Enhanced Biological Activity of a Novel Preparation of Lavandula angustifolia Essential Oil
Abstract
:1. Introduction
2. Results
2.1. Identification of Lavender Oils
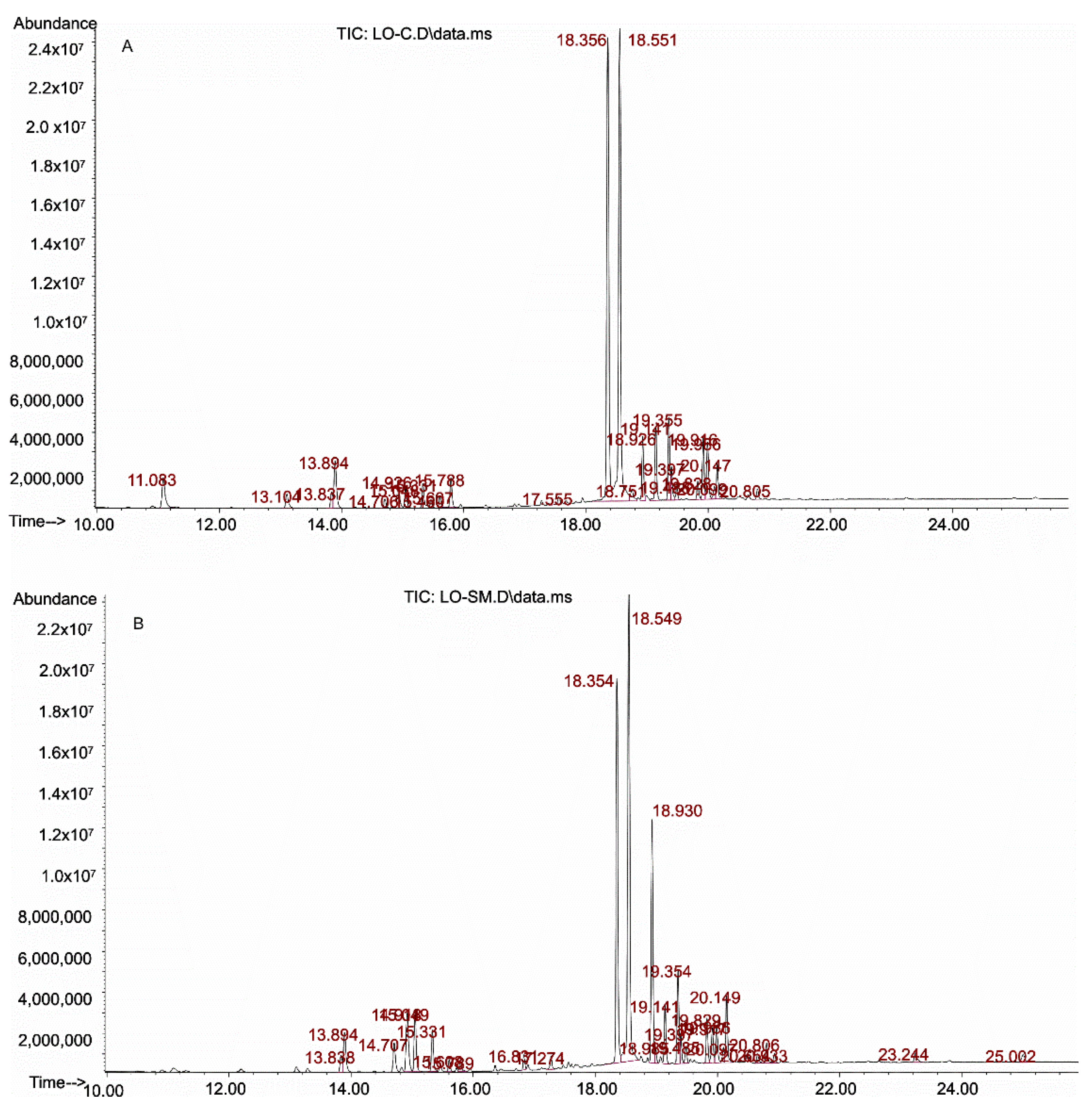
2.1.1. Qualitative Analysis of Essential Oils
