]
- Antoszczak, M.; Maj, E.; Borgström, B.; Oredsson, S.; Huczyski, A.; Wietrzyk, J.; Strand, D. Bivalent polyether ionophores: Synthesis and biological evaluation of C2-symmetric salinomycin dimers. Tetrahedron Lett. 2017, 58, 2396–2399. [Google Scholar] [CrossRef]
- Machado, L.; Spengler, G.; Evaristo, M.; Handzlik, J.; Molnár, J.; Viveiros, M.; Kiec-Kononowicz, K.; Amaral, L. Biological activity of twenty-three hydantoin derivatives on intrinsic efflux pump system of Salmonella enterica serovar Enteritidis NCTC 13349. In Vivo 2011, 25, 769–772. [Google Scholar]
- Sumoto, K.; Furutachi, M.; Fujisaki, F.; Tsuru, R.; Ejima, A.; Gondo, T.; Goto, S.; Ito, M.; Nakamura, M.; Aki, H.; et al. Synthesis and Antibacterial Evaluation of Some New 5-Substituted Hydantoins and Novel Twin-Drug Type Derivatives. Heterocycles 2016, 92, 1111–1120. [Google Scholar] [CrossRef][Green Version]
- Lakshmi, R.; Nusrin, K.S.; Georgy, S.A.; Sreelakshmi, K.S. Role of Beta Lactamases in Antibiotic Resistance: A review. Int. Res. J. Pharm. 2014, 5, 37–40. [Google Scholar] [CrossRef]
- Banik, I.; Becker, A.F.F.; Banik, B.K. Stereoselective Synthesis of β-Lactams with Polyaromatic Imines: Entry to New and Novel Anticancer Agents. J. Med. Chem. 2003, 46, 12–15. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Manickam, M.F.; Vinayagam, V. Synthesis, antibacterial and anticancer activity of novel bis-azetidinones. J. Chem. Pharm. Res. 2016, 8, 733–742. [Google Scholar]
