Contribution of Attenuation of TNF-α and NF-κB in the Anti-Epileptic, Anti-Apoptotic and Neuroprotective Potential of Rosa webbiana Fruit and Its Chitosan Encapsulation
Abstract
1. Introduction
2. Results
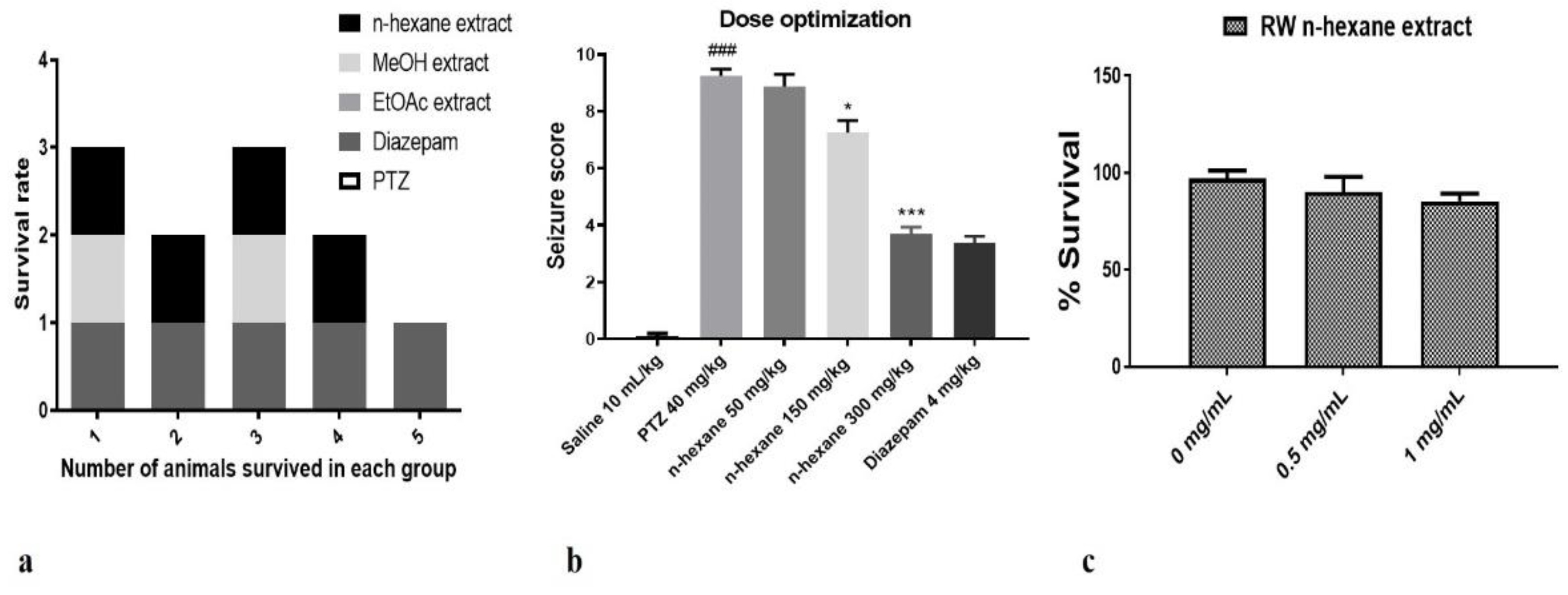
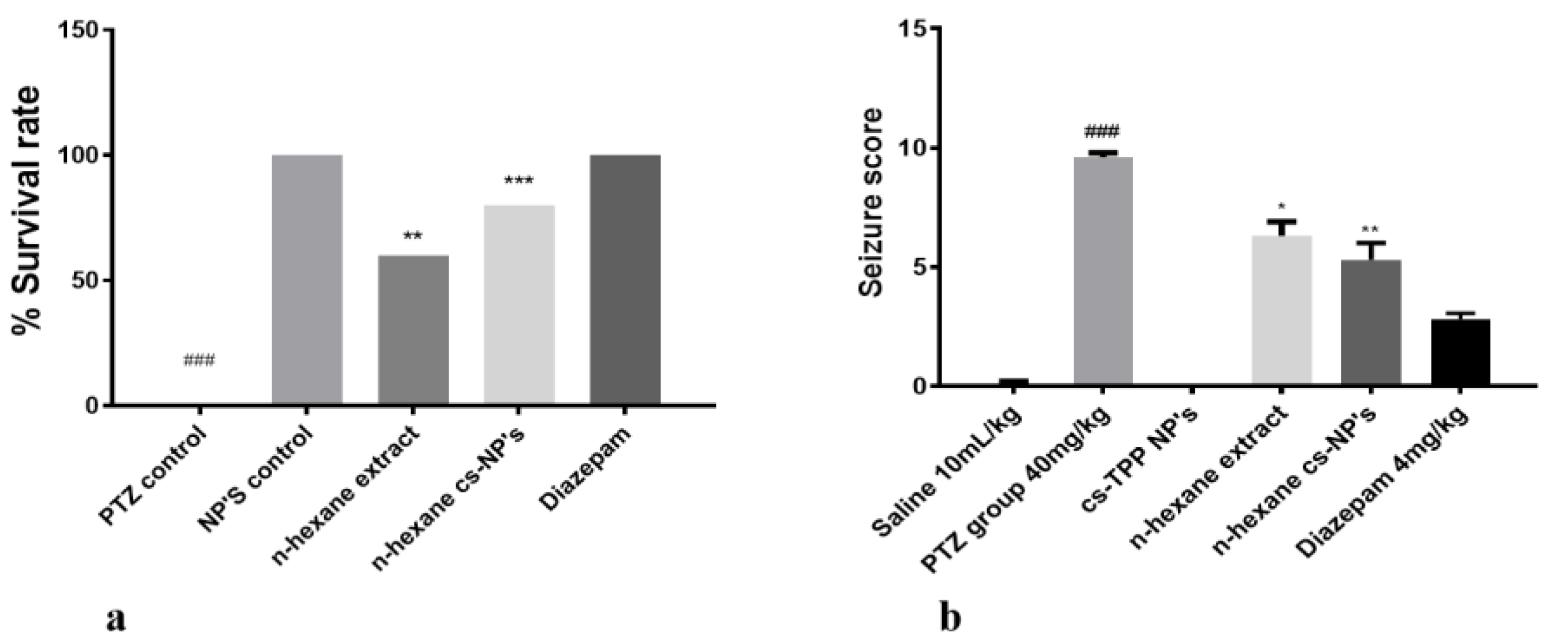
2.1. Anti-Convulsant Effect and Cytotoxicity of N-Hexane, Ethyl Acetate and Methanol Extracts of Fruit of Rosa webbiana
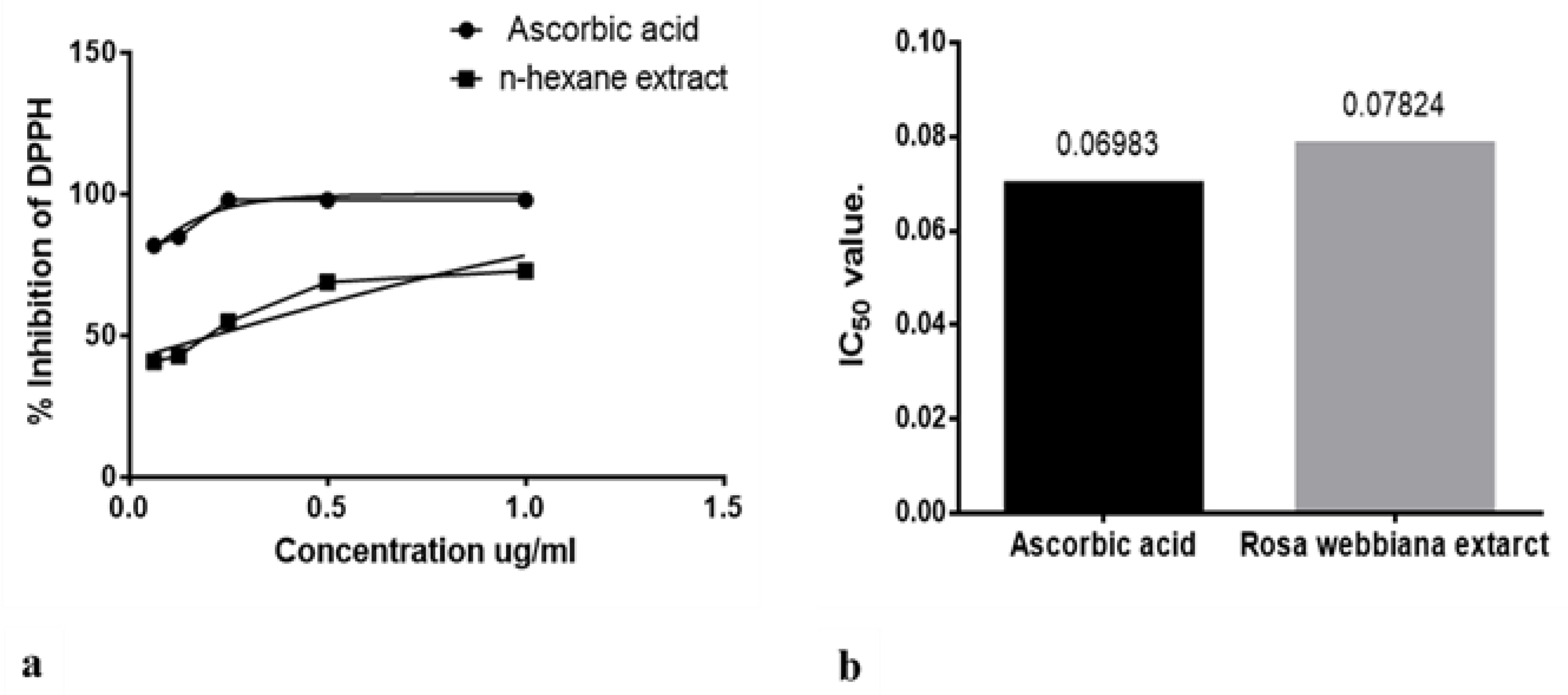
2.2. Anti-Oxidant Potential of the N-Hexane Extract of Fruit of Rosa webbiana
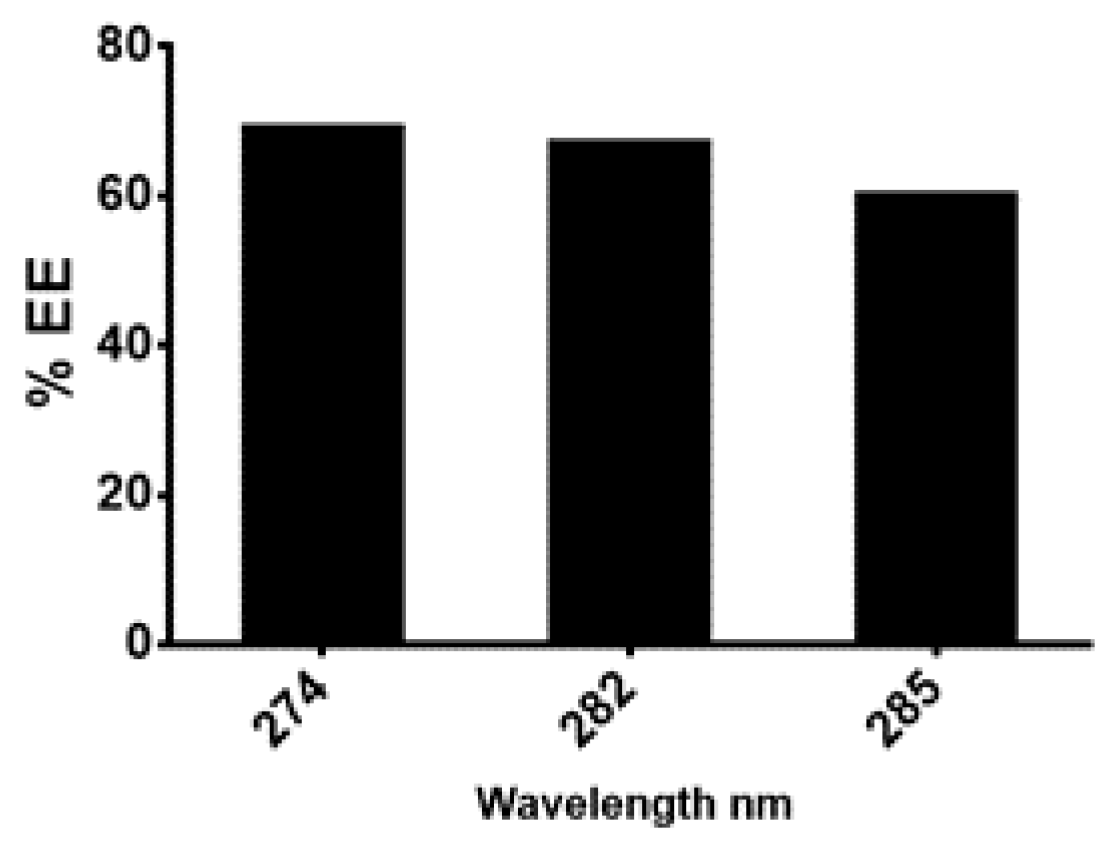
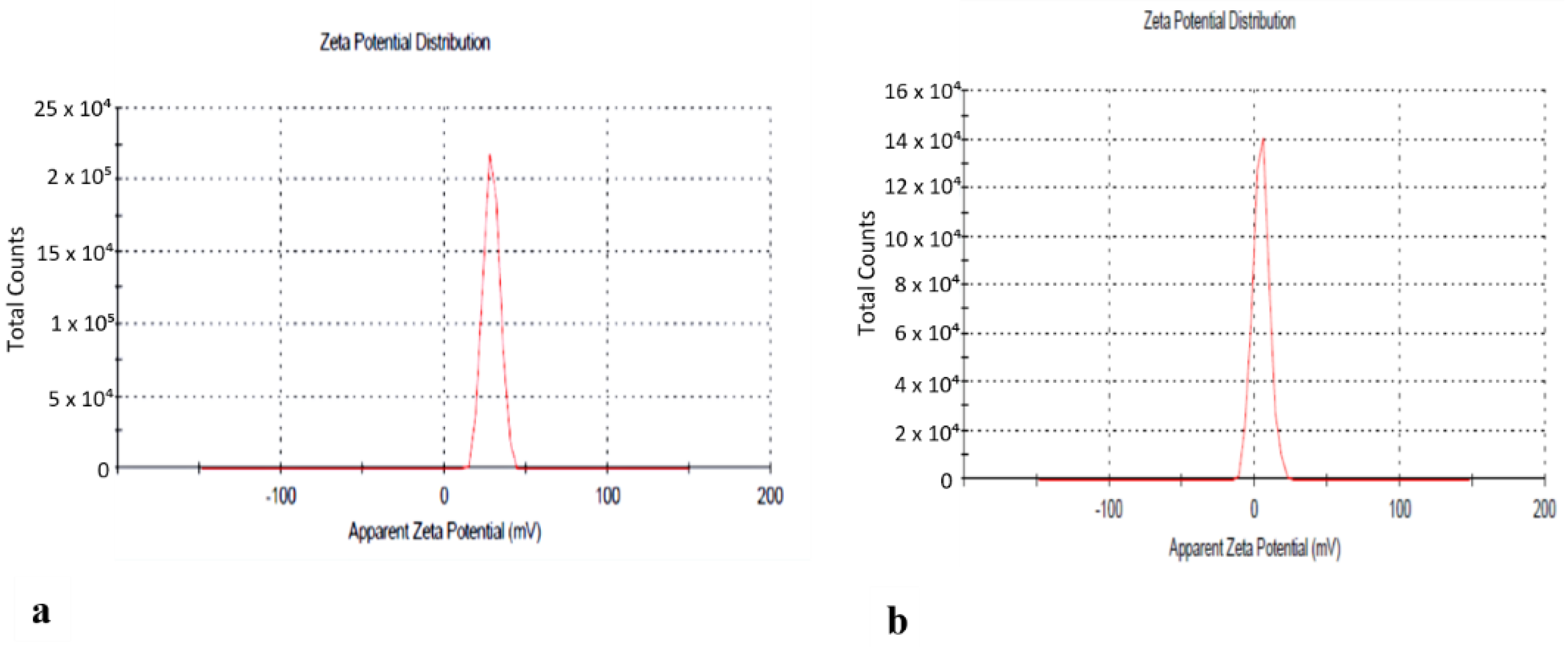
2.3. Development of N-Hexane Extract into Chitosan Nanoformulation
2.4. Evaluation of Chitosan Nanoformulation for Anti-Convulsant Effects
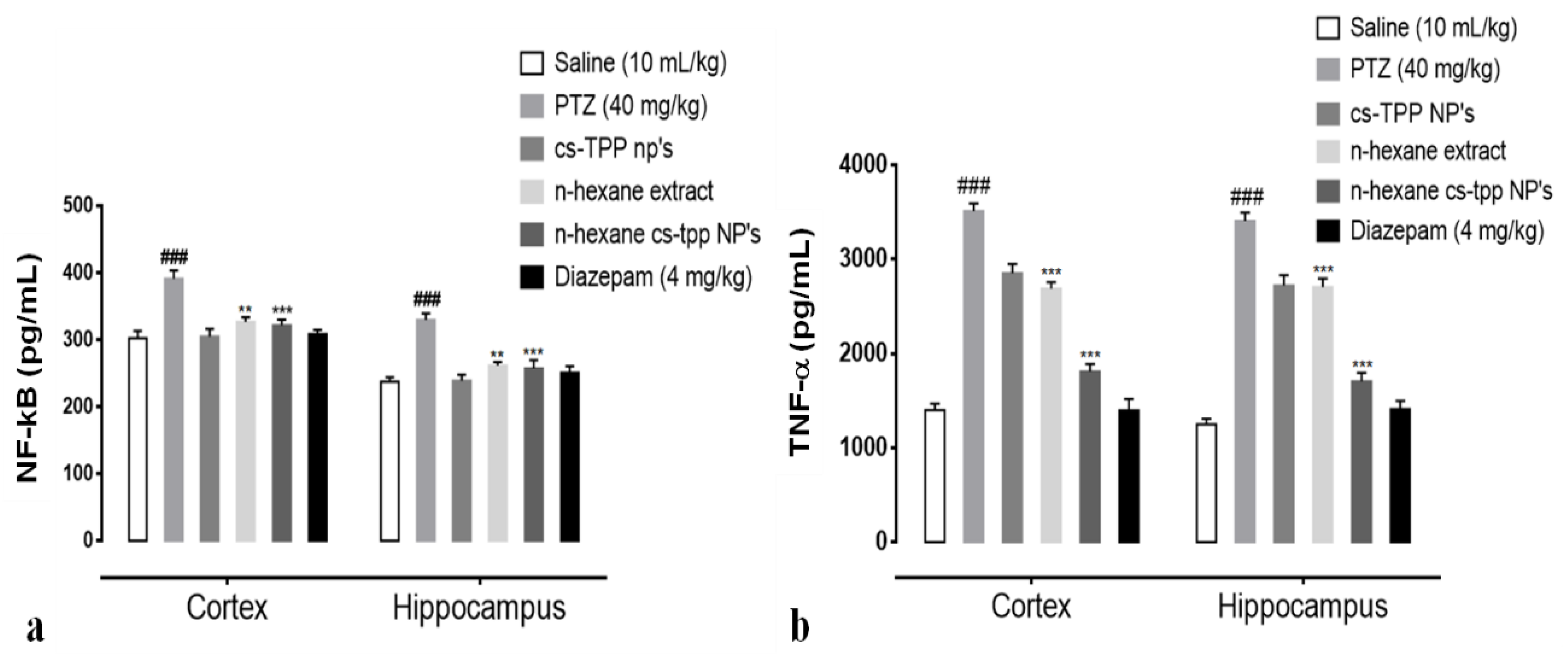
2.5. Effect of N-Hexane Extract and Its Nanoformulation on the Tnf-α and NF-KB Expression Detected Through Enzyme Linked Immunosorbent Assay
2.6. Cytoprotective Effect of N-Hexane Extract and Its Chitosan Nanoformulation on the Neurons in the Hippocampus and Cortex
2.7. Detection of Constituents in N-Hexane Extracts of Rosa webbiana
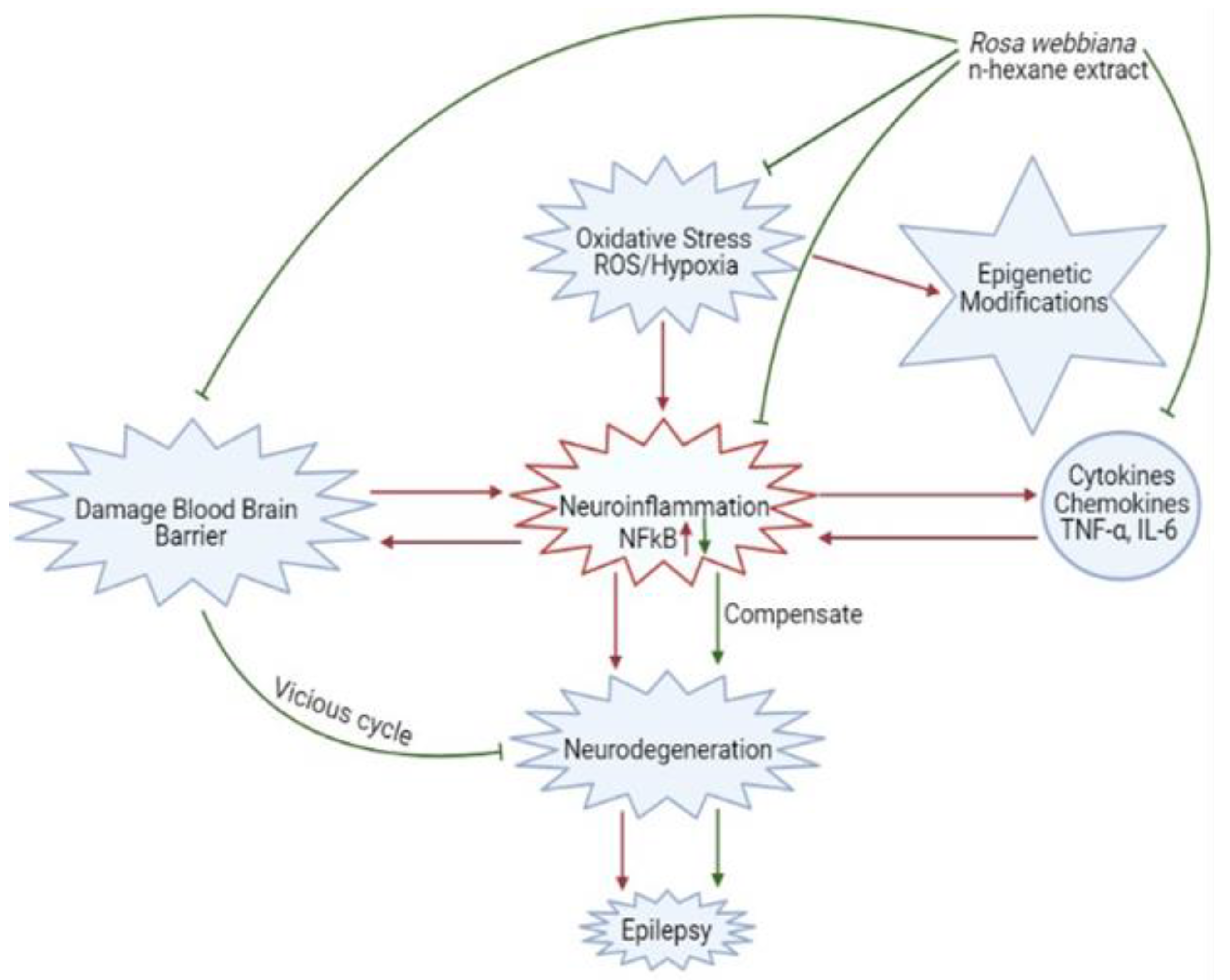
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection and Extraction
4.2. Pharmacological Studies
4.2.1. Toxicity Study
4.2.2. Cytotoxicity
