Impact of the Type of Crosslinking Agents on the Properties of Modified Sodium Alginate/Poly(vinyl Alcohol) Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
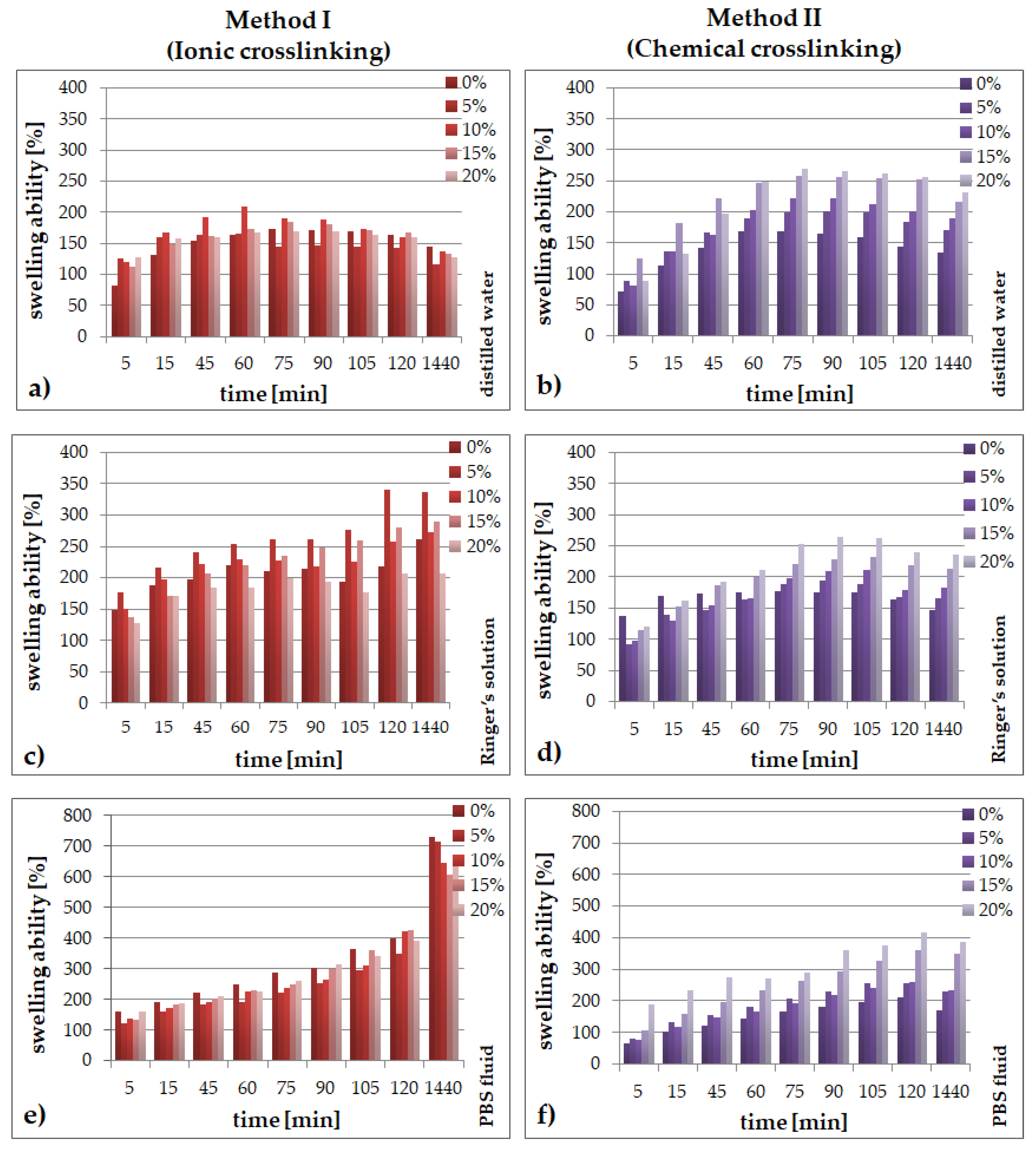
2.1. Swelling Abilities
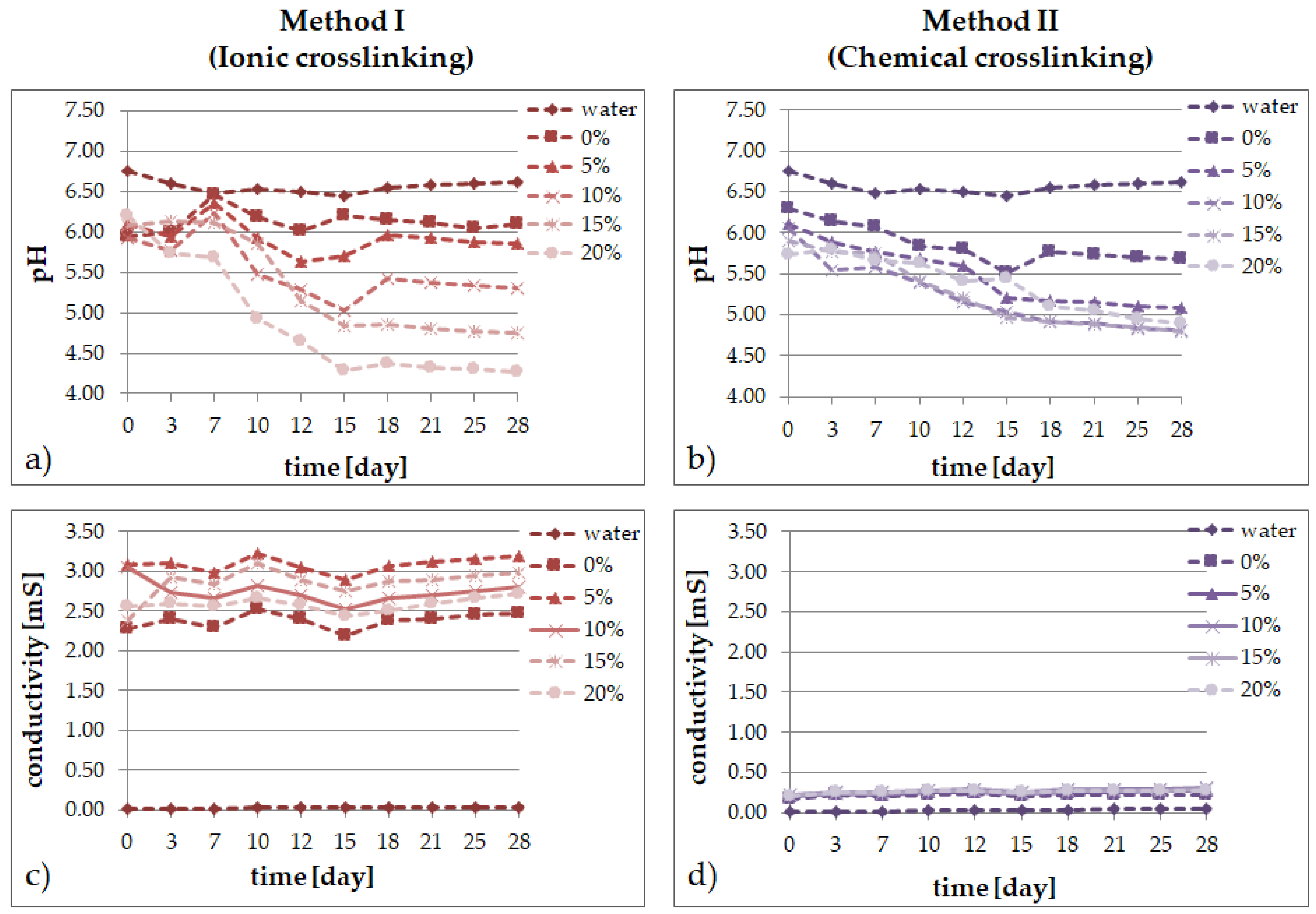
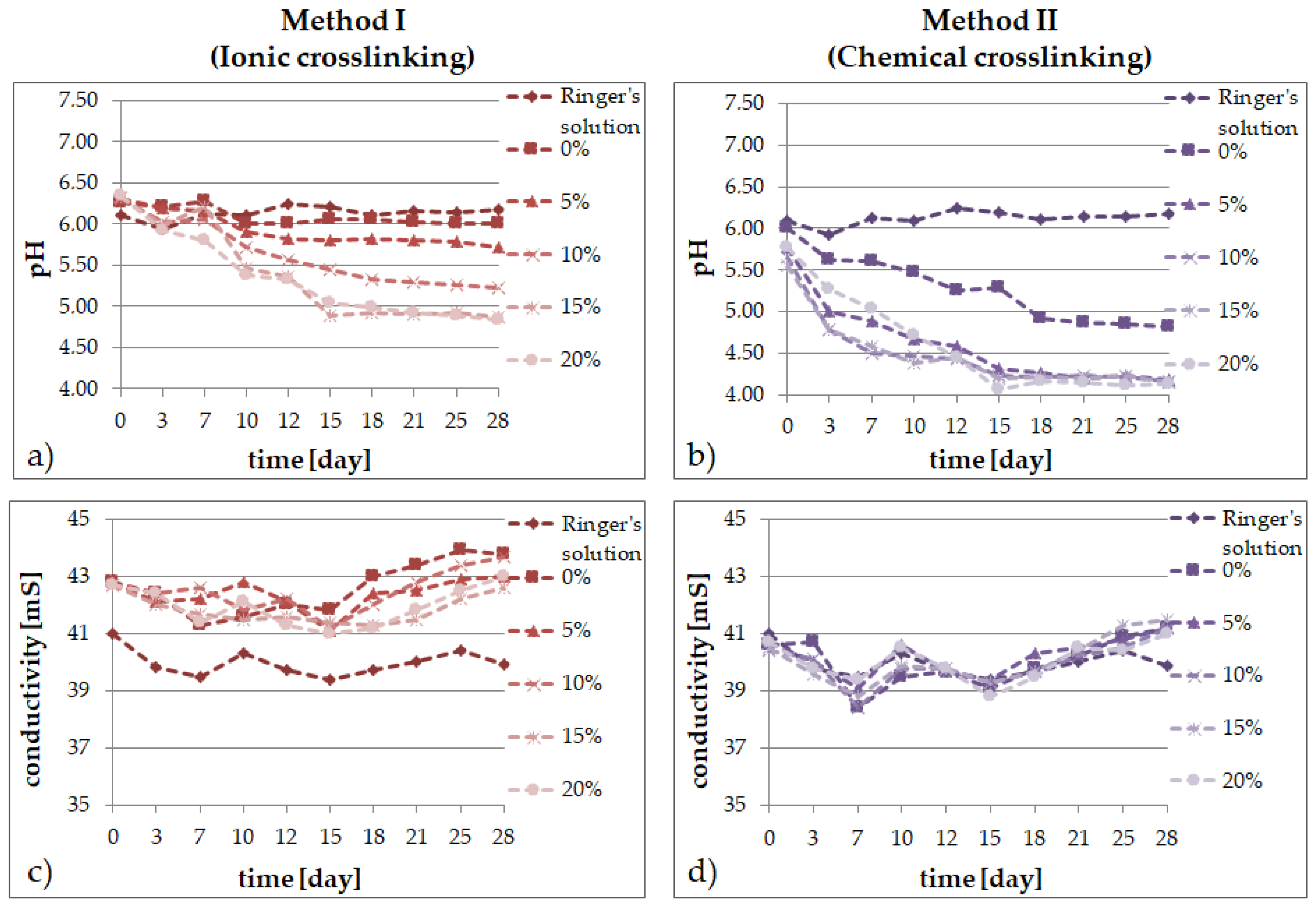
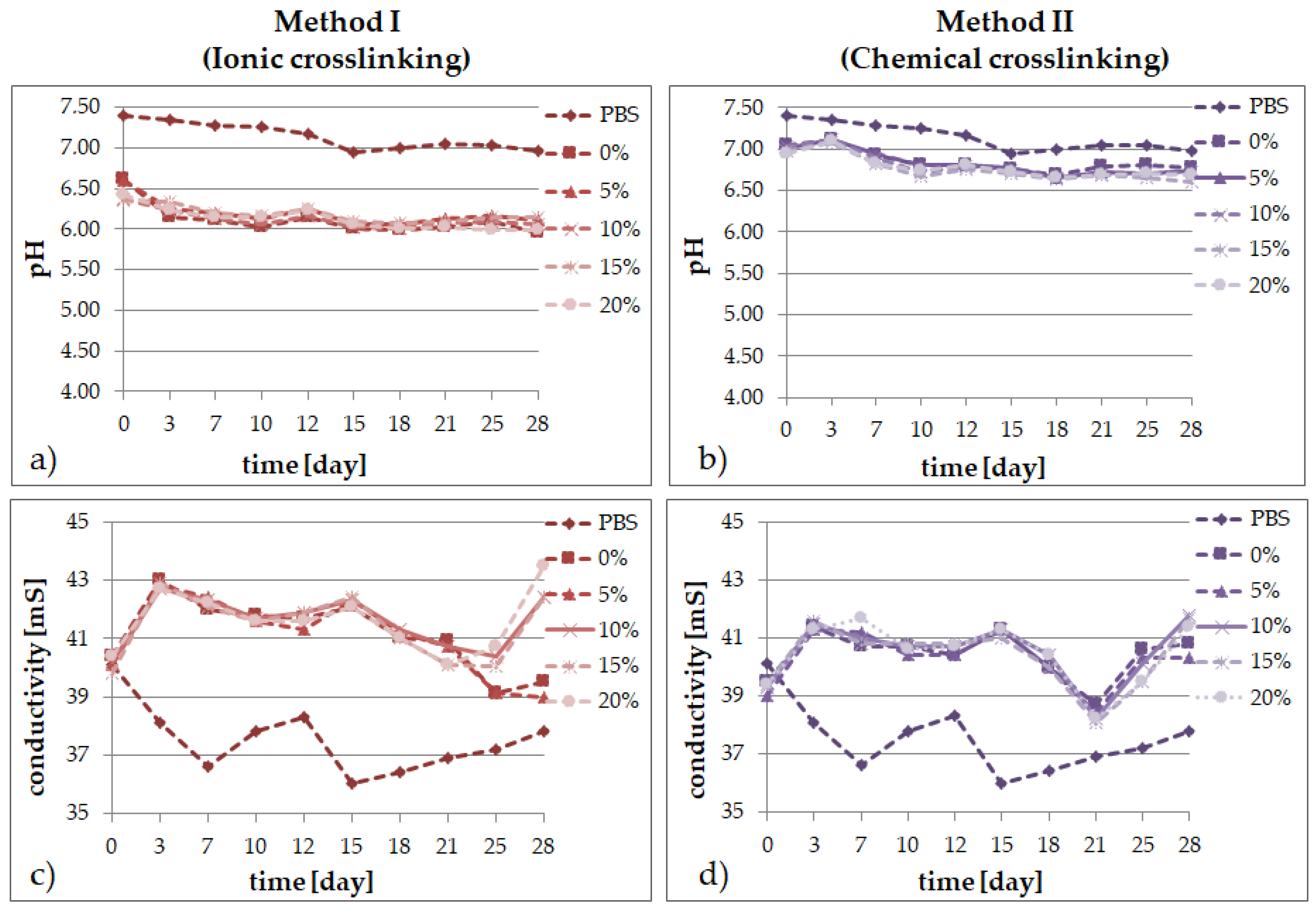
2.2. Degradation Tests
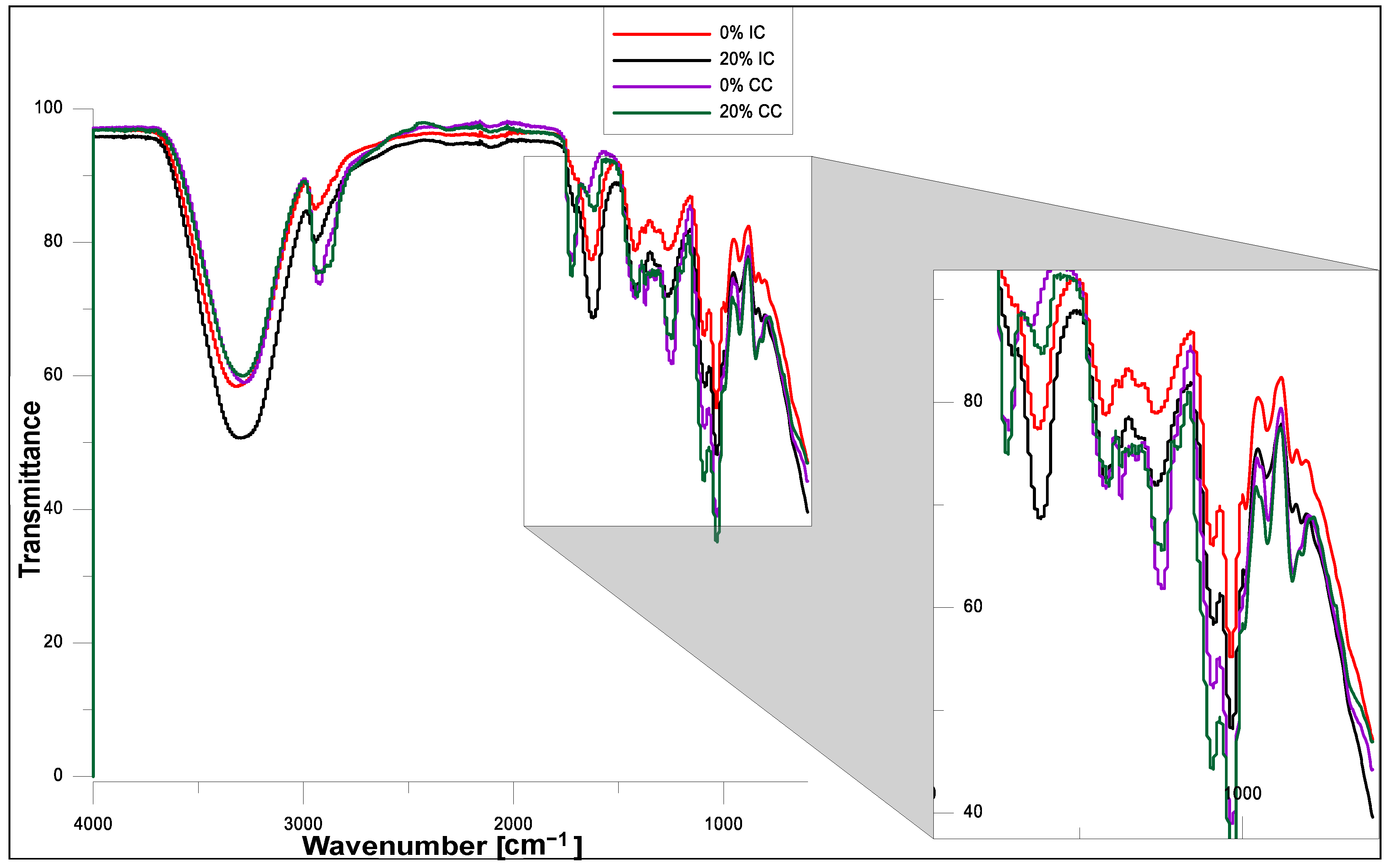
2.3. FT-IR Analysis
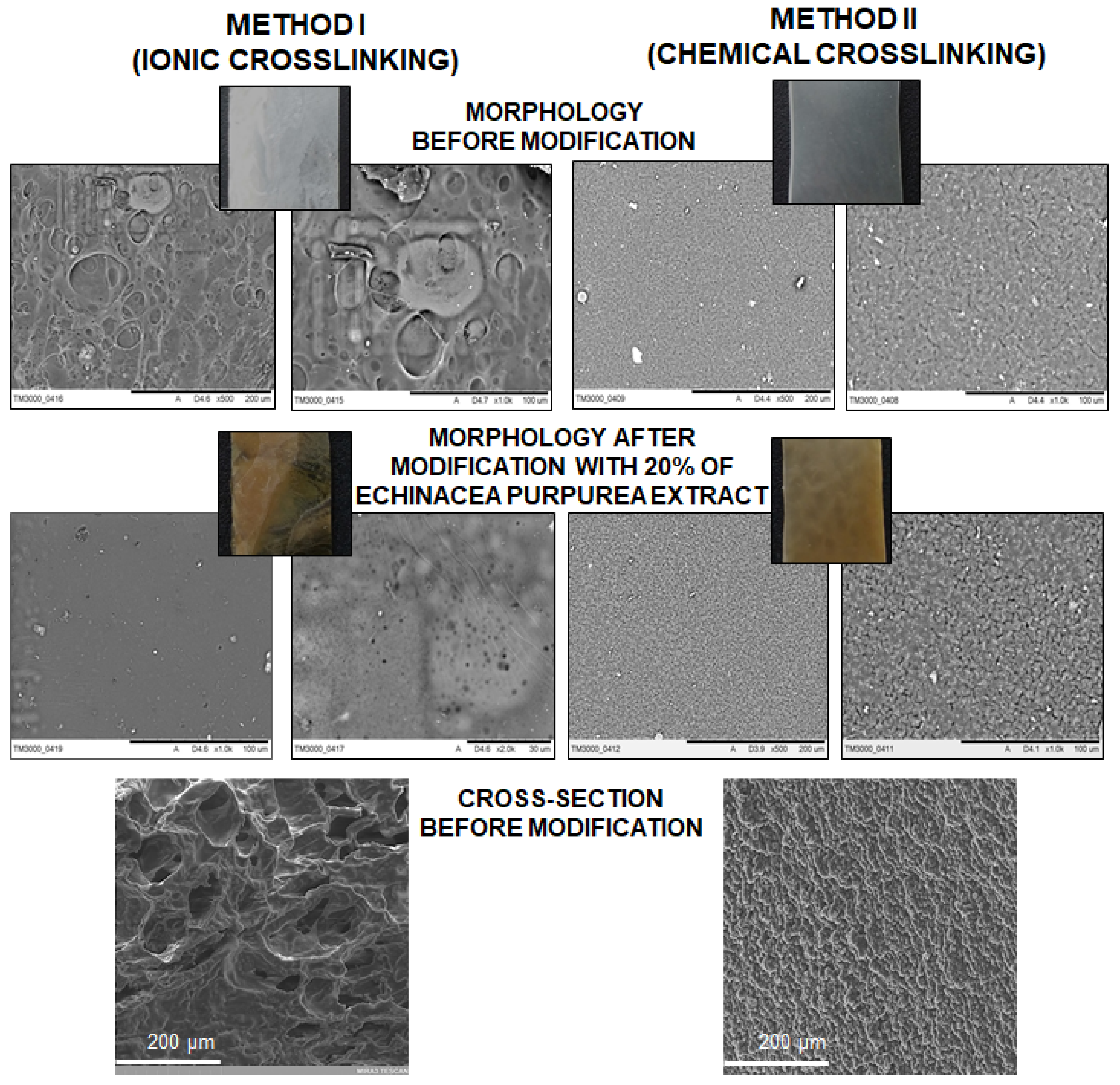
2.4. SEM Analysis
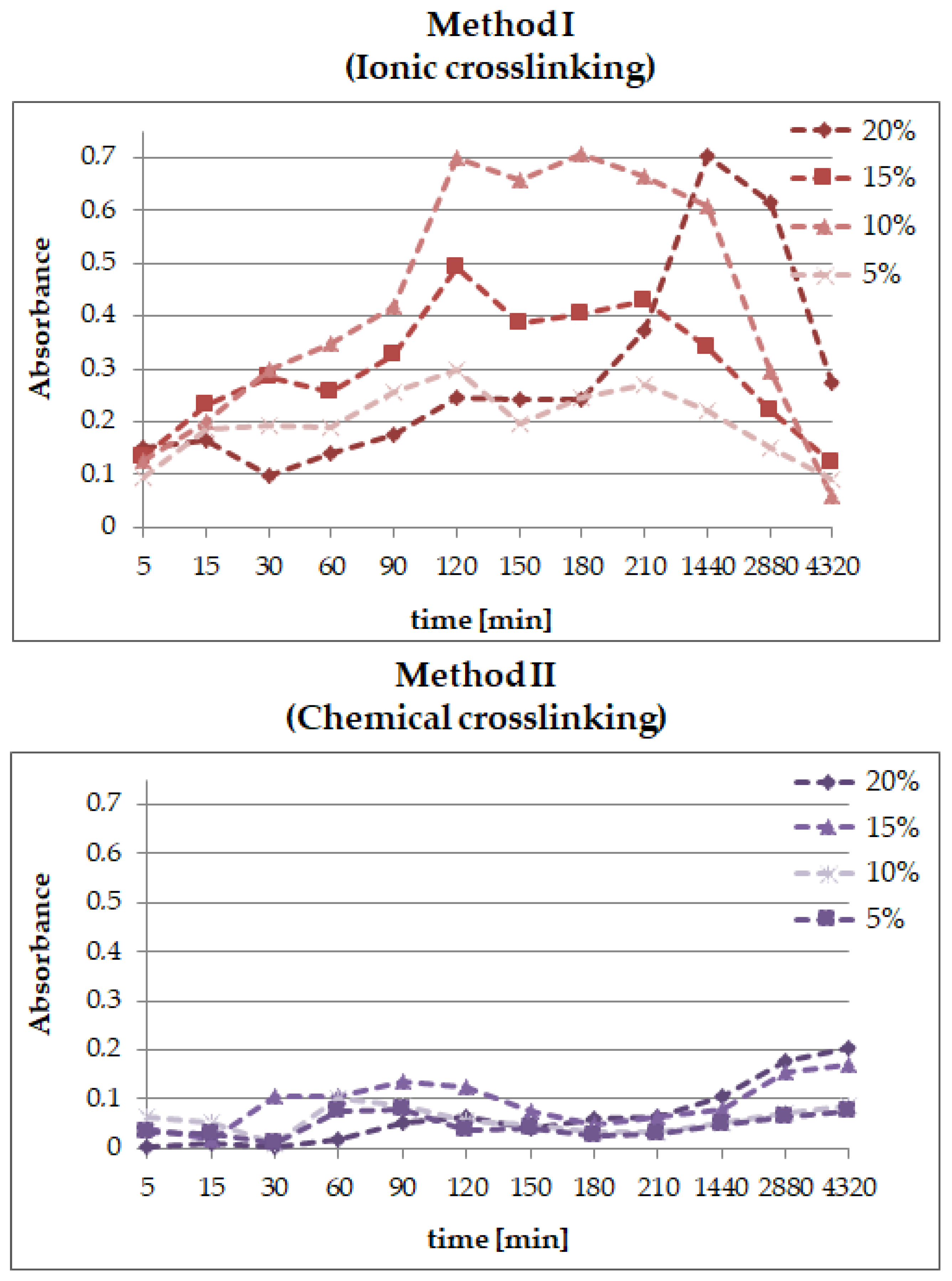
2.5. The Release Profile of Echinocoside from SA/PVA/EP Hydrogels
3. Experimental Part
3.1. Materials
3.2. Preparation of E. purpurea Extract
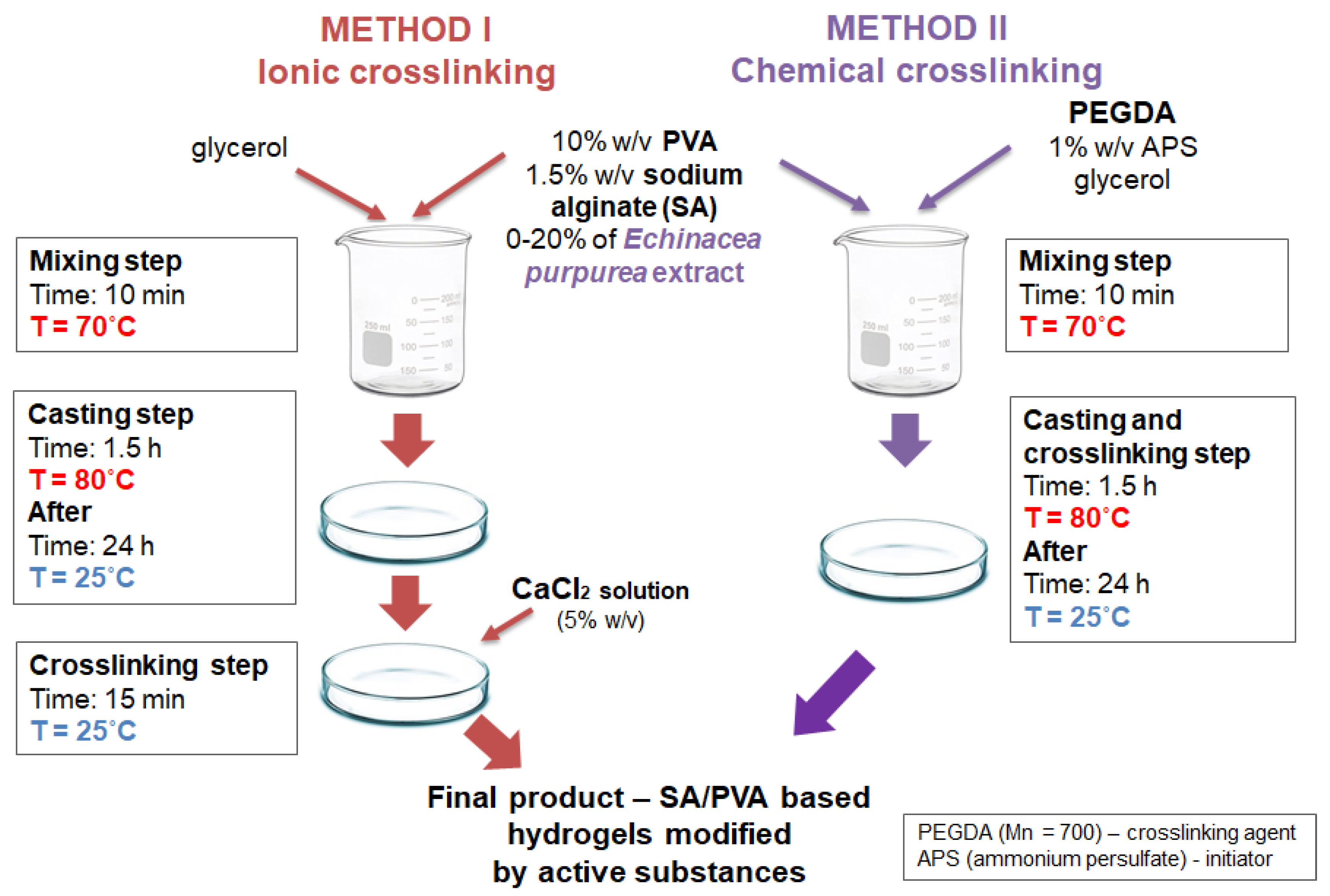
3.3. Preparation of SA/PVA/Echinacea Purpurea Hydrogels
3.4. Determination of Swelling Abilities
3.5. Degradation Test
3.6. ATR-FT-IR
3.7. SEM Analysis
3.8. Studies of Echinacoside Release
4. Conclusions
- the type of the crosslinking method strongly influences the ability of the materials to absorb simulated fluids;
- an increase of the amount of the Echinacea purpurea extract caused an increase of the swelling ability of the modified materials regardless of the crosslinking method used;
- the highest degree of swelling was observed in the PBS fluid, after 24 h, but only in the case of the hydrogels prepared through ionic crosslinking;
- the results of the incubation investigations suggest that the modified hydrogels can degrade faster, especially after ionic crosslinking;
