Protective Effect of Cudrania tricuspidata Extract against High-Fat Diet Induced Nonalcoholic Fatty Liver Disease through Nrf-2/HO-1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cudrania tricuspidata (CT) Extract Preparation
2.2. Chemicals and Reagents
2.3. Experimental Animals
2.4. Induction of Fatty Liver and Treatment with CT Extract
2.5. Cell Culture
2.6. MTT Cell Viability Assay
2.7. Metabolic Parameters of Blood and Liver Extract
2.8. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.9. Histology and Oil-Red O Staining
2.10. Estimation of Thiobarbituric Acid Reactive Substances (TBARS) Level for Lipid Peroxidation
2.11. qRT-PCR and Analysis of mRNA Expression
2.12. Western Blot Analysis
2.13. Fluorescence Microscopy for Analysis of ROS
2.14. Statistical Analysis
3. Results
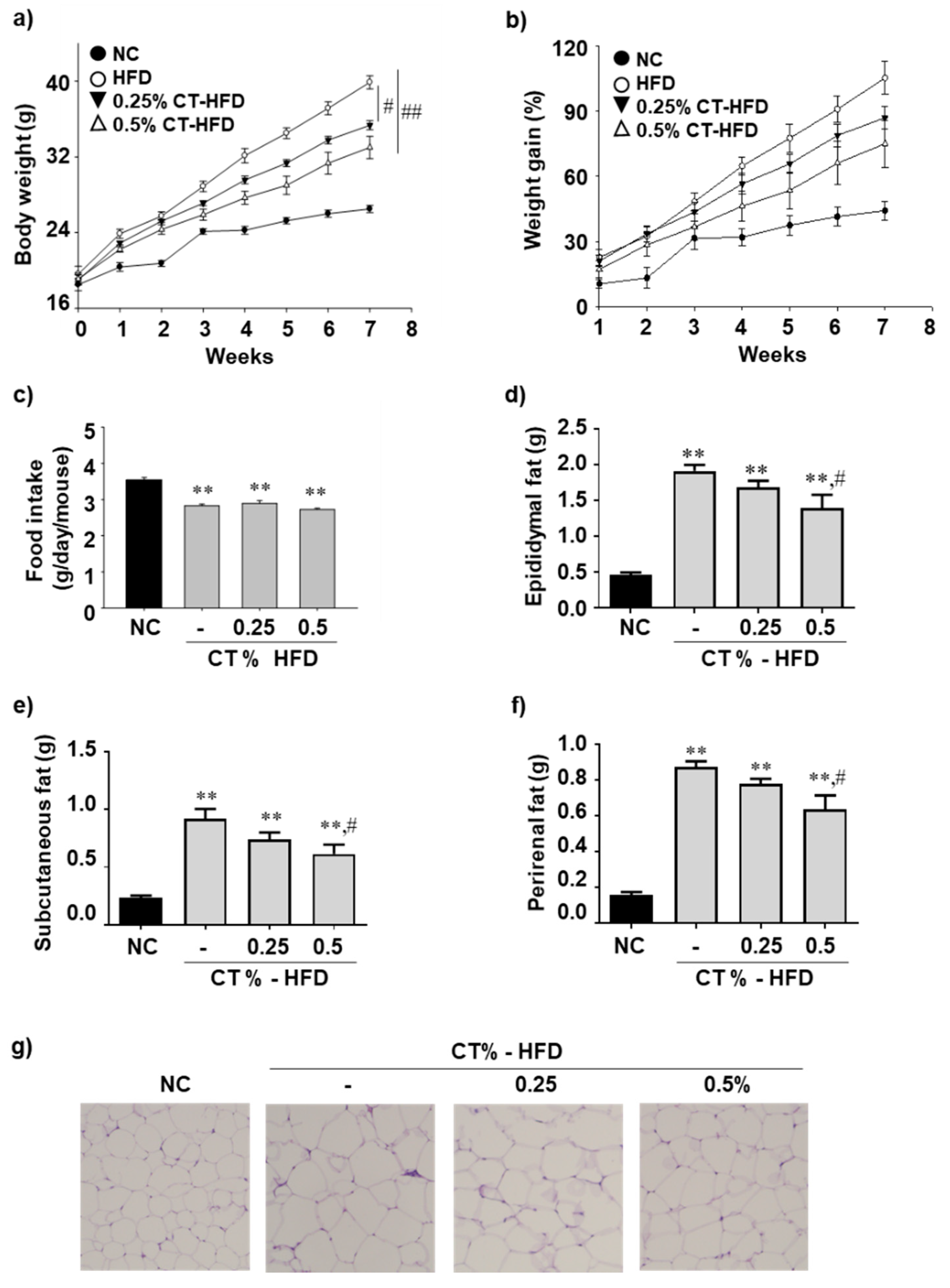
3.1. CT Treatment Reduces the Adiposity and Restores Plasma TG in HFD-Fed Mice
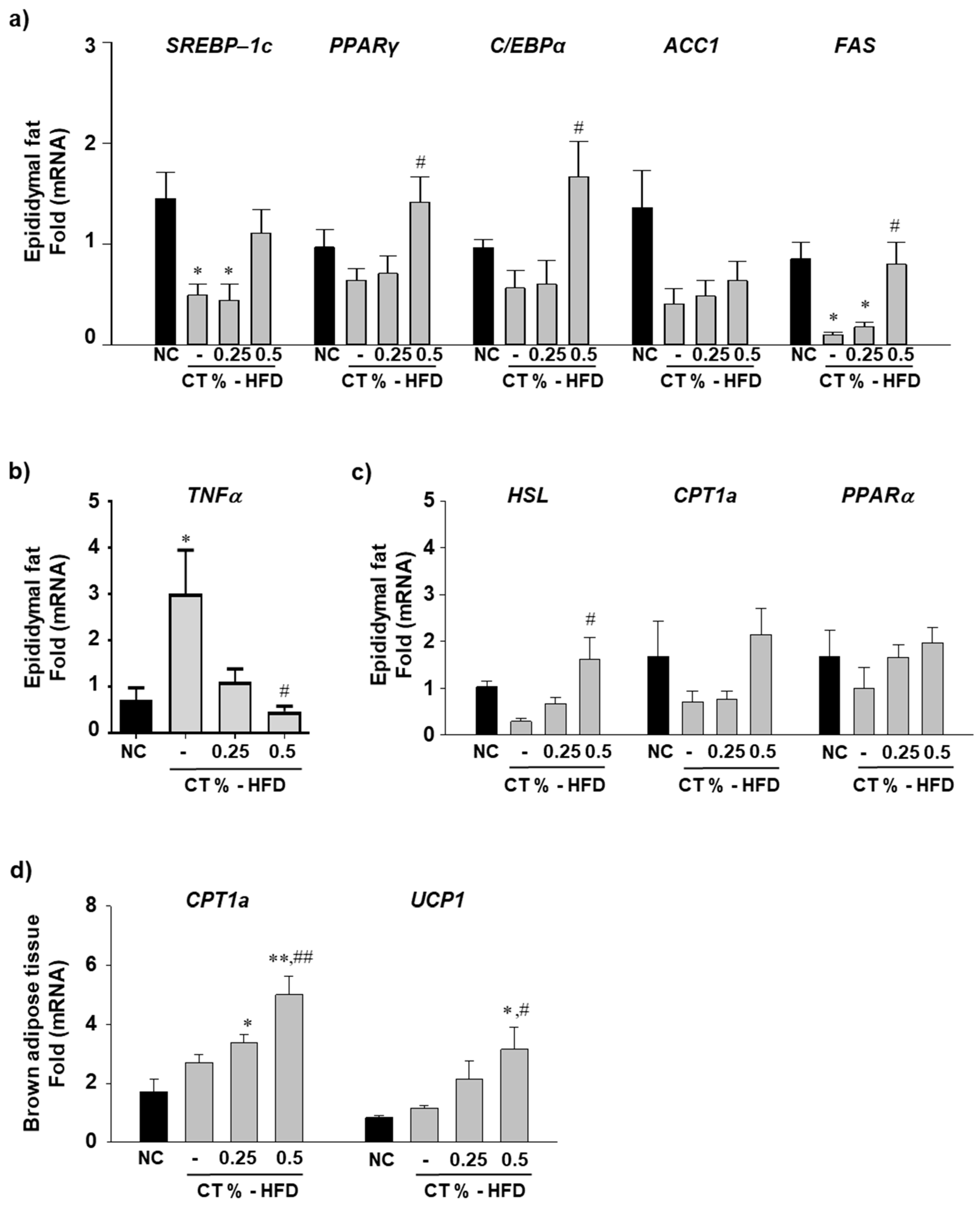
3.2. CT Treatment Increases the mRNA Expression Level of Epididymal Lipogenic and Beta-Oxidation Genes and Decreases Inflammatory Gene Expression
3.3. Blood Glucose Level and Glucose Tolerance Improved by CT Supplementation
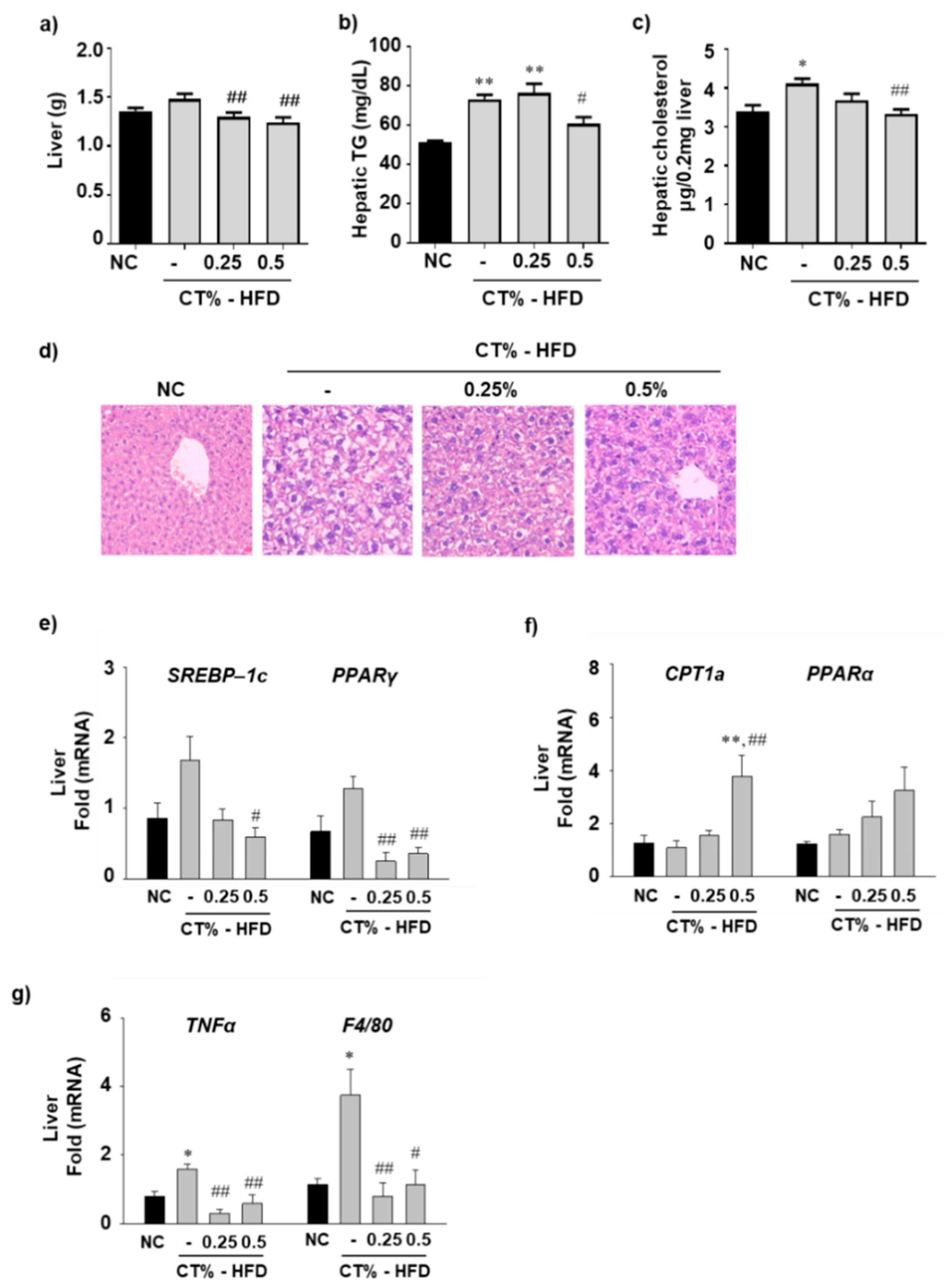
3.4. Effect of CT Extract on Hepatic Fat Accumulation, Lipogenesis, Beta-Oxidation and Inflammation in HFD Fed Mice
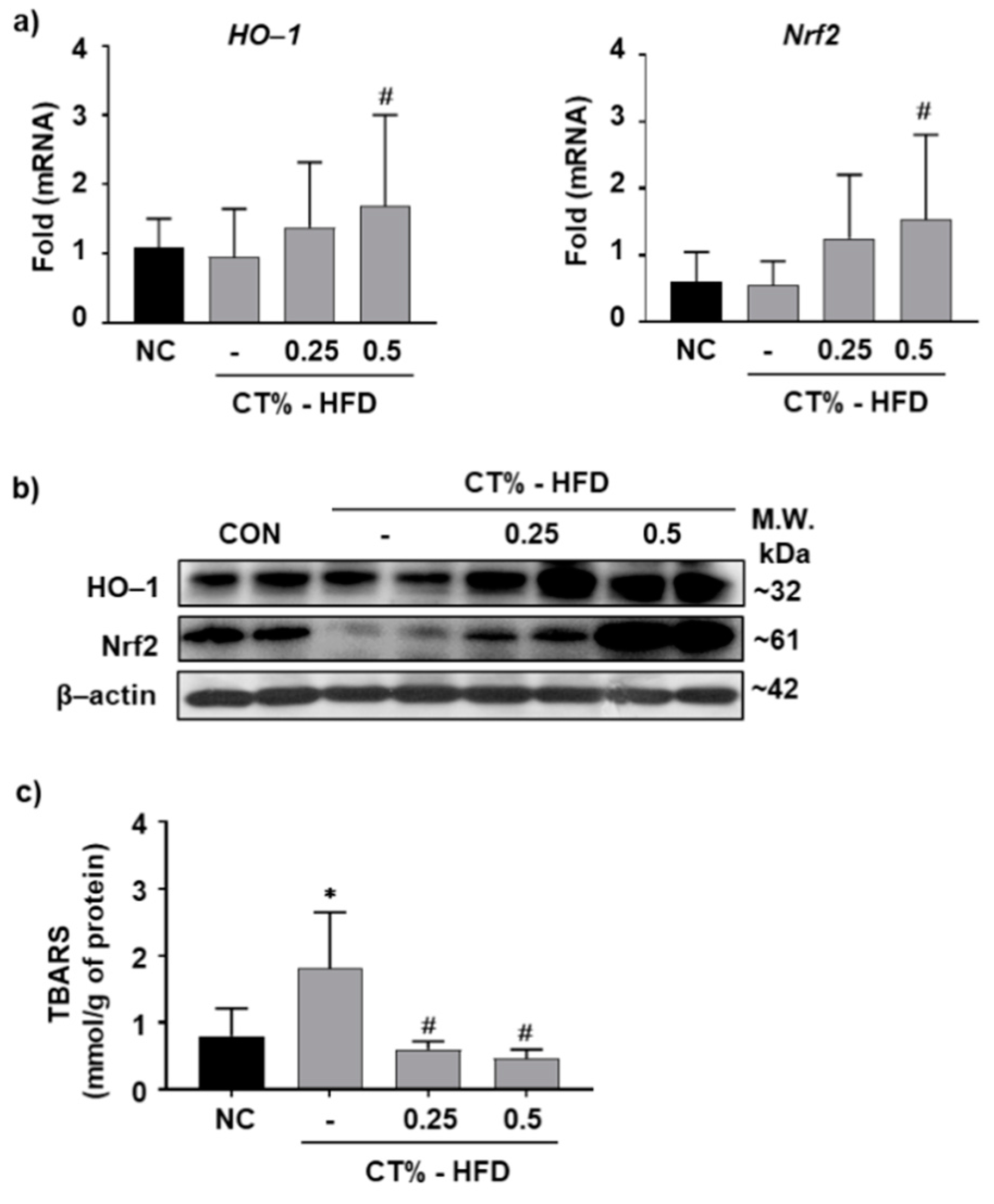
3.5. Antioxidant Role of CT Extract in Protecting Hepatocytes from HFD-Induced Hypertrophy through Nrf2/HO-1 Mediated Pathway
3.6. CT Extract Attenuates In Vitro Lipid Accumulation in Mice Hepatocyte AML-12 Cells
