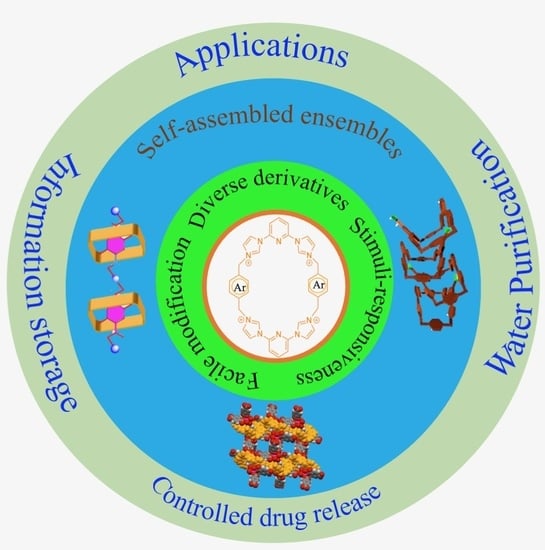
“Texas-Sized” Molecular Boxes: From Chemistry to Applications
Abstract
:1. Introduction
2. Synthesis of “Texas-Sized” Molecular Box
3. Host–Guest Chemistry of “Texas-Sized” Molecular Box
4. Self-Assembled Ensembles Containing a “Texas-Sized” Molecular Box
5. Applications of “Texas-Sized” Molecular Box
5.1. Hydrogel for Removal of Anions from Water
5.2. Multifluorescent Hydrogels for Encoding, Reading, and Transforming Information
5.3. TxSB-Based Amphiphilic Copolymer for Controlled Release
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fyfe, M.C.; Stoddart, J.F. Synthetic Supramolecular Chemistry. Acc. Chem. Res. 1997, 30, 393–401. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.-A.; Nakamoto, Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as Ion Pair Receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar] [CrossRef]
- Wang, X.; Jia, F.; Yang, L.-P.; Zhou, H.; Jiang, W. Conformationally adaptive macrocycles with flipping aromatic sidewalls. Chem. Soc. Rev. 2020, 49, 4176–4188. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Z.Y.; Yu, C.; Wang, B.; Dong, M.; Zeng, X.; Gou, R.; Cui, L.; Li, C. A Modular Synthetic Strategy for Functional Macrocycles. Angew. Chem. Int. Ed. 2020, 59, 7214–7218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, F.; Dong, S.; Huang, F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef]
- Price, T.L., Jr.; Wessels, H.R.; Slebodnick, C.; Gibson, H.W. High-Yielding Syntheses of Crown Ether-Based Pyridyl Cryptands. J. Org. Chem. 2017, 82, 8117–8122. [Google Scholar] [CrossRef] [PubMed]
- Wenz, G. Cyclodextrins as Building Blocks for Supramolecular Structures and Functional Units. Angew. Chem. Int. Ed. 1994, 33, 803–822. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef]
- Dale, E.J.; Vermeulen, N.A.; Juricek, M.; Barnes, J.C.; Young, R.M.; Wasielewski, M.R.; Stoddart, J.F. Supramolecular Explorations: Exhibiting the Extent of Extended cationic cyclophanes. Acc. Chem. Res. 2016, 49, 262–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, S.E.; Beer, P.D. Calixarene-Based Anion Receptors. Supramol. Chem. 2005, 17, 411–435. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.-S.; Liu, Y. Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 2012, 41, 5907–5921. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular Chemistry at Interfaces: Host–Guest Interactions for Fabricating Multifunctional Biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef]
- Isaacs, L. Cucurbit [n] urils: From mechanism to structure and function. Chem. Commun. 2009, 619–629. [Google Scholar] [CrossRef]
- Chi, X.; Ji, X.; Xia, D.; Huang, F. A Dual-Responsive Supra-Amphiphilic Polypseudorotaxane Constructed from a Water-Soluble Pillar[7]arene and an Azobenzene-Containing Random Copolymer. J. Am. Chem. Soc. 2015, 137, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, C.N.; Zeng, H.; Ji, X.; Xie, T.; Yan, X.; Wu, Z.L.; Huang, F. Reversible Ion-Conducting Switch in a Novel Single-Ion Supramolecular Hydrogel Enabled by Photoresponsive Host–Guest Molecular Recognition. Adv. Mater. 2019, 31, 1807328. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, L.; Li, G.; Liu, K.; Zhang, Z.; Li, P.; Dong, S.; Yu, W.; Huang, F.; Yan, X. A Self-Cross-Linking Supramolecular Polymer Network Enabled by Crown-Ether-Based Molecular Recognition. J. Am. Chem. Soc. 2020, 142, 2051–2058. [Google Scholar] [CrossRef]