- the surface of the hydrogels synthesized using chemical crosslinking is more regular and dense in comparison to the materials prepared using the second method; SEM analysis of the cross-section of hydrogels allow to conclude that the type of crosslinking agents influence directly on their network; in the case of ionically crosslinked hydrogels, the structure is more porous due to the release of echinacoside occurred faster;
- the release profiles show that echinacoside was delivered in a controlled manner for an extended time, but only in the case of the alginate-based hydrogels prepared using chemical crosslinking; however, in the second method, an undesirable phenomenon called the “burst effect” was observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Verhulsel, M.; Vignes, M.; Descroix, S.; Malaquin, L.; Vignjevic, D.M.; Viovy, J.-L. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials 2014, 35, 1816–1832. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Martin, N.; Youssef, G. Dynamic properties of hydrogels and fiber-reinforced hydrogels. J. Mech. Behav. Biomed. Mater. 2018, 85, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Correa, P.C.; Lui, A.C.F.; Silva, C.B.; Gracitelli, C.P.B.; Mimica, L.M.; Sasagawa, S.M.; Netto, A.L. Study of the Effectiveness of Multipurpose Solutions on the Bacterial Disinfection of Silicone Hydrogel Contact Lenses In Vitro. Eye Contact Lens Sci. Clin. Pract. 2018, 44, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Taubner, T.; Marounek, M.; Synytsya, A. Preparation and characterization of amidated derivatives of alginic acid. Int. J. Biol. Macromol. 