Evaluation of Seven Essential Oils as Seed Treatments against Seedborne Fungal Pathogens of Cucurbita maxima
Abstract
:1. Introduction
2. Results
2.1. Efficacy of Essential Oils in the Control of Seed Infections
2.2. Effects of 0.5 mg/mL Essential Oils on Seed Germination, Radicle Length, and Individual Fungal Seed Infections
2.3. Effects of Cymbopogon citratus Essential Oil on Plantlets
3. Discussion
4. Materials and Methods
4.1. Collection of Squash Seed Samples
4.2. Essential Oils
4.3. Seed Treatments with the Essential Oils
4.4. Effects of Cymbopogon citratus Essential Oil on Emergence of Seedlings and Disease Incidence of S. cucurbitacearum of Squash Plantlets
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gannibal, P.B. Alternaria cucumerina causing leaf spot of pumpkin newly reported in north caucasus (Russia). New Dis. Rep. 2011, 23, 36. [Google Scholar] [CrossRef] [Green Version]
- Keinath, A.P. From native plants in central Europe to cultivate crops worldwide: The emergence of Didymella bryoniae as a cucurbit pathogen. HortScience 2011, 46, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Mehl, H.L.; Epstein, L. Identification of Fusarium solani f. sp. cucurbitae race 1 and race 2 with PCR and production of disease-free pumpkin seeds. Plant Dis. 2007, 91, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Moumni, M.; Allagui, M.B.; Mancini, V.; Murolo, S.; Tarchoun, N.; Romanazzi, G. Morphological and molecular identification of seedborne fungi in squash (Cucurbita maxima, Cucurbita moschata). Plant Dis. 2020, 104, 1335–1350. [Google Scholar] [CrossRef]
- Zhang, X.; Babadoost, M. Characteristics of Xanthomonas cucurbitae isolates from pumpkins and survival of the bacterium in pumpkin seeds. Plant. Dis. 2018, 9, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Gao, S.; Berendsen, S.; Fei, Z.; Ling, K.S. Complete genome sequence of a novel genotype of Squash mosaic virus infecting squash in Spain. Genome Announc. 2015, 3, e01583-14. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, L.; Pathak, N.; Zaidi, R.K. Antifungal potential of plant extracts against seed-borne fungi isolated from barley seeds (Hordeum vulgare L.). J. Plant Pathol. Microbiol. 2016, 7, 350. [Google Scholar] [CrossRef] [Green Version]
- Özer, N.; Coşkuntuna, A. The Biological Control Possibilities of Seed-Borne Fungi. In Current Trends in Plant Disease Diagnostics and Management Practices; Kumar, P., Gupta, V.K., Tiwari, A.K., Kamle, M., Eds.; Springer: Cham, Germany, 2016; pp. 383–403. [Google Scholar]