4.2.3. Anticonvulsant Activity
4.2.4. Detection of Expression of p-NF-kB and p-TNF-α through ELISA
4.2.5. Cytoprotective Effect Determined through Hematoxylin and Eosin (H&E) Staining
4.3. Antioxidant Assay
4.4. GC-MS Analysis of Extract
4.5. Synthesis of Chitosan Nanoparticles (cs-NPs)
Characterization of Nanoparticles
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Juźwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.; Garcia-Gimeno, M.A. Reactive glia inflammatory signaling pathways and epilepsy. Int. J. Mol. Sci. 2020, 21, 4096. [Google Scholar] [CrossRef]
- Iori, V.; Frigerio, F.; Vezzani, A. Modulation of neuronal excitability by immune mediators in epilepsy. Curr. Opin. Pharmacol. 2016, 26, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Shandra, A.; Godlevsky, L.; Vastyanov, R.; Oleinik, A.; Konovalenko, V.; Rapoport, E.; Korobka, N. The role of TNF-α in amygdala kindled rats. Neurosci. Res. 2002, 42, 147–153. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Pan, Z.; Leng, Y.; Lv, J.; Li, B. The effects of herbal medicine on epilepsy. Oncotarget 2017, 8, 48385. [Google Scholar] [CrossRef]
- Schachter, S.C. Botanicals and herbs: A traditional approach to treating epilepsy. Neurotherapeutics 2009, 6, 415–420. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, traditional uses and pharmacological profile of rose hip: A review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef]