3.7. CT Extract Attenuates Reactive Oxygen Species in AML-12 Cells by Inducing Nrf2 and HO-1 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. 1), 81–84. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Mishra, A.; Younossi, Z.M. Epidemiology and Natural History of Non-alcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2012, 2, 135–144. [Google Scholar] [CrossRef]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Brunetti, L.; Leone, S.; Orlando, G.; Recinella, L.; Ferrante, C.; Chiavaroli, A.; Di Nisio, C.; Di Michele, P.; Vacca, M. Effects of obestatin on feeding and body weight after standard or cafeteria diet in the rat. Peptides 2009, 30, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.; Anstee, Q.M.; Day, C.P. Nonalcoholic fatty liver disease: New treatments. Curr. Opin. Gastroenterol. 2015, 31, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Kim, D.-C.; Quang, T.H.; Oh, H.; Kim, Y.-C. Steppogenin Isolated from Cudrania tricuspidata Shows Antineuroinflammatory Effects via NF-κB and MAPK Pathways in LPS-Stimulated BV2 and Primary Rat Microglial Cells. Molecules 2017, 22, 2130. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Jang, H.W.; Shibamoto, T. Antioxidant activities of extracts from teas prepared from medicinal plants, Morus alba L., Camellia sinensis L., and Cudrania tricuspidata, and their volatile components. J. Agric. Food Chem. 2012, 60, 9097–9105. [Google Scholar] [CrossRef]
- Xin, L.-T.; Yue, S.-J.; Fan, Y.-C.; Wu, J.-S.; Yan, D.; Guan, H.-S.; Wang, C.-Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017, 7, 31807–31832. [Google Scholar] [CrossRef]