- Smolyanitsky, A.; Paulechka, E.; Kroenlein, K. Aqueous Ion Trapping and Transport in Graphene-Embedded 18-Crown-6 Ether Pores. ACS Nano 2018, 12, 6677–6684. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-F.; Li, Z.; Lin, Q.; Yang, Y.-W. Functional supramolecular gels based on pillar[n]arene macrocycles. Nanoscale 2020, 12, 2180–2200. [Google Scholar] [CrossRef]
- Liu, K.; Yao, Y.; Kang, Y.; Liu, Y.; Han, Y.; Wang, Y.; Li, Z.; Zhang, X. A supramolecular approach to fabricate highly emissive smart materials. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Schalley, C.A. Exploring Macrocycles in Functional Supramolecular Gels: From Stimuli Responsiveness to Systems Chemistry. Acc. Chem. Res. 2014, 47, 2222–2233. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional Supramolecular Polymeric Networks: The Marriage of Covalent Polymers and Macrocycle-Based Host–Guest Interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Guo, D.-S.; Liu, Y. Supramolecular Chemistry of p-Sulfonatocalix[n]arenes and Its Biological Applications. Acc. Chem. Res. 2014, 47, 1925–1934. [Google Scholar] [CrossRef]
- Kolesnichenko, I.V.; Anslyn, E.V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 2385–2390. [Google Scholar] [CrossRef]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xu, B. Supramolecular medicine. Chem. Soc. Rev. 2017, 46, 6430–6432. [Google Scholar] [CrossRef]
- Sauvage, J.P. From Chemical Topology to Molecular Machines (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11080–11093. [Google Scholar] [CrossRef] [Green Version]
- Pezzato, C.; Cheng, C.; Stoddart, J.F.; Astumian, R.D. Mastering the non-equilibrium assembly and operation of molecular machines. Chem. Soc. Rev. 2017, 46, 5491–5507. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Feringa, B.L. Towards artificial molecular factories from framework-embedded molecular machines. Nat. Rev. Chem. 2020, 4, 550–562. [Google Scholar] [CrossRef]
- Guo, Q.H.; Fu, Z.D.; Zhao, L.; Wang, M.X. Synthesis, Structure, and Properties of O6-Corona[3]arene[3]tetrazines. Angew. Chem. Int. Ed. 2014, 53, 13548–13552. [Google Scholar] [CrossRef]
- Jia, F.; He, Z.; Yang, L.-P.; Pan, Z.-S.; Yi, M.; Jiang, R.-W.; Jiang, W. Oxatub[4]arene: A smart macrocyclic receptor with multiple interconvertible cavities. Chem. Sci. 2015, 6, 6731–6738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Tropp, J.; Qiao, B.; Pink, M.; Azoulay, J.D.; Flood, A.H. Tunable Adhesion from Stoichiometry-Controlled and Sequence-Defined Supramolecular Polymers Emerges Hierarchically from Cyanostar-Stabilized Anion–Anion Linkages. J. Am. Chem. Soc. 2020, 142, 2579–2591. [Google Scholar] [CrossRef] [PubMed]