2.1.2. Quantitative Analysis
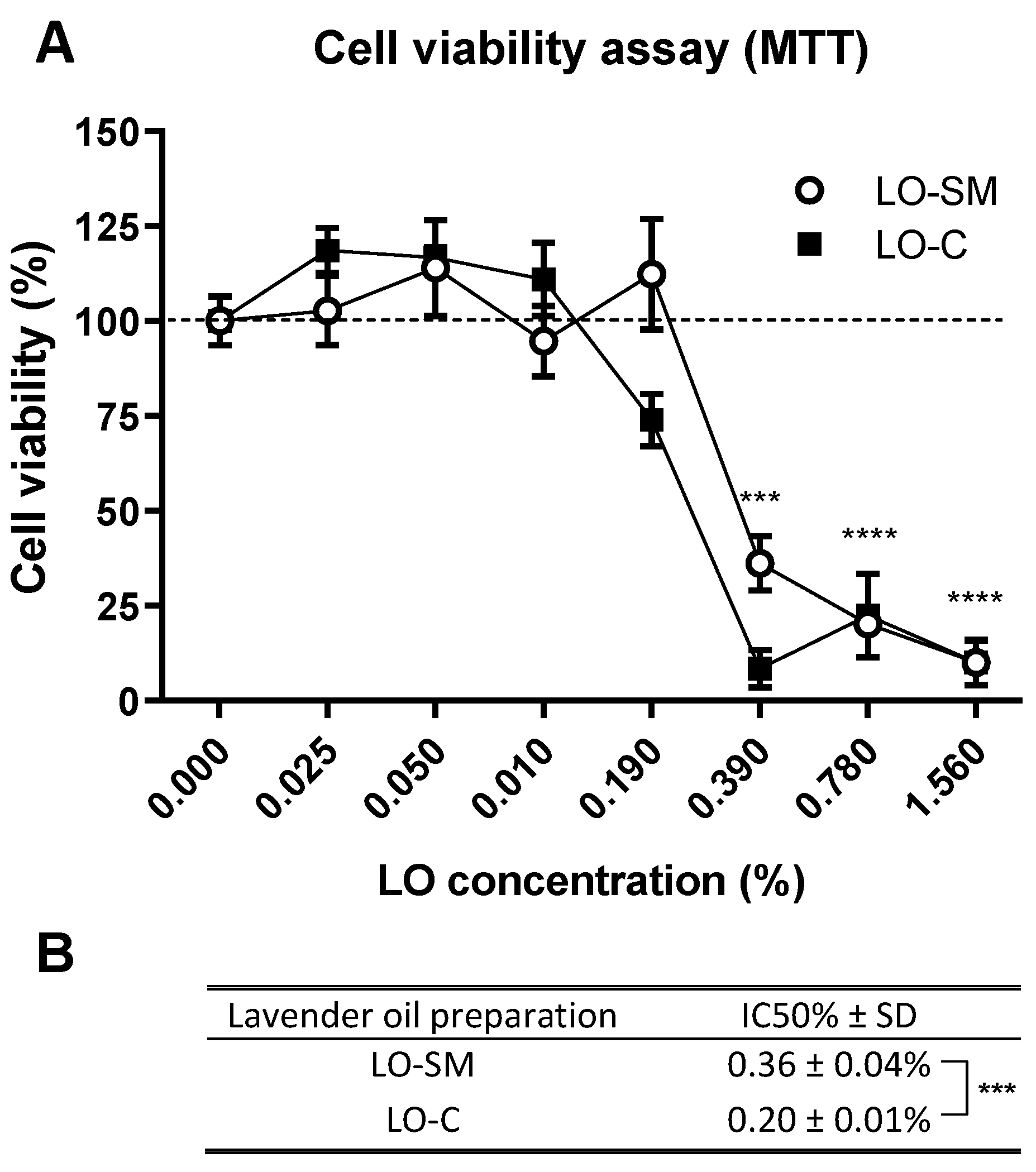
2.2. Lavender Oil Cytotoxicity
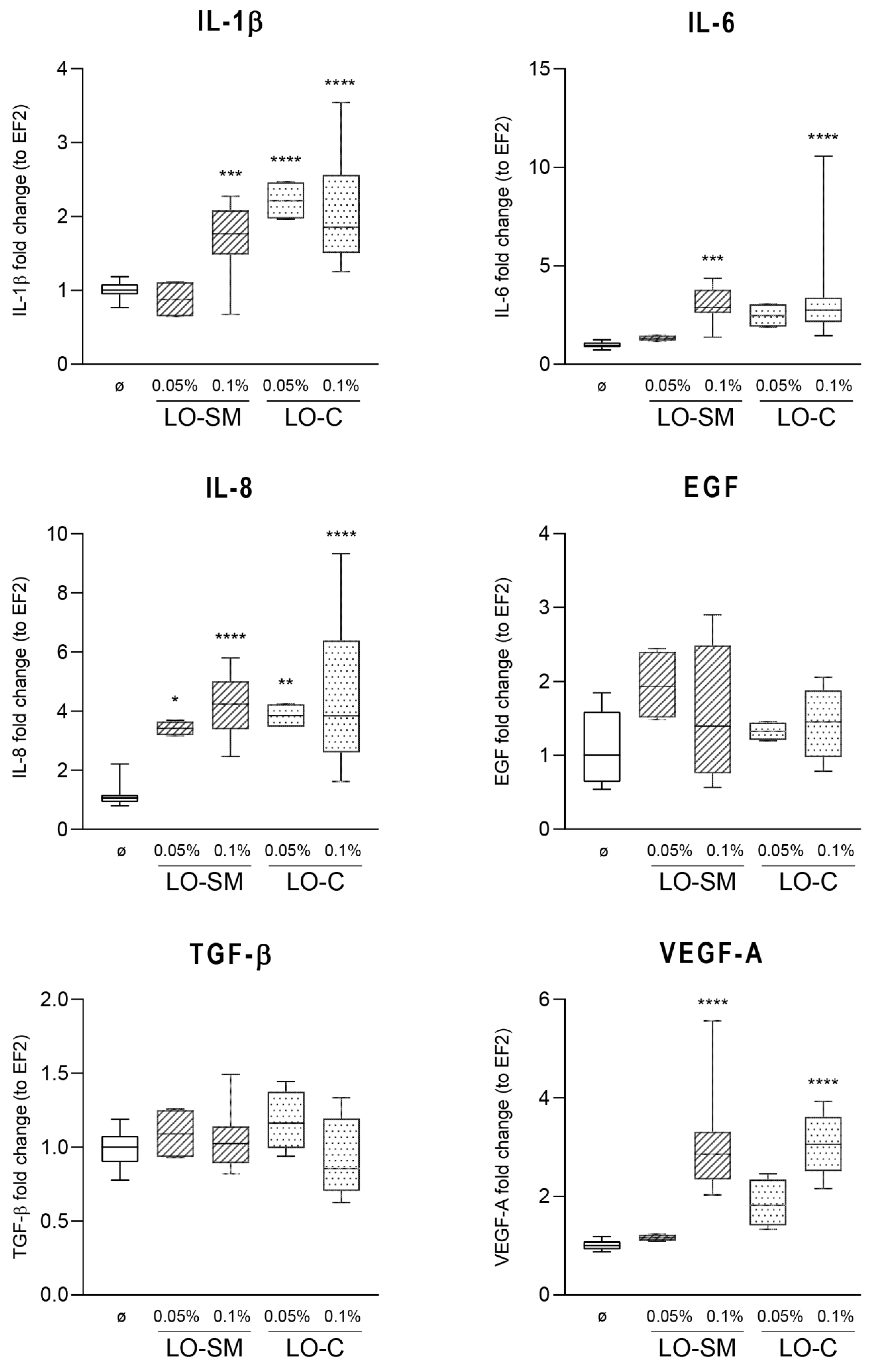