- Abbas, Z.; Khan, S.M.; Alam, J.; Khan, S.W.; Abbasi, A.M. Medicinal plants used by inhabitants of the Shigar Valley, Baltistan region of Karakorum range-Pakistan. J. Ethnobiol. Ethnomed. 2017, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Bano, A.; Ullah, F. Traditional drug therapies from various medicinal plants of central karakoram national park, Gilgit-Baltistan Pakistan. Pak. J. Bot. 2011, 43, 79–84. [Google Scholar]
- Akhter, N.; Akhtar, S.; Kazim, S.; Khan, T. Ethnomedicinal study of important medicinal plants used for gynecological issues among rural women folk in district gilgit. Nat. Sci. 2016, 14, 30–34. [Google Scholar]
- Ovais, M.; Zia, N.; Ahmad, I.; Khalil, A.T.; Raza, A.; Ayaz, M.; Sadiq, A.; Ullah, F.; Shinwari, Z.K. Phyto-therapeutic and nanomedicinal approaches to cure Alzheimer’s disease: Present status and future opportunities. Front. Aging Neurosci. 2018, 10, 284. [Google Scholar] [CrossRef]
- Haddad-Tóvolli, R.; Dragano, N.R.; Ramalho, A.F.; Velloso, L.A. Development and function of the blood-brain barrier in the context of metabolic control. Front. Neurosci. 