- Jeon, S.M.; Lee, D.S.; Jeong, G.S. Cudraticusxanthone A isolated from the roots of Cudrania tricuspidata inhibits metastasis and induces apoptosis in breast cancer cells. J. Ethnopharmacol. 2016, 194, 57–62. [Google Scholar] [CrossRef]
- Kwon, S.B.; Kim, M.J.; Yang, J.M.; Lee, H.P.; Hong, J.T.; Jeong, H.S.; Kim, E.S.; Yoon, D.Y. Cudrania tricuspidata Stem Extract Induces Apoptosis via the Extrinsic Pathway in SiHa Cervical Cancer Cells. PLoS ONE 2016, 11, e0150235. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Kwon, J.; Hiep, N.T.; Kim, D.W.; Hwang, B.Y.; Lee, H.J.; Mar, W.; Lee, D. Neuroprotective Xanthones from the Root Bark of Cudrania tricuspidata. J. Nat. Prod. 2014, 77, 1893–1901. [Google Scholar] [CrossRef]
- Kim, T.-J.; Han, H.-J.; Hong, S.-S.; Hwang, J.-H.; Hwang, B.-Y.; Yoo, H.-S.; Jin, Y.-R.; Lee, J.-J.; Yu, J.-Y.; Lee, K.-H. Cudratricusxanthone A isolated from the root bark of Cudrania tricuspidata inhibits the proliferation of vascular smooth muscle cells through the suppression of PDGF-receptor beta tyrosine kinase. Biol. Pharm. Bull. 2007, 30, 805–809. [Google Scholar] [CrossRef]
- Kim, O.K.; Nam, D.E.; Jun, W.; Lee, J. Cudrania tricuspidata water extract improved obesity-induced hepatic insulin resistance in db/db mice by suppressing ER stress and inflammation. Food Nutr. Res. 2015, 59, 29165. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Kiyosawa, T.; Nakamura, S.; Osada, K. Effects of isoflavone supplementation on disturbances in lipid metabolism and antioxidant system due to exogenous cholesterol oxidation products in rats. J. Funct. Foods 2014, 7, 212–218. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.; Chung, Y.W.; Kim, B.M.; Kim, H.; Kim, K.; Yang, K.M. Antiobesity and Antidiabetes Effects of a Cudrania tricuspidata Hydrophilic Extract Presenting PTP1B Inhibitory Potential. BioMed Res. Int. 2016, 2016, 8432759. [Google Scholar] [PubMed]