- Chen, S.; Wang, J.; Lin, X.; Zhao, B.; Wei, X.; Li, G.; Kaliaperumal, K.; Liao, S.; Yang, B.; Zhou, X.; et al. Chrysamides A–C, Three Dimeric Nitrophenyl trans-Epoxyamides Produced by the Deep-Sea-Derived Fungus Penicillium chrysogenum SCSIO41001. Org. Lett. 2016, 18, 3650–3653. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Pereira, R.B.; Andrade, P.B.; Valentão, P. Double the Chemistry, Double the Fun: Structural Diversity and Biological Activity of Marine-Derived Diketopiperazine Dimers. Mar. Drugs 2019, 17, 551. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Sedlock, D.M.; Barrow, C.J.; Brownell, J.E.; Hong, A.; Gillum, A.M.; Houck, D.R. WIN 64821, a novel neurokinin antagonist produced by an Aspergillus sp. I. Fermentation and isolation. J. Antibiot. 1994, 47, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, F.C.; Qin, M.; Qin, S.; Kelter, G.; Fiebig, H.H.; Laatsch, H. Anti-tumor compounds isolated from marine Aspergillus sp. Chin. J. Nat. Med. 2008, 6, 421–424. [Google Scholar] [CrossRef]
- Barrow, C.J.; Musza, L.L.; Cooper, R. Structure-activity studies of the natural product substance P antagonist win 64821. Bioorg. Med. Chem. Lett. 1995, 5, 377–380. [Google Scholar] [CrossRef]