2017, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Vishnudas, D.; Mitra, B.; Sant, S.B.; Annamalai, A. Green-synthesis and characterization of Silver Nanoparticles by aqueous Leaf extracts of Cardiospermum helicacabum L. Drug Invent. Today 2012, 4, 340–344. [Google Scholar]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Harris, R.; Lecumberri, E.; Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2011, 84, 803–806. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Naseer, M.I.; Ullah, I.; Al-Qahtani, M.H.; Karim, S.; Ullah, N.; Ansari, S.A.; Kim, M.O.; Bibi, F. Decreased GABA BR expression and increased neuronal cell death in developing rat brain after PTZ-induced seizure. Neurol. Sci. 2013, 34, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Garcia Alai, M.M.; Kapadnis, P.B.; Neumann, H.; Chin, J.W. Genetically encoding N ϵ-methyl-l-lysine in recombinant histones. J. Am. Chem. Soc. 2009, 131, 14194–14195. [Google Scholar] [CrossRef]
- Mari, A.; Lyon, D.; Fragner, L.; Montoro, P.; Piacente, S.; Wienkoop, S.; Egelhofer, V.; Weckwerth, W. Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC-MS and LC-MS metabolomics platform. Metabolomics 2013, 9, 599–607. [Google Scholar] [CrossRef]
- Mileva, M.; Krumova, E.; Miteva-Staleva, J.; Kostadinova, N.; Dobreva, A.; Galabov, A.S. Chemical compounds, in vitr o antioxidant and antifungal activities of some plant essentia l oils belonging to Rosaceae