- Svec, J.; Necas, M.; Sindelar, V. Bambus[6]uril. Angew. Chem. Int. Ed. 2010, 49, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Fahrenbach, A.C.; Sampath, S.; Late, D.J.; Barnes, J.C.; Kleinman, S.L.; Valley, N.; Hartlieb, K.J.; Liu, Z.; Dravid, V.P.; Schatz, G.C.; et al. A Semiconducting Organic Radical Cationic Host–Guest Complex. ACS Nano 2012, 6, 9964–9971. [Google Scholar] [CrossRef]
- Flood, A.H.; Stoddart, J.F.; Steuerman, D.W.; Heath, J.R. Whence Molecular Electronics? Science 2004, 306, 2055–2056. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.F. Molecular Machines. Acc. Chem. Res. 2001, 34, 410–411. [Google Scholar] [CrossRef]
- Gong, H.-Y.; Rambo, B.M.; Karnas, E.; Lynch, V.M.; Sessler, J.L. A ‘Texas-sized’molecular box that forms an anion-induced supramolecular necklace. Nat. Chem. 2010, 2, 406–409. [Google Scholar] [CrossRef]
- Wu, R.-T.; Chi, X.; Hirao, T.; Lynch, V.M.; Sessler, J.L. Supramolecular Properties of a Monocarboxylic Acid-Functionalized “Texas-Sized” Molecular Box. J. Am. Chem. Soc. 2018, 140, 6823–6831. [Google Scholar] [CrossRef]
- Ji, X.; Chi, X.; Ahmed, M.; Long, L.; Sessler, J.L. Soft Materials Constructed Using Calix[4]pyrrole- and “Texas-Sized” Box-Based Anion Receptors. Acc. Chem. Res. 2019, 52, 1915–1927. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, S.K.; Singh, N.J.; Kim, K.S. Imidazolium receptors for the recognition of anions. Chem. Soc. Rev. 2006, 35, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Odell, B.; Reddington, M.V.; Slawin, A.M.; Spencer, N.; Stoddart, J.F.; Williams, D.J. Cyclobis(paraquat-p-phenylene): A Tetracationic Multipurpose Receptor. Angew. Chem. Int. Ed. 1988, 27, 1547–1550. [Google Scholar] [CrossRef]
- Gong, H.-Y.; Rambo, B.M.; Lynch, V.M.; Keller, K.M.; Sessler, J.L. “Texas-Sized” Molecular Boxes: Building Blocks for the Construction of Anion-Induced Supramolecular Species via Self-Assembly. J. Am. Chem. Soc. 2013, 135, 6330–6337. [Google Scholar] [CrossRef]
- Chi, X.; Cen, W.; Queenan, J.A.; Long, L.; Lynch, V.M.; Khashab, N.M.; Sessler, J.L. Azobenzene-Bridged Expanded “Texas-sized” Box: A Dual-Responsive Receptor for Aryl Dianion Encapsulation. J. Am. Chem. Soc. 2019, 141, 6468–6472. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jie, K.; Huang, F. Supramolecular Amphiphiles Based on Host–Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xue, C.; Ma, X.; Gao, M.; Tian, H.; Li, Q. Light-Driven Linear Helical Supramolecular Polymer Formed by Molecular-Recognition-Directed Self-Assembly of Bis(p-sulfonatocalix[4]arene) and Pseudorotaxane. J. Am. Chem. Soc. 2013, 135, 5990–5993. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.-J.; Shen, M.-J.; Xu, L.-J.; Gong, H.-Y. The complexation between ‘Texas sized’ molecular box and linear n-aliphate dianion: En route to supramolecular organic frameworks (SOFs) for selectively CO2 absorption. Tetrahedron 2016, 72, 431–435. [Google Scholar] [CrossRef]
- Tang, F.; Cao, R.; Gong, H.-Y. Aromatic plane effect study in pseudorotaxane construction between ‘Texas-sized’molecular box and carboxylate anions. Tetrahedron Lett. 2015, 56, 820–823. [Google Scholar] [CrossRef]