- Son, B.W.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. New Cytotoxic Epidithiodioxopiperazines Related to Verticillin A from a Marine Isolate of the Fungus Penicillium. Nat. Prod. Lett. 1999, 13, 213–222. [Google Scholar] [CrossRef]
- Paschall, A.V.; Yang, D.; Lu, C.; Choi, J.-H.; Li, X.; Liu, F.; Figueroa, M.; Oberlies, N.H.; Pearce, C.J.; Bollag, W.B.; et al. H3K9 Trimethylation Silences Fas Expression to Confer Colon Carcinoma Immune Escape and 5-Fluorouracil Chemoresistance. J. Immunol. 2015, 195, 1868–1882. [Google Scholar] [CrossRef]
- Lu, C.; Paschall, A.V.; Shi, H.; Savage, N.; Waller, J.L.; Sabbatini, M.E.; Oberlies, N.H.; Pearce, C.; Liu, K. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. J. Natl. Cancer Inst. 2017, 109, djw283. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Chen, Y.; Guo, X.-N.; Zhang, X.-W.; Zhao, W.-M.; Zhong, L.; Zhou, J.; Xi, Y.; Lin, L.-P.; Ding, J. 11,11′-Dideoxy-verticillin: A natural compound possessing growth factor receptor tyrosine kinase-inhibitory effect with anti-tumor activity. Anticancer Drugs 2005, 16, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.R.; Ratnam, J.; Ang, K.-H.; Tenney, K.; Compton, J.E.; McKerrow, J.; Crews, P. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines from two deep water marine-derived fungi. Bioorg. Med. Chem. 2010, 18, 2566–2574. [Google Scholar] [CrossRef]