- Pellegrino, C.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Detection of Phoma valerianellae in lamb’s lettuce seeds. Phytoparasitica 2010, 38, 159–165. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Ning, H.; Li, W.; Bai, Y.; Li, Y. Evaluation and management of fungal-infected carrot seeds. Sci. Rep. 2020, 10, 10808. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, J.; Zhang, L.; Yan, C.; Jiang, S.; Li, Z.; Sun, D.; Lai, Y.; Gong, Z. Genome-scale analyses and characteristics of putative pathogenicity genes of Stagonosporopsis cucurbitacearum, a pumpkin gummy stem blight fungus. Sci. Rep. 2020, 10, 18065. [Google Scholar] [CrossRef]
- Chang, X.; Li, H.; Naeem, M.; Wu, X.; Yong, T.; Song, C.; Liu, T.; Chen, W.; Yang, W. Diversity of the seed-borne fungi and pathogenicity of fusarium species associated with intercropped soybean. Pathogens 2020, 9, 531. [Google Scholar] [CrossRef]
- Gaur, A.; Kumar, A.; Kiran, R.; Kumari, P. Importance of seed-borne diseases of agricultural crops: Economic losses and impact on society. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis and Management, 1st ed.; Kumar, R., Gupta, A., Eds.; Springer: Singapore; Karnal, India, 2020; pp. 3–23. [Google Scholar] [CrossRef]
- Abdulsalaam, S.; Shenge, K.C. Seed-borne pathogens on farmer-saved sorghum (Sorghum bicolor L.) seeds. J. Stored Prod. Postharvest Res. 2011, 2, 24–28. [Google Scholar]
- Mathur, S.B.; Kongsdal, O. Common Laboratory Seed Health Testing Methods for Detecting Fungi, 1st ed.; International Seed Testing Association: Bassersdorf, Switzerland, 2003; pp. 1–317. [Google Scholar]
- Mancini, V.; Romanazzi, G. Seed treatments to control seed-borne fungal pathogens of vegetable crops. Pest. Manage. Sci. 2014, 70, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens 2020, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Thomas-Sharma, S.; Andrade-Piedra, J.; Carvajal Yepes, M.; Hernandez Nopsa, J.F.; Jeger, M.J.; Jones, R.A.C.; Kromann, P.; Legg, J.P.; Yuen, J.; Forbes, G.A. A risk assessment framework for seed degeneration: Informing an integrated seed health strategy for vegetatively propagated crops. Phytopathology 2017, 107, 1123–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Castellanos, P.P.; Bishop, C.D.; Pascual-Villalobos, M.J. Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry 2001, 57, 99–102. [Google Scholar] [CrossRef]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar] [CrossRef]
- Romagnoli, C.; Bruni, R.; Andreotti, E.; Rai, M.K.; Vicentini, C.B.; Mares, D. Chemical characterization and antifungal activity of essential oil of capitula from wild Indian Tagetes patula L. Protoplasma 2005, 225, 57–65. [Google Scholar] [CrossRef]
- Moumni, M.; Romanazzi, G.; Najar, B.; Pistelli, L.; Ben Amara, H.; Mezrioui, K.; Karous, O.; Chaieb, I.; Allagui, M.B. Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of cucurbits. Antibiotics 2021, 10, 104. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef] [Green Version]