family. Comptes Rendus de I Acad. Bulg. des Sci. 2014, 67, 1363–1368. [Google Scholar]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Antioxidant and anti-cholinesterase activity of Sargassum wightii. Pharm. Biol. 2013, 51, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, L.; Yang, D.; Li, T.; Li, W. Biochemical composition and antioxidant capacity of extracts from Podophyllum hexandrum rhizome. BMC Complement. Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Therap. 2020, 216, 107695. [Google Scholar] [CrossRef]
- Homayoun, M.; Seghatoleslam, M.; Pourzaki, M.; Shafieian, R.; Hosseini, M.; Bideskan, A.E. Anticonvulsant and neuroprotective effects of Rosa damascena hydro-alcoholic extract on rat hippocampus. Avicenna J. Phytomed. 2015, 5, 260–270. [Google Scholar]
- Nade, V.S.; Kawale, L.A.; Dwivedi, S.; Yadav, A.V. Neuroprotective effect of Hibiscus rosa sinensis in an oxidative stress model of cerebral post-ischemic reperfusion injury in rats. Pharm. Biol. 2010, 48, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv. Colloid Interface Sci. 2015, 221, 60–76. [Google Scholar] [CrossRef]
- Mourtas, S.; Lazar, A.N.; Markoutsa, E.; Duyckaerts, C.; Antimisiaris, S.G. Multifunctional nanoliposomes with curcumin–lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur. J. Med. Chem. 2014, 80, 175–183. [Google Scholar] [CrossRef]