- Jo, Y.H.; Choi, K.M.; Liu, Q.; Kim, S.B.; Ji, H.J.; Kim, M.; Shin, S.K.; Do, S.G.; Shin, E.; Jung, G.; et al. Anti-Obesity Effect of 6,8-Diprenylgenistein, an Isoflavonoid of Cudrania tricuspidata Fruits in High-Fat Diet-Induced Obese Mice. Nutrients 2015, 7, 10480–10490. [Google Scholar] [CrossRef]
- Tian, Y.-H.; Kim, H.-C.; Cui, J.-M.; Kim, Y.-C. Hepatoprotective constituents of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ha, H.; Lee, J.K.; Seo, C.S.; Lee, N.H.; Jung, D.Y.; Park, S.J.; Shin, H.K. The fruits of Cudrania tricuspidata suppress development of atopic dermatitis in NC/Nga mice. Phytother. Res. PTR 2012, 26, 594–599. [Google Scholar] [CrossRef]
- Oyeleye, S.I.; Ojo, O.R.; Oboh, G. Moringa oleifera leaf and seed inclusive diets influenced the restoration of biochemicals associated with erectile dysfunction in the penile tissue of STZ-induced diabetic male rats treated with/without Acarbose drug. J. Food Biochem. 2020, 45, e13323. [Google Scholar] [PubMed]
- Lin, X.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, F.; Ai, L. Lactobacillus plantarum AR501 Alleviates the Oxidative Stress of D-Galactose-Induced Aging Mice Liver by Upregulation of Nrf2-Mediated Antioxidant Enzyme Expression. J. Food Sci. 2018, 83, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, J.; Ki, S.H.; Shin, S.M.; Kim, S.W.; Lee, J.-Y.; Jun, H.-S.; Lee, T.; Kim, S.; Baek, D.J.; Park, E.-Y. Synthesis of novel FTY720 analogs with anticancer activity through PP2A activation. Molecules 2018, 23, 2750. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.H.; Sohn, U.D.; Kim, H.C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef]