2017, 103, 202–207. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Vazirian, M.; Dianat, S.; Manayi, A.; Ziari, R.; Mousazadeh, A.; Habibi, E. Anti-inflammatory effect, total polysaccharide, total phenolics content and antioxidant activity of the aqueous extract of three basidiomycetes. Res. J. Phcog. 2014, 1, 15–21. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties†. J. Pharm. Pharmacol. 2010, 57, 929–954. [Google Scholar] [CrossRef] [Green Version]
- Maggini, V.; De Leo, M.; Granchi, C.; Tuccinardi, T.; Mengoni, A.; Gallo, E.R.; Biffi, S.; Fani, R.; Pistelli, L.; Firenzuoli, F.; et al. The influence of Echinacea purpurea leaf microbiota on chicoric acid level. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Darnell, M.C.; Sun, J.-Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-R.; Wang, S.-H.; Chiang, C.-C.; Juang, Y.-C.; Yu, F.-A.; Tsai, L. High strain-rate response of injectable PAA hydrogel. J. Biomater. Sci. Polym. Ed. 2015, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Emami, Z.; Ehsani, M.; Zandi, M.; Foudazi, R. Controlling alginate oxidation conditions for making alginate-gelatin hydrogels. Carbohydr. Polym. 2018, 198, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J. Drug Deliv. Sci. Technol. 2020, 55, 101379. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Ghanaat, F. Antimicrobial alginate/PVA silver nanocomposite hydrogel, synthesis and characterization. J. Polym. Res. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Ghanem, S. Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. J. Polym. Res. 2009, 16, 1–10. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J.; Sankar, V.; Gopal, R.K. Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloids Surf. B Biointerfaces 2013, 107, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat. Plants 2015, 4, 3. [Google Scholar]
- Ditta, L.A.; Rao, E.; Provenzano, F.; Sánchez, J.L.; Santonocito, R.; Passantino, R.; Costa, M.A.; Sabatino, M.A.; Dispenza, C.; Giacomazza, D.; et al. Agarose/κ-carrageenan-based hydrogel film enriched with natural plant extracts for the treatment of cutaneous wounds. Int. J. Biol. Macromol. 2020, 164, 2818–2830. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose–lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Impacts of nanowhisker on formation kinetics and properties of all-cellulose composite gels. Carbohydr. Polym. 2011, 83, 1937–1946. [Google Scholar] [CrossRef]
- Elashmawy, N.E.; El-Zamarany, E.; Salem, M.; Elbahrawy, H.A.; AlAshmawy, G.M. In vitro and in vivo studies of the immunomodulatory effect of Echinacea purpurea on dendritic cells. J. Genet. Eng. Biotechnol. 2015, 13, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Acuna, R.; Garcia, A.J. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interac-tions. Matrix Biol. 2017, 57, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Calhelha, R.C.; Santos, P.F.; Ferreira, I.C. Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals 2020, 13, 125. [Google Scholar] [CrossRef]