- Riccioni, L.; Orzali, L.; Romani, M.; Annicchiarico, P.; Pecetti, L. Organic seed treatments with essential oils to control ascochyta blight in pea. Eur. J. Plant Pathol. 2019, 155, 831–840. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The use of essential oils for postharvest decay control. Flavour. Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Djioua, T.; Charles, F.; Freire, M., Jr.; Filgueiras, H.; Ducamp-Collin, M.; Sallanon, H. Combined effects of postharvest heat treatment and chitosan coating on quality of fresh-cut mangoes (Mangifera indica L.). Int. J. Food Sci.Technol. 2010, 45, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Shahi, S.K.; Patra, M.; Shukla, A.C.; Dikshit, A. Use of essential oil as botanical-pesticide against postharvest spoilage in Malus pumilo fruits. Biocontrol 2003, 48, 223–232. [Google Scholar] [CrossRef]
- Newark, M.J.; Li, P.; Yang, X.P.; Paret, M.L.; Dufault, N.S. Comparing Stagonosporopsis spp. fungicide resistance profiles in Florida and East China cucurbit production systems. Plant Dis. 2020, 104, 129–136. [Google Scholar] [CrossRef]
- Mao, X.; Wu, Z.; Bi, C.; Wang, J.; Zhao, F.; Gao, J.; Hou, Y.; Zhou, M. Molecular and biochemical characterization of pydiflumetofen-resistant mutants of Didymella bryoniae. J. Agric. Food Chem. 2020, 68, 9120–9130. [Google Scholar] [CrossRef] [PubMed]
- Moumni, M.; Mancini, V.; Allagui, M.B.; Murolo, S.; Romanazzi, G. Black rot of squash (Cucurbita moschata Duchesne) caused by Stagonosporopsis cucurbitacearum reported in Italy. Phytopathol. Mediterr. 2019, 58, 381–385. [Google Scholar] [CrossRef]
- Gimode, W.; Bao, K.; Fei, Z.; McGregor, C. QTL associated with gummy stem blight resistance in watermelon. Theor. Appl. Genet. 2020, 134, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D.; Romanazzi, G. Use of essential oils to improve postharvest quality and control postharvest decay of tropical, subtropical, and temperate fruits. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press: New York, NY, USA, 2019; pp. 5–19. [Google Scholar] [CrossRef]
- Fiori, A.C.G.; Schwan-Estrada, K.R.F.; Stangarlin, J.R.; Vida, J.B.; Scapim, C.A.; Cruz, M.E.S.; Pascholati, S.F. Antifungal activity of leaf extracts and essential oils of some medicinal plants against Didymella bryoniae. J. Phytopathol. 2000, 148, 483–487. [Google Scholar] [CrossRef]
- Dalcin, M.S.; Cafee-Filho, A.C.; de Almeida Sarmento, R.; do Nascimento, I.R.; de Souza Ferreira, T.P.; de Sousa Aguiar, R.W.; dos Santos, G.R. Evaluation of essential oils for preventive or curative management of melon gummy stem blight and plant toxicity. J. Med. Plant. Res. 2017, 11, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Baka, Z.A.M. Biological control of the predominant seed-borne fungi of tomato by using plant extracts. Phytopathol. Pest. Manag. 2014, 1, 10–22. [Google Scholar]