- Greiner, D.; Bonaldi, T.; Eskeland, R.; Roemer, E.; Imhof, A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9. Nat. Chem. Biol. 2005, 1, 143–145. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chen, J.-Y.; Tsai, H.-J.; Chen, T.-Y.; Hung, W.-C. The SUV39H1 inhibitor chaetocin induces differentiation and shows synergistic cytotoxicity with other epigenetic drugs in acute myeloid leukemia cells. Blood Cancer J. 2015, 5, e313. [Google Scholar] [CrossRef] [PubMed]
- Isham, C.R.; Tibodeau, J.D.; Bossou, A.R.; Merchan, J.R.; Bible, K.C. The anticancer effects of chaetocin are independent of programmed cell death and hypoxia, and are associated with inhibition of endothelial cell proliferation. Br. J. Cancer 2011, 106, 314–323. [Google Scholar] [CrossRef]
- Zofou, D.; Ntie-Kang, F.; Sippl, W.; Efange, S.M.N. Bioactive natural products derived from the Central African flora against neglected tropical diseases and HIV. Nat. Prod. Rep. 2013, 30, 1098–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Seupel, R.; Bruhn, T.; Feineis, D.; Kaiser, M.; Brun, R.; Mudogo, V.; Awale, S.; Bringmann, G. Jozilebomines A and B, Naphthylisoquinoline Dimers from the Congolese Liana Ancistrocladus ileboensis, with Antiausterity Activities against the PANC-1 Human Pancreatic Cancer Cell Line. J. Nat. Prod. 2017, 80, 2807–2817. [Google Scholar] [CrossRef]
- Bringmann, G.; Zhang, G.; Büttner, T.; Bauckmann, G.; Kupfer, T.; Braunschweig, H.; Brun, R.; Mudogo, V. Jozimine A2: The First Dimeric Dioncophyllaceae-Type Naphthylisoquinoline Alkaloid, with Three Chiral Axes and High Antiplasmodial Activity. Chem. A Eur. J. 2012, 19, 916–923. [Google Scholar] [CrossRef]