- Park, E.Y.; Choi, H.; Yoon, J.Y.; Lee, I.Y.; Seo, Y.; Moon, H.S.; Hwang, J.H.; Jun, H.S. Polyphenol-Rich Fraction of Ecklonia cava Improves Nonalcoholic Fatty Liver Disease in High Fat Diet-Fed Mice. Mar. Drugs 2015, 13, 6866–6883. [Google Scholar] [CrossRef]
- Duval, C.; Thissen, U.; Keshtkar, S.; Accart, B.; Stienstra, R.; Boekschoten, M.V.; Roskams, T.; Kersten, S.; Muller, M. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes 2010, 59, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Jia, Y.; Yang, G.; Zhang, X.; Boddu, P.C.; Petersen, B.; Narsingam, S.; Zhu, Y.J.; Thimmapaya, B.; Kanwar, Y.S.; et al. PPARalpha-Deficient ob/ob Obese Mice Become More Obese and Manifest Severe Hepatic Steatosis Due to Decreased Fatty Acid Oxidation. Am. J. Pathol. 2015, 185, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, D.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury-Sayegh, N.; Trak-Smayra, V.; Khazzaka, A.; Esseily, F.; Obeid, O.; Lahoud-Zouein, M.; Younes, H. Characteristics of nonalcoholic fatty liver disease induced in wistar rats following four different diets. Nutr. Res. Pract. 2015, 9, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Larter, C.Z.; Yeh, M.M.; Van Rooyen, D.M.; Brooling, J.; Ghatora, K.; Farrell, G.C. Peroxisome proliferator-activated receptor-alpha agonist, Wy 14,643, improves metabolic indices, steatosis and ballooning in diabetic mice with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2012, 27, 341–350. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Choi, E.M. Kaempferol protects MC3T3-E1 cells through antioxidant effect and regulation of mitochondrial function. Food Chem. Toxicol. 2011, 49, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- M Calderon-Montano, J.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, Y.; Kim, J.; Sohn, E.; Kim, C.S.; Lee, Y.M.; Jo, K.; Shin, S.; Song, Y.; Kim, J.H.; et al. Inhibitory Activities of Cudrania tricuspidata Leaves on Pancreatic Lipase In Vitro and Lipolysis In Vivo. Evid. Based Complement. Alternat. Med. 2012, 2012, 878365. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Villani, R.; Facciorusso, A.; Vendemiale, G.; Serviddio, G. Lipid oxidation products in the pathogenesis of non-alcoholic steatohepatitis. Free Radic. Biol. Med. 2017, 111, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Choi, A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. J. Lab. Clin. Med. 2016, 167, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Demirel, T.; Batirel, S. Palmitic acid induces endoplasmic reticulum stress in AML12 liver cells. Marmara Med. J. 2018, 31, 40–46. [Google Scholar] [CrossRef]
- Kim, K.W.; Quang, T.H.; Ko, W.; Kim, D.C.; Yoon, C.S.; Oh, H.; Kim, Y.C. Anti-neuroinflammatory effects of cudraflavanone A isolated from the chloroform fraction of Cudrania tricuspidata root bark. Pharm. Biol. 2018, 56, 192–200. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Min, S.; Lee, Y.-H.; Hwang, K.; Jun, W. Hepatoprotective effect of 10% ethanolic extract from Curdrania tricuspidata leaves against ethanol-induced oxidative stress through suppression of CYP2E1. Food Chem. Toxicol. 2017, 108, 298–304. [Google Scholar] [CrossRef]