- Sahab, A.F.; Aly, S.; Hathout, A.S.; Ziedan, E.S.H.; Sabry, B.A. Application of some plant essential oils to control Fusarium isolates associated with freshly harvested maize in Egypt. J. Essent. Oil-Bear. Plants 2014, 17, 1146–1155. [Google Scholar] [CrossRef]
- Szczerbanik, M.; Jobling, J.; Morris, S.; Holford, P. Essential oil vapours control some common postharvest fungal pathogens. Aust. J. Exp. Agric. 2007, 47, 103–109. [Google Scholar] [CrossRef]
- Kumar, K.N.; Venkataramana, M.; Allen, J.A.; Chandranayaka, S.; Murali, H.S.; Batra, H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT 2016, 69, 522–528. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crop. Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Ferreira, F.M.D.; Hirooka, E.Y.; Ferreira, F.D.; Silva, M.V.; Mossini, S.A.G.; Machinski Jr, M. Effect of Zingiber officinale Roscoe essential oil in fungus control and deoxynivalenol production of Fusarium graminearum Schwabe in vitro. Food Addit. Contam. Part. A 2018, 35, 2168–2174. [Google Scholar] [CrossRef]
- Perczak, A.; Gwiazdowska, D.; Marchwińska, K.; Juś, K.; Gwiazdowski, R.; Waśkiewicz, A. Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch. Microbiol. 2019, 201, 1085–1097. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.; El-Hefny, M.; El-Shanhorey, N.A.; Ali, H.M. Foliar application of bio-stimulants enhancing the production and the toxicity of Origanum majorana essential oils against four rice seed-borne fungi. Molecules 2020, 25, 2363. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wolf, J.M.; Birnbaum, Y.; Van Der Zouwen, P.S.; Groot, S.P.C. Disinfection of vegetable seed by treatment with essential oils, organic acids and plant extracts. Seed Sci. Technol. 2008, 36, 76–88. [Google Scholar] [CrossRef]
- Xu, S.; Ni, Z.; Ma, L.; Zheng, X. Control of alternaria rot of cherry tomatoes by food-grade Laurus nobilis essential oil microemulsion. J. Food Saf. 2017, 37, e12286. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Yan, F.; Ni, Z.; Chen, Q.; Zhang, H.; Zheng, X. In vitro and in vivo control of Alternaria alternata in cherry tomato by essential oil from Laurus nobilis of Chinese origin. J. Sci. Food Agric. 2014, 94, 1403–1408. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. Mich. J. Crop. Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Džamić, A.; Soković, M.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; kMarin, P.D. Chemical composition and antifungal activity of Salvia sclarea (Lamiaceae) essential oil. Arch. Biol. Sci. 2008, 60, 233–237. [Google Scholar] [CrossRef]
- Kishore, G.K.; Pande, S.; Harish, S. Evaluation of essential oils and their components for broad-spectrum antifungal activity and control of late leaf spot and crown rot diseases in peanut. Plant Dis. 2007, 91, 375–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Xu, S.; Wu, T.; Guo, J.; Sha, S.; Zheng, X.; Yu, T. Effect of citronella essential oil on the inhibition of postharvest Alternaria alternata in cherry tomato. J. Sci. Food Agric. 2014, 94, 2441–2447. [Google Scholar] [CrossRef]