- Cheng, J.; Jia, Z.; Li, T. A constitutive model of microfiber reinforced anisotropic hydrogels: With applications to wood-based hydrogels. J. Mech. Phys. Solids 2020, 138, 103893. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Li, J.; Zhou, Y.; Li, D.-Q.; Du, G.-M. Chemical cross-linking approach for prolonging diclofenac sodium release from pectin-based delivery system. Int. J. Biol. Macromol. 2019, 137, 512–520. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling Mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-Linker Type and Cross-Linking Density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Echalier, C.; Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun. 2019, 20, 100536. [Google Scholar] [CrossRef] [Green Version]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef]
- Rezende, R.A.; Jorge, B.P.; Mendes, A.; Filho, R.M. Rheological Behavior of Alginate Solutions for Biomanufacturing. J. Appl. Polym. Sci. 2009, 113, 3866–3871. [Google Scholar] [CrossRef]
- Lee, C. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocom-patibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Kaklamani, G.; Cheneler, D.; Grover, L.M.; Adams, M.J.; Bowen, J. Mechanical properties of alginate hydrogels manufac-tured using external gelation. J. Mech. Behav. Biomed. Mater. 2014, 36, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Chun-hui, L.; Chang-hai, W.; Zhi-liang, X.; Yi, W. Isolation, chemical characterization and antioxidant activities of two polysaccha-rides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process. Biochem. 