- Rambo, B.M.; Gong, H.-Y.; Oh, M.; Sessler, J.L. The “Texas-Sized” Molecular Box: A Versatile Building Block for the Construction of Anion-Directed Mechanically Interlocked Structures. Acc. Chem. Res. 2012, 45, 1390–1401. [Google Scholar] [CrossRef]
- Yang, Y.-D.; Sessler, J.L.; Gong, H.-Y. Flexible imidazolium macrocycles: Building blocks for anion-induced self-assembly. Chem. Commun. 2017, 53, 9684–9696. [Google Scholar] [CrossRef]
- Gong, H.-Y.; Rambo, B.M.; Karnas, E.; Lynch, V.M.; Keller, K.M.; Sessler, J.L. Environmentally Responsive Threading, Dethreading, and Fixation of Anion-Induced Pseudorotaxanes. J. Am. Chem. Soc. 2011, 133, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.-Y.; Rambo, B.M.; Cho, W.; Lynch, V.M.; Oh, M.; Sessler, J.L. Anion-directed assembly of a three-dimensional metal–organic rotaxane framework. Chem. Commun. 2011, 47, 5973–5975. [Google Scholar] [CrossRef]
- Gong, H.-Y.; Rambo, B.M.; Nelson, C.A.; Cho, W.; Lynch, V.M.; Zhu, X.; Oh, M.; Sessler, J.L. Multi component self-assembly: Supramolecular organic frameworks containing metal–rotaxane subunits (RSOFs). Dalton Trans. 2012, 41, 1134–1137. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-L.; Shen, Y.-J.; Gao, C.; Yang, J.; Sun, X.; Zhang, X.; Yang, Y.-D.; Wei, G.-P.; Xiang, J.-F.; Sessler, J.L.; et al. Regulating the Structures of Self-Assembled Mechanically Interlocked Moleculecular Constructs via Dianion Precursor Substituent Effects. J. Am. Chem. Soc. 2020, 142, 7443–7455. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wu, R.T.; Long, L.; Guo, C.; Khashab, N.M.; Huang, F.; Sessler, J.L. Physical Removal of Anions from Aqueous Media by Means of a Macrocycle-Containing Polymeric Network. J. Am. Chem. Soc. 2018, 140, 2777–2780. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wu, R.T.; Long, L.; Ke, X.S.; Guo, C.; Ghang, Y.J.; Lynch, V.M.; Huang, F.; Sessler, J.L. Encoding, Reading, and Transforming Information Using Multifluorescent Supramolecular Polymeric Hydrogels. Adv. Mater. 2018, 30, 1705480. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, H.; Li, Y.; Xia, D.; Li, H.; Tang, G.; Sessler, J.L.; Huang, F. Controlling amphiphilic copolymer self-assembly morphologies based on macrocycle/anion recognition and nucleotide-induced payload release. Chem. Sci. 2016, 7, 6006–6014. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Guest | Binding Site | H:G Binding Ratio | Equilibrium | Association Constant |
|---|---|---|---|---|
6 | Outside Cavity | 2:3 | [H] + [G] [H–G] 2[H–G] + [G] [H2–G3] | K1 = (8.3 ± 0.4) × 103 M−1 K2 = (2.3 ± 0.1) ×106 M−2 |
7 | Threaded through Cavity | 1:2 | [H] + [G] [H–G] [H–G] + [G] [H–G2] | K1 = (1.0 ± 0.1) × 104 M−1 K2 = (3.5 ± 0.2) × 103 M−1 |
8 | Threaded through Cavity | 1:2 | [H] + [G] [H–G] [H–G] + [G] [H–G2] | K1 = (8.3 ± 0.4) × 103 M−1 K2 = (2.3 ± 0.2) × 103 M−1 |
9 | Threaded through Cavity | 2:3 | [H] + [G] [H–G] 2[H–G] + [G] [H2–G3] | K1 = (9.1 ± 0.5) × 103 M−1 K2 = (8.1 ± 0.4) × 105 M−2 |
10 | Threaded through Cavity | 1:2 | [H] + [G] [H–G] [H–G] + [G] [H–G2] | K1 = (6.1 ± 0.3) × 102 M−1 K2 = (7.2 ± 0.4) × 102 M−1 |