- Bilonda, M.K.; Mammino, L. Computational Study of Jozimine A2, a Naphthylisoquinoline Alkaloid with Antimalarial Activity. In Concepts, Methods and Applications of Quantum Systems in Chemistry and Physics, 1st ed.; Wang, Y.A., Thachuk, M., Krems, R., Maruani, J., Eds.; Springer: Vancouver, BC, Canada, 2018; pp. 305–328. [Google Scholar]



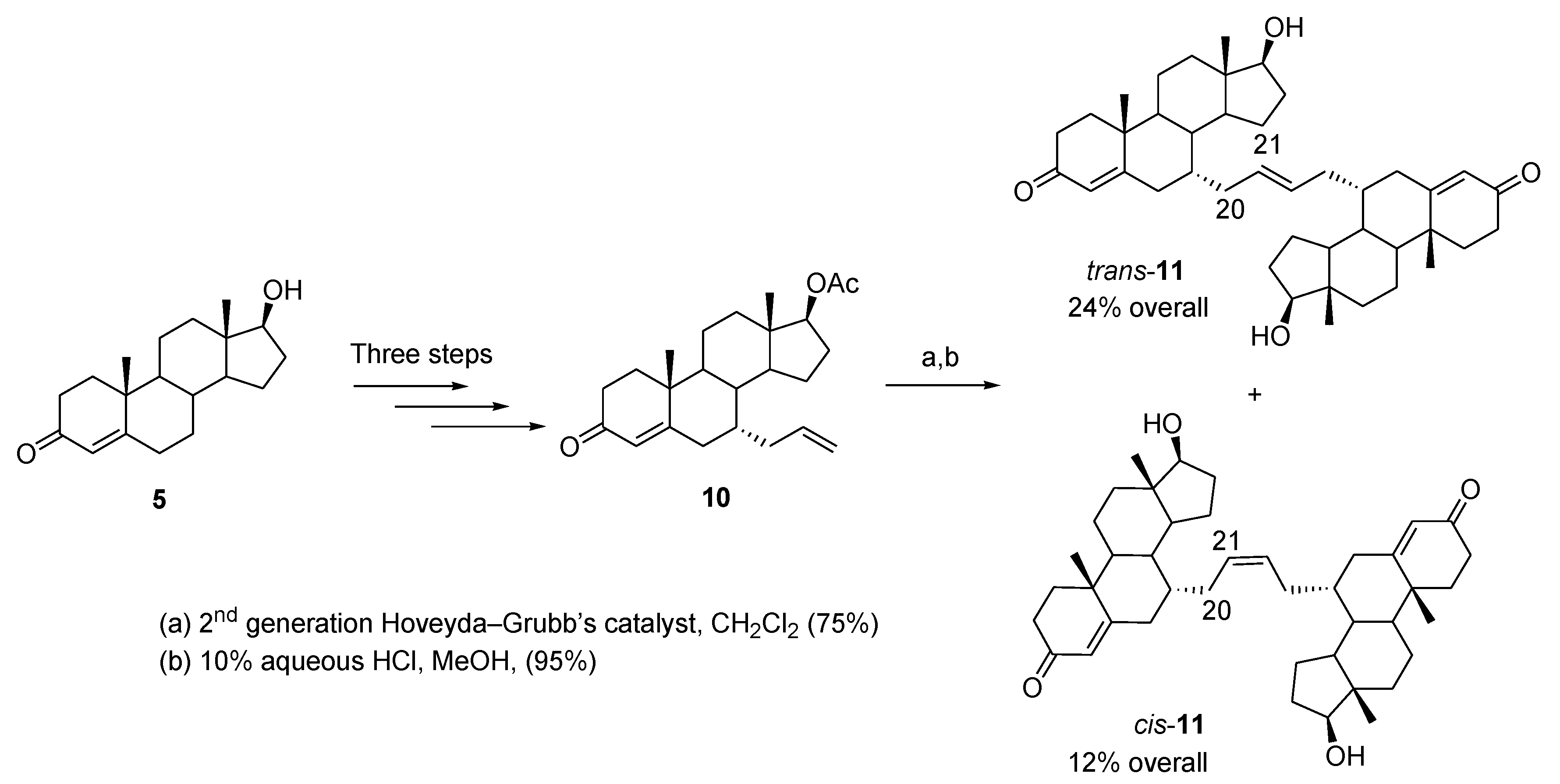



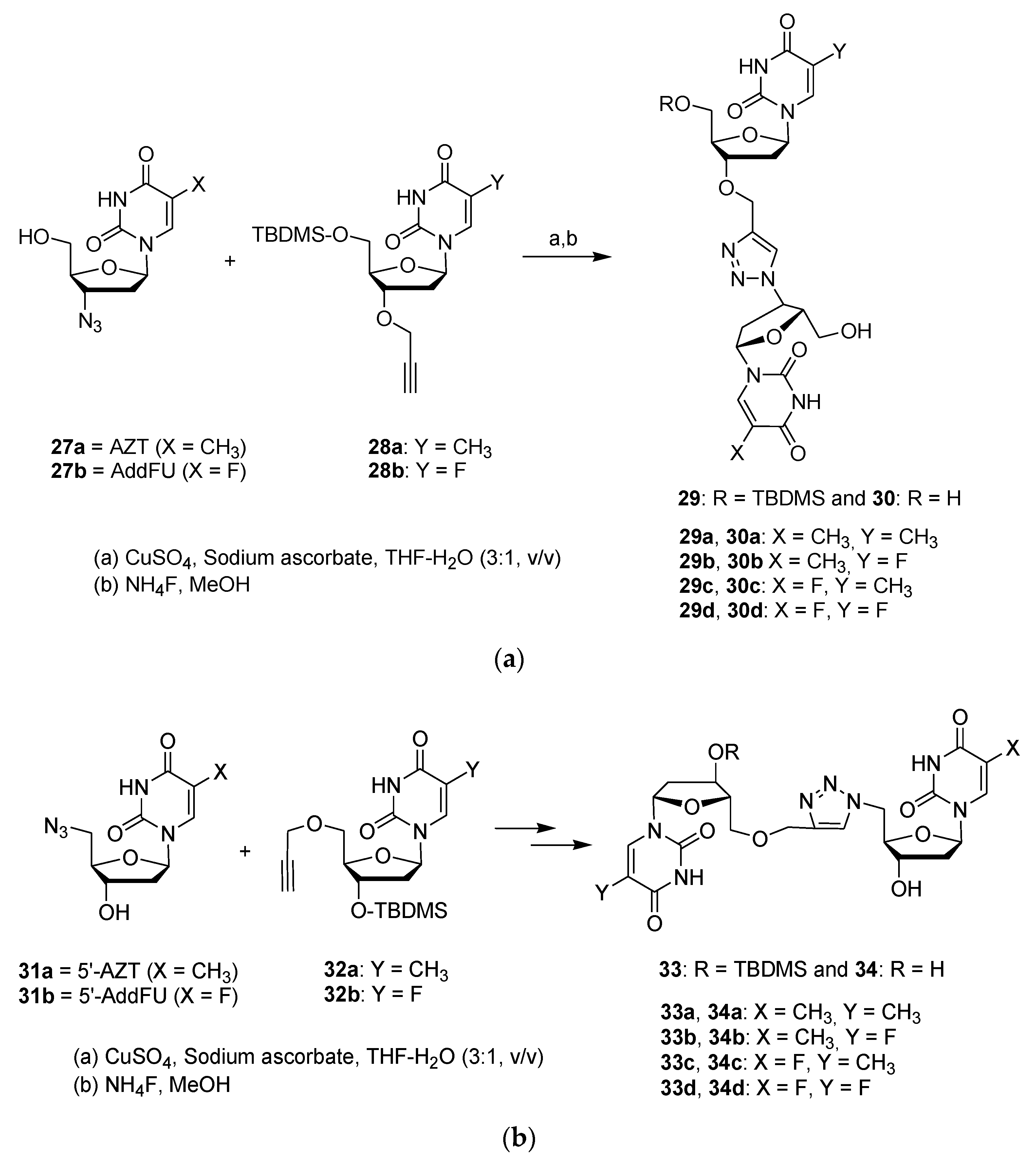



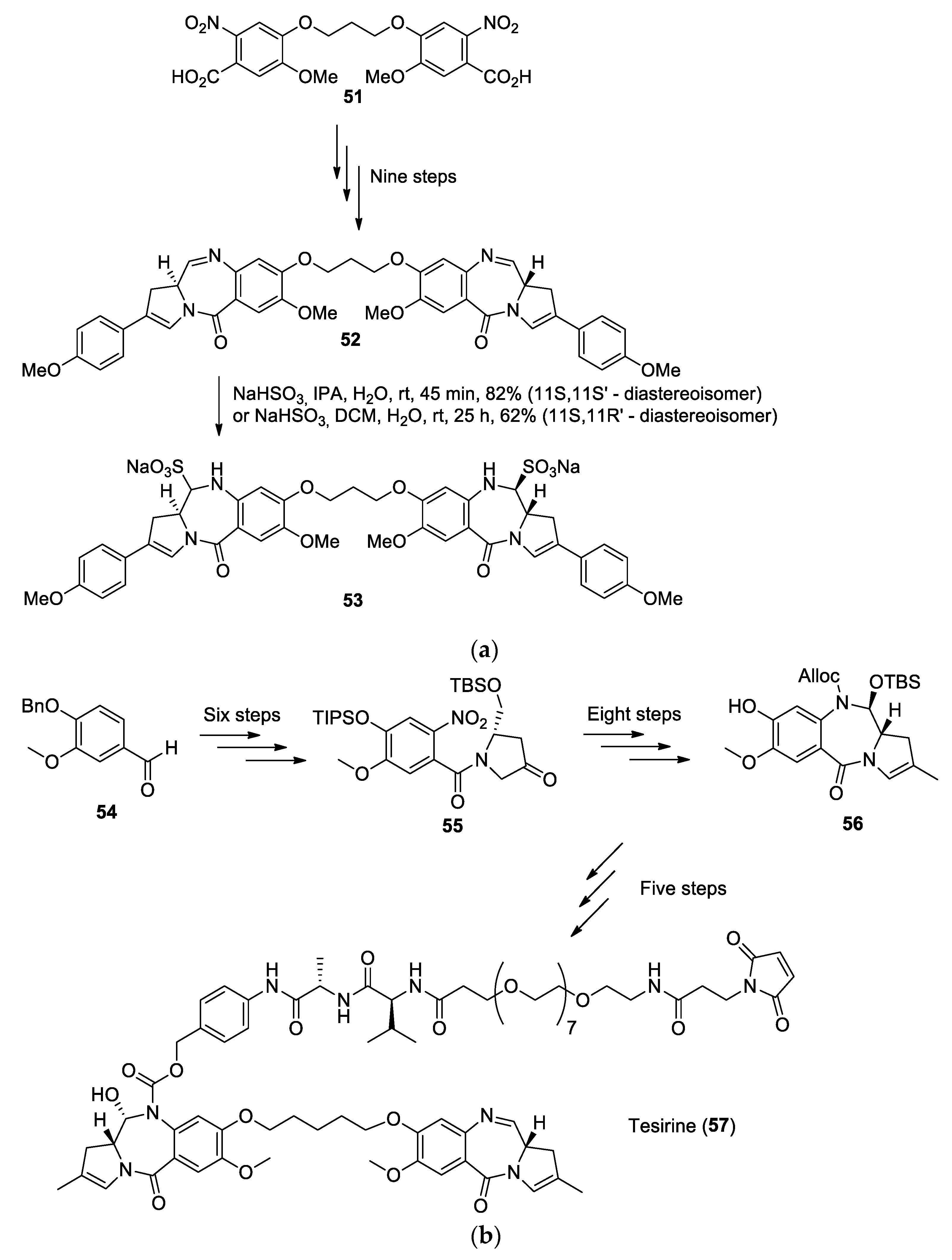













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paquin, A.; Reyes-Moreno, C.; Bérubé, G. Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules. Molecules 2021, 26, 2340. https://doi.org/10.3390/molecules26082340
Paquin A, Reyes-Moreno C, Bérubé G. Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules. Molecules. 2021; 26(8):2340. https://doi.org/10.3390/molecules26082340
Chicago/Turabian StylePaquin, Alexis, Carlos Reyes-Moreno, and Gervais Bérubé. 2021. "Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules" Molecules 26, no. 8: 2340. https://doi.org/10.3390/molecules26082340
APA StylePaquin, A., Reyes-Moreno, C., & Bérubé, G. (2021). Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules. Molecules, 26(8), 2340. https://doi.org/10.3390/molecules26082340