- Vezzani, A.; Friedman, A.; Dingledine, R.J. The role of inflammation in epileptogenesis. Neuropharmacology 2013, 69, 16–24. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Münch, G. Plant polyphenols as inhibitors of NF-κB induced cytokine production—A potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Zhang, K.; Yang, W.; Li, B. The anticonvulsant effects of ketogenic diet on epileptic seizures and potential mechanisms. Curr. Neuropharmacol. 2018, 16, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Van Erum, J.; Van Dam, D.; De Deyn, P.P. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav. 2019, 95, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ezhilan, B.P.; Neelamegam, R. GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacogn. Res. 2012, 4, 11–14. [Google Scholar]
- Arulpriya, P.; Calitha, P.; Hemalatha, S. In vitro antioxidant testing of the extracts of Samanea saman (Jacq.) Merr. Chem. Sin. 2010, 1, 73–79. [Google Scholar]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
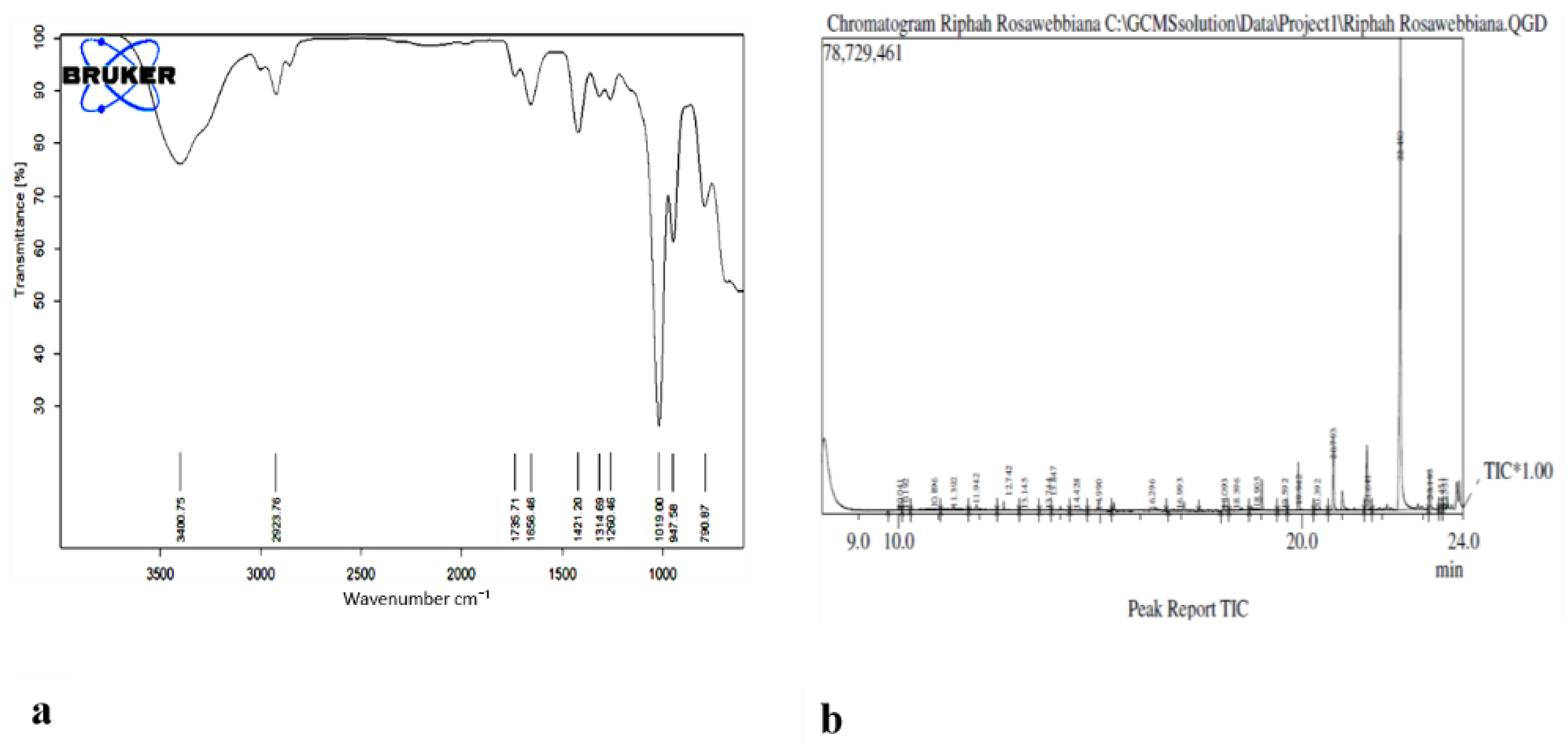

| Frequencies | Functional Groups |
|---|---|
| 3400 cm−1 | C=0, O-H, C-H and N-H of amines, alkanes and alcohols. |
| 2923 cm−1 | C-H, CH3 band of alkanes |
| 1735 cm−1 | C=0 of ester and acids |