2007, 42, 961–970. [Google Scholar] [CrossRef]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel contain-ing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Xu, F.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery. Carbohydr. Polym. 2017, 174, 904–914. [Google Scholar] [CrossRef]
- Lin, T.; Bai, Q.; Peng, J.; Xu, L.; Li, J.; Zhai, M. One-step radiation synthesis of agarose/polyacrylamide double-network hy-drogel with extremely excellent mechanical properties. Carbohydr. Polym. 2018, 200, 72–81. [Google Scholar] [CrossRef]
- Singh, B.; Varshney, L.; Francis, S. Rajneesh Synthesis and characterization of tragacanth gum based hydrogels by radiation method for use in wound dressing application. Radiat. Phys. Chem. 2017, 135, 94–105. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Majka, T.M. Alginate/PVA-based hydrogel matrices with Echinacea purpurea extract as a new approach to dermal wound healing. Int. J. Polym. Mater. 2021, 70, 195–206. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef]
- Choi, Y.; Hong, S.; Lee, Y.; Song, K.; Park, M.; Nam, Y. Study on gelatin-containing artificial skin: I. Preparation and charac-teristics of novel gelatin-alginate sponge. Biomaterials 1999, 20, 409–417. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Bialik-Was, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hy-drogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C 2021, 120, 111667. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shi, J.; Xia, Y. Effect of SiO2, PVA and glycerol concentrations on chemical and mechanical properties of algi-nate-based films. Int. J. Biol. Macromol. 2018, 107, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identyfication of Organic Compounds; John Willey & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. Ionically cross-linked carrageenan-alginate hydrogel beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Ghosal, K.; Das, A.; Kumar, S.; Mahmood, S.; Abdel, M.; Ramadan, M.; Thomas, S. Synthesis and characterization of inter-penetrating polymeric networks based bio-composite alginate film: A well-designed drug delivery platform. Int. J. Biol. Macromol. 2019, 130, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Govoni, P.; Guizzardi, S.; Renzulli, R.; Guerra, M.C. Anti-inflammatory and cicatrizing activity of Echinacea pal-lida Nutt. root extract. J. Ethnopharmacol. 2002, 79, 265–272. [Google Scholar] [CrossRef]
- Hu, O.; Chen, G.; Gu, J.; Lu, J.; Zhang, J.; Zhang, X.; Hou, L.; Jiang, X. A facile preparation method for anti-freezing, tough, transparent, conductive and thermoplastic poly(vinyl alcohol)/sodium alginate/glycerol organohydrogel electrolyte. Int. J. Biol. Macromol. 2020, 164, 2512–2523. [Google Scholar] [CrossRef]
- Saraydın, D.; Işıkver, Y.; Karadağ, E.; Sahiner, N.; Güven, O. In vitro dynamic swelling behaviors of radiation synthesized polyacrylamide with crosslinkers in the simulated physiological body fluids. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2002, 187, 340–344. [Google Scholar] [CrossRef]