- Eke, P.; Adamou, S.; Fokom, R.; Nya, V.D.; Fokou, P.V.T.; Wakam, L.N.; Nwagab, D.; Boyom, F.F. Arbuscular mycorrhizal fungi alter antifungal potential of lemongrass essential oil against Fusarium solani, causing root rot in common bean (Phaseolus vulgaris L.). Heliyon 2020, 6, e05737. [Google Scholar] [CrossRef] [PubMed]
- Orzali, L.; Valente, M.T.; Scala, V.; Loreti, S.; Pucci, N. Antibacterial activity of essential oils and Trametes versicolor extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for seed treatment and development of a rapid in vivo assay. Antibiotics 2020, 9, 628. [Google Scholar] [CrossRef]
- Orzali, L.; Forni, C.; Riccioni, L. Effect of chitosan seed treatment as elicitor of resistance to Fusarium graminearum in wheat. Seed Sci. Technol. 2014, 42, 132–149. [Google Scholar] [CrossRef]
- Somda, I.; Leth, V.; Sereme, P. Antifungal effect of Cymbopogon citratus, Eucalyptus camaldulensis and Azadirachta indica oil extracts on sorghum seed-borne fungi. Asian J. Plant. Sci. 2007, 6, 1182–1189. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Muthukumar, A.; Sangeetha, G.; Mohanapriya, R. Developing eco-friendly biofungicide for the management of major seed-borne diseases of rice and assessing their physical stability and storage life. Comptes Rendus Biol. 2017, 340, 214–225. [Google Scholar] [CrossRef]
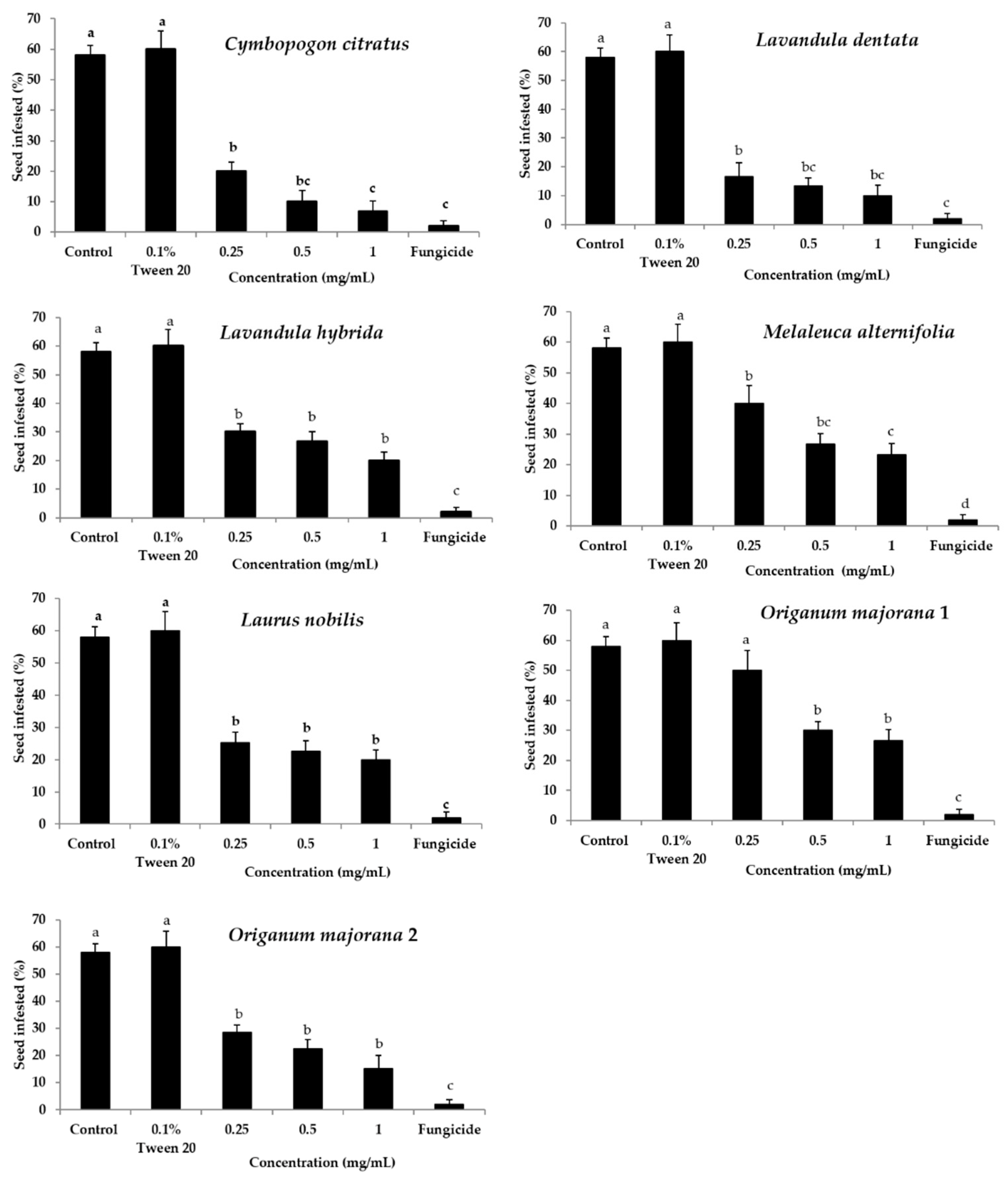


| Treatment/ Essential Oil | Germination (%) | Radicle Length (cm) | Incidence of Seed Infection (%) c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S.c. | A.a. | F.f. | F.s. | P.r. | A.v. | C.s. | R.s. | |||
| Control a | 85 ± 1.4 a | 20.9 ± 0.7 b | 16.1 ± 2.3 a | 9.3 ± 1.8 a | 22.6 ± 2.4 a | 4.6 ± 1.0 a | 5.8 ± 1.6 a | 3.3 ± 1.6 a | 3.3 ± 1.7 a | 16.4 ± 3.9 a |
| C. citratus | 86 ± 1.9 a | 21.0 ± 0.9 b | 4.5 ± 0.9 b | 1.1 ± 0.5 c | 5.3 ± 1.2 c,d | 0.5 ± 0.3 b | 1.7 ± 0.6 b | 0.5 ± 0.3 b | 0.9 ± 0.4 b | 4.2 ± 1.4 b,c |
| L. dentata | 86 ± 1.7 a | 15.0 ± 0.7 c | 5.2 ± 1.2 b | 1.0 ± 0.4 c | 2.4 ± 0.8 c,d | 0.5 ± 0.5 b | 1.0 ± 0.0 b | 1.7 ± 0.6 a,b | 0.0 ± 0.0 b | 3.7 ± 1.1 b,c |
| L. hybrida | 85 ± 2.2 a | 16.0 ± 0.6 c | 4.3 ± 0.9 b | 2.3 ± 0.8 c | 8.0 ± 1.4 c | 3.0 ± 1.6 a | 2.3 ± 0.8 b | 0.2 ± 0.2 b | 1.0 ± 0.6 b | 6.1 ± 1.9 b,c |
| M. alternifolia | 85 ± 2.4 a | 13.7 ± 0.7 c | 3.9 ± 1.2 b,c | 1.7 ± 0.6 c | 7.9 ± 1.3 c | 0.0 ± 0.0 b | 0.7 ± 0.4 b | 1.7 ± 0.8 a,b | 0.2 ± 0.2 b | 7.6 ± 2.0 b,c |
| L. nobilis | 86 ±1.9 a | 15.5 ± 0.6 c | 5.3 ± 1.1 b | 1.0 ± 0.4 c | 9.3 ± 1.4 b,c | 0.5 ± 0.3 b | 1.2 ± 0.5 b | 1.0 ±0.4 b | 0.7 ± 0.4 b | 6.3 ± 1.8 b,c |
| O. majorana | 87 ± 1.5 a | 20.5 ± 0.9 b | 5.0 ± 1.3 b | 2.7 ± 0.7 c | 8.7 ± 1.6 b,c | 0.5 ± 0.3 b | 1.0 ± 0.0 b | 1.7 ± 0.6 a,b | 1.2 ± 0.5 b | 8.1 ± 1.9 b |