| 1656 cm−1 | C=O, C=C of aryl ketones, arenes and alkenes. |
| 1421 cm−1 | CH2 group |
| 1314 cm−1 | C-N, C-O bonds |
| 1260 cm−1 | CH2 rocking and S=0 bond |
| 790 cm−1 | C-X bond |
| Peak # | R. Time | Constituents | Activity | Area% |
|---|---|---|---|---|
| 1 | 11.392 | Heptadecane, Pentadecane | - | 2.28 |
| 2 | 11.942 | Pentanoic acid or valeric acid | Neuroprotection, GABA-ergic effect | 2 |
| 3 | 12.742 | N-epsilon-methyl-L-lysine | Control transcription and translation and effect expression of proteins [20] | 1.33 |
| 4 | 16.296 | Nortriptyline | Neuroprotective, anti-inflammatory [21] | 1.67 |
| 5 | 16.993 | N-methyl epsilon-C-parolactam | - | 1.34 |
| 6 | 18.905 | Nitro-L-arginine | Amino acid, anti-oxidant and anti-inflammatory [22] | 4.13 |
| 7 | 19.942 | 2,4-Bis (hydroxylamino)-5-nitropyrimidine | Anti-depressant, neuroprotective [22] | 4.74 |
| 8 | 20.793 | Tetracosane | - | 9.24 |
| 9 | 22.450 | 1,2-Benzenedicarboxylic acid, di-iso octyl ester or some other ester | Cytotoxic, antioxidant and fungicidal effects [23,24] | 55.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firdous, A.; Sarwar, S.; Shah, F.A.; Tabasum, S.; Zeb, A.; Nadeem, H.; Alamro, A.; Alghamdi, A.A.; Alvi, A.M.; Naeem, K.; et al. Contribution of Attenuation of TNF-α and NF-κB in the Anti-Epileptic, Anti-Apoptotic and Neuroprotective Potential of Rosa webbiana Fruit and Its Chitosan Encapsulation. Molecules 2021, 26, 2347. https://doi.org/10.3390/molecules26082347