11 | Threaded through Cavity | 2:3 | [H] + [G] [H–G] 2[H–G] + [G] [H2–G3] | K1 = (2.1 ± 0.5) × 103 M−1 K2 = (2.4 ± 0.2) × 105 M−2 |
12 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (3.3 ± 0.1) × 103 M−1 |
13 | Threaded through Cavity | 1:1 | [H] + [G] [H–G] | Ka= (2.1 ± 0.1) × 103 M−1 |
14 | Threaded through Cavity | 1:1 | [H] + [G] [H–G] | Ka= (3.5 ± 0.2) × 103 M−1 |
15 | Threaded through Cavity | 2:3 | [H] + [G] [H–G] 2[H–G] + [G] [H2–G3] | K1 = (1.5 ± 0.1) × 103 M−1 K2 = (1.8 ± 0.2) × 107 M−2 |
16 | Threaded through Cavity | 2:3 | [H] + [G] [H–G] 2[H–G] + [G] [H2–G3] | K1 = (1.8 ± 0.2) × 103 M−1 K2 = (1.1 ± 0.1) × 107 M−2 |
17 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (1.7 ± 0.1) × 103 M−1 |
18 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (3.6 ± 0.2) × 103 M−1 |
19 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (4.8 ± 0.2) × 103 M−1 |
20 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (7.1 ± 0.4) × 103 M−1 |
21 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (1.0 ± 0.1) × 104 M−1 |
22 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (2.6 ± 0.1) × 104 M−1 |
23 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= 4.9 ± 0.2) × 103 M−1 |
24 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (4.6 ± 0.2) × 103 M−1 |
25 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (6.9 ± 0.4) × 103 M−1 |
26  | Outside Cavity | 1:2 | [H] + [G] [H–G] [H–G] + [G] [H–G2] | K1 = (3.4 ± 0.3) × 103 M−1 K2 = (2.5 ± 0.2) × 103 M−1 |
27 | Outside Cavity | 1:1 | [H] + [G] [H–G] | Ka= (3.4 ± 0.1) × 103 M−1 |
28 | Outside Cavity | 2:3 | [H] + [G] [H–G] 2[H–G] + [G] [H2–G3] | K1 = (6.5 ± 0.1) × 103 M−1 K2 = (8.5 ± 0.2) × 103 M−2 |
29 | Sandwich | 2:3 | [H] + [G] [H–G] [H–G] + [G] [H–G2] [H–G] + [H–G2] [H2–G3] | K1 = (4.3 ± 0.1) × 103 M−1 K2 = (2.9 ± 0.2) × 103 M−2 K3 = (2.5 ± 0.2) × 103 M−2 |
30 | Sandwich | 2:3 | [H] + [G] [H–G] [H–G] + [G] [H–G2] [H–G] + [H–G2] [H2–G3] | K1 = (5.1 ± 0.2) × 103 M−1 K2 = (3.2 ± 0.3) × 103 M−2 K3 = (3.2 ± 0.2) × 103 M−2 |
31 | Sandwich | 2:3 | [H] + [G] [H–G] [H–G] + [G] [H · G2] [H–G] + [H–G2] [H2–G3] | K1 = (4.3 ± 0.1) × 103 M−1 K2 = (3.0 ± 0.3) × 103 M−2 K3 = (3.6 ± 0.1) × 103 M−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, X.; Tian, J.; Luo, D.; Gong, H.-Y.; Huang, F.; Sessler, J.L. “Texas-Sized” Molecular Boxes: From Chemistry to Applications. Molecules 2021, 26, 2426. https://doi.org/10.3390/molecules26092426
Chi X, Tian J, Luo D, Gong H-Y, Huang F, Sessler JL. “Texas-Sized” Molecular Boxes: From Chemistry to Applications. Molecules. 2021; 26(9):2426. https://doi.org/10.3390/molecules26092426
Chicago/Turabian StyleChi, Xiaodong, Jinya Tian, Dan Luo, Han-Yuan Gong, Feihe Huang, and Jonathan L. Sessler. 2021. "“Texas-Sized” Molecular Boxes: From Chemistry to Applications" Molecules 26, no. 9: 2426. https://doi.org/10.3390/molecules26092426
APA StyleChi, X., Tian, J., Luo, D., Gong, H.-Y., Huang, F., & Sessler, J. L. (2021). “Texas-Sized” Molecular Boxes: From Chemistry to Applications. Molecules, 26(9), 2426. https://doi.org/10.3390/molecules26092426