| O. majorana | 85 ± 1.4 a | 15.1 ± 0.7 c | 2.5 ± 0.7 b,c | 1.2 ± 0.5 c | 10.1 ± 1.6 b | 0.0 ± 0.0 b | 1.7 ± 0.6 b | 1.0 ± 0.4 b | 0.3 ± 0.3 b | 8.3 ± 2.4 b |
| Fungicides b | 75 ± 2.2 b | 26.0 ± 1.1 a | 0.2 ± 0.2 c | 0.0 ± 0.0 c | 1.21 ± 0.5 d | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 1.0 ± 0.0 b | 1.0 ± 0.0 c |
| Significance (p) | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.025 | 0.032 | <0.001 |
| Treatment | Plantlet | Disease Incidence of Stagonosporopsis cucurbitacearum on the Plantlets (%) | |||
|---|---|---|---|---|---|
| Length (cm) | Infection (%) | Leaves | Stems | Roots | |
| Control a | 23.3 ± 1.2 a | 49.9 ± 2.9 c | 30.3 ± 5.2 b | 43.0 ± 4.4 b | 43.1 ± 3.2 b |
| C. citratus | 30.5 ± 1.1 b | 30.0 ± 4.4 b | 18.7 ± 3.3 a | 28.3 ± 4.5 a | 18.1 ± 4.3 a |
| Fungicide b | 30.2 ± 1.2 b | 16.8 ± 1.9 a | 9.7 ± 1.6 a | 17.3 ± 1.9 a | 17.0 ± 2.1 a |
| Species | Common Name | Source | Two Main Components | |
|---|---|---|---|---|
| Compound | (%) | |||
| Cymbopogon citratus | Lemongrass | CRRHAB | α-Citral | 51.6 |
| 𝛽-Citral | 26.0 | |||
| Lavandula dentata | Lavender | CRRHAB | Eucalyptol | 63.5 |
| β-Selinene | 4.1 | |||
| Lavandula hybrida | Lavandin | FLORA s.r.l. | Linalool | 33.7 |
| (Batch N° 161808) | Camphor | 9.3 | ||
| Melaleuca alternifolia | Tea tree | FLORA s.r.l. | Terpinen-4-ol | 41.1 |
| (Batch N° 161960) | γ-Terpinene | 16.0 | ||
| Laurus nobilis | Bay laurel | INAT | Eucalyptol | 47.9 |
| 𝛼-Terpinyl acetate | 10.2 | |||
| Origanum majorana | Marjoram#1 | INAT | Terpinen-4-ol | 32.4 |
| γ-Terpinene | 12.6 | |||
| Origanum majorana | Marjoram#2 | CRRHAB | Terpinen-4-ol | 50.1 |
| p-Cymene | 17.8 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moumni, M.; Allagui, M.B.; Mezrioui, K.; Ben Amara, H.; Romanazzi, G. Evaluation of Seven Essential Oils as Seed Treatments against Seedborne Fungal Pathogens of Cucurbita maxima. Molecules 2021, 26, 2354. https://doi.org/10.3390/molecules26082354
Moumni M, Allagui MB, Mezrioui K, Ben Amara H, Romanazzi G. Evaluation of Seven Essential Oils as Seed Treatments against Seedborne Fungal Pathogens of Cucurbita maxima. Molecules. 2021; 26(8):2354. https://doi.org/10.3390/molecules26082354
Chicago/Turabian StyleMoumni, Marwa, Mohamed Bechir Allagui, Kaies Mezrioui, Hajer Ben Amara, and Gianfranco Romanazzi. 2021. "Evaluation of Seven Essential Oils as Seed Treatments against Seedborne Fungal Pathogens of Cucurbita maxima" Molecules 26, no. 8: 2354. https://doi.org/10.3390/molecules26082354
APA StyleMoumni, M., Allagui, M. B., Mezrioui, K., Ben Amara, H., & Romanazzi, G. (2021). Evaluation of Seven Essential Oils as Seed Treatments against Seedborne Fungal Pathogens of Cucurbita maxima. Molecules, 26(8), 2354. https://doi.org/10.3390/molecules26082354