| Method I (Ionic Crosslinking) | ||||
| PVA:A Ratio [v/v] | Glycerol [%, v/v] | Echinacea purpurea Extract [%, v/v] | CaCl2 [%, v/v] | |
| 1:2 | 3.5 | 0 | 35 | |
| 5 | ||||
| 10 | ||||
| 15 | ||||
| 20 | ||||
| Method II (Chemical Crosslinking) | ||||
| PVA:A Ratio [v/v] | Glycerol [%, v/v] | Echinacea purpurea Extract [%, v/v] | PEGDA [%, v/v] | APS [%, v/v] |
| 1:2 | 3.5 | 0 | 7.75 | 4.3 |
| 5 | ||||
| 10 | ||||
| 15 | ||||
| 20 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bialik-Wąs, K.; Królicka, E.; Malina, D. Impact of the Type of Crosslinking Agents on the Properties of Modified Sodium Alginate/Poly(vinyl Alcohol) Hydrogels. Molecules 2021, 26, 2381. https://doi.org/10.3390/molecules26082381
Bialik-Wąs K, Królicka E, Malina D. Impact of the Type of Crosslinking Agents on the Properties of Modified Sodium Alginate/Poly(vinyl Alcohol) Hydrogels. Molecules. 2021; 26(8):2381. https://doi.org/10.3390/molecules26082381
Chicago/Turabian StyleBialik-Wąs, Katarzyna, Ewelina Królicka, and Dagmara Malina. 2021. "Impact of the Type of Crosslinking Agents on the Properties of Modified Sodium Alginate/Poly(vinyl Alcohol) Hydrogels" Molecules 26, no. 8: 2381. https://doi.org/10.3390/molecules26082381
APA StyleBialik-Wąs, K., Królicka, E., & Malina, D. (2021). Impact of the Type of Crosslinking Agents on the Properties of Modified Sodium Alginate/Poly(vinyl Alcohol) Hydrogels. Molecules, 26(8), 2381. https://doi.org/10.3390/molecules26082381