| Treatment | Triglyceride | Cholesterol | AST | ALT |
|---|---|---|---|---|
| NC | 138.2 ± 11 | 135.8 ± 10 | 133.0 ± 6.4 | 38.8 ± 2.50 |
| HFD | 189.3 ± 40 ** | 170.3 ± 12 ** | 146.5 ± 8.3 | 40.3 ± 3.41 |
| 0.25% CT-HFD | 151.9 ± 13 # | 179.5 ± 8 ** | 111.3 ± 15.2 # | 32.9 ± 2.01 |
| 0.5% CT- HFD | 151.2 ± 21 # | 183.1 ± 10 ** | 124.6 ± 10.6 | 31.4 ± 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, J.; Baek, D.-J.; Oh, Y.-S.; Cho, S.-S.; Ki, S.-H.; Park, E.-Y. Protective Effect of Cudrania tricuspidata Extract against High-Fat Diet Induced Nonalcoholic Fatty Liver Disease through Nrf-2/HO-1 Pathway. Molecules 2021, 26, 2434. https://doi.org/10.3390/molecules26092434
Shrestha J, Baek D-J, Oh Y-S, Cho S-S, Ki S-H, Park E-Y. Protective Effect of Cudrania tricuspidata Extract against High-Fat Diet Induced Nonalcoholic Fatty Liver Disease through Nrf-2/HO-1 Pathway. Molecules. 2021; 26(9):2434. https://doi.org/10.3390/molecules26092434
Chicago/Turabian StyleShrestha, Jitendra, Dong-Jae Baek, Yoon-Sin Oh, Sam-Seok Cho, Sung-Hwan Ki, and Eun-Young Park. 2021. "Protective Effect of Cudrania tricuspidata Extract against High-Fat Diet Induced Nonalcoholic Fatty Liver Disease through Nrf-2/HO-1 Pathway" Molecules 26, no. 9: 2434. https://doi.org/10.3390/molecules26092434
APA StyleShrestha, J., Baek, D.-J., Oh, Y.-S., Cho, S.-S., Ki, S.-H., & Park, E.-Y. (2021). Protective Effect of Cudrania tricuspidata Extract against High-Fat Diet Induced Nonalcoholic Fatty Liver Disease through Nrf-2/HO-1 Pathway. Molecules, 26(9), 2434. https://doi.org/10.3390/molecules26092434