Firdous A, Sarwar S, Shah FA, Tabasum S, Zeb A, Nadeem H, Alamro A, Alghamdi AA, Alvi AM, Naeem K, et al. Contribution of Attenuation of TNF-α and NF-κB in the Anti-Epileptic, Anti-Apoptotic and Neuroprotective Potential of Rosa webbiana Fruit and Its Chitosan Encapsulation. Molecules. 2021; 26(8):2347. https://doi.org/10.3390/molecules26082347
Chicago/Turabian StyleFirdous, Anum, Sadia Sarwar, Fawad Ali Shah, Sobia Tabasum, Alam Zeb, Humaira Nadeem, Abir Alamro, Amani Ahmed Alghamdi, Arooj Mohsin Alvi, Komal Naeem, and et al. 2021. "Contribution of Attenuation of TNF-α and NF-κB in the Anti-Epileptic, Anti-Apoptotic and Neuroprotective Potential of Rosa webbiana Fruit and Its Chitosan Encapsulation" Molecules 26, no. 8: 2347. https://doi.org/10.3390/molecules26082347
APA StyleFirdous, A., Sarwar, S., Shah, F. A., Tabasum, S., Zeb, A., Nadeem, H., Alamro, A., Alghamdi, A. A., Alvi, A. M., Naeem, K., & Khalid, M. S. (2021). Contribution of Attenuation of TNF-α and NF-κB in the Anti-Epileptic, Anti-Apoptotic and Neuroprotective Potential of Rosa webbiana Fruit and Its Chitosan Encapsulation. Molecules, 26(8), 2347. https://doi.org/10.3390/molecules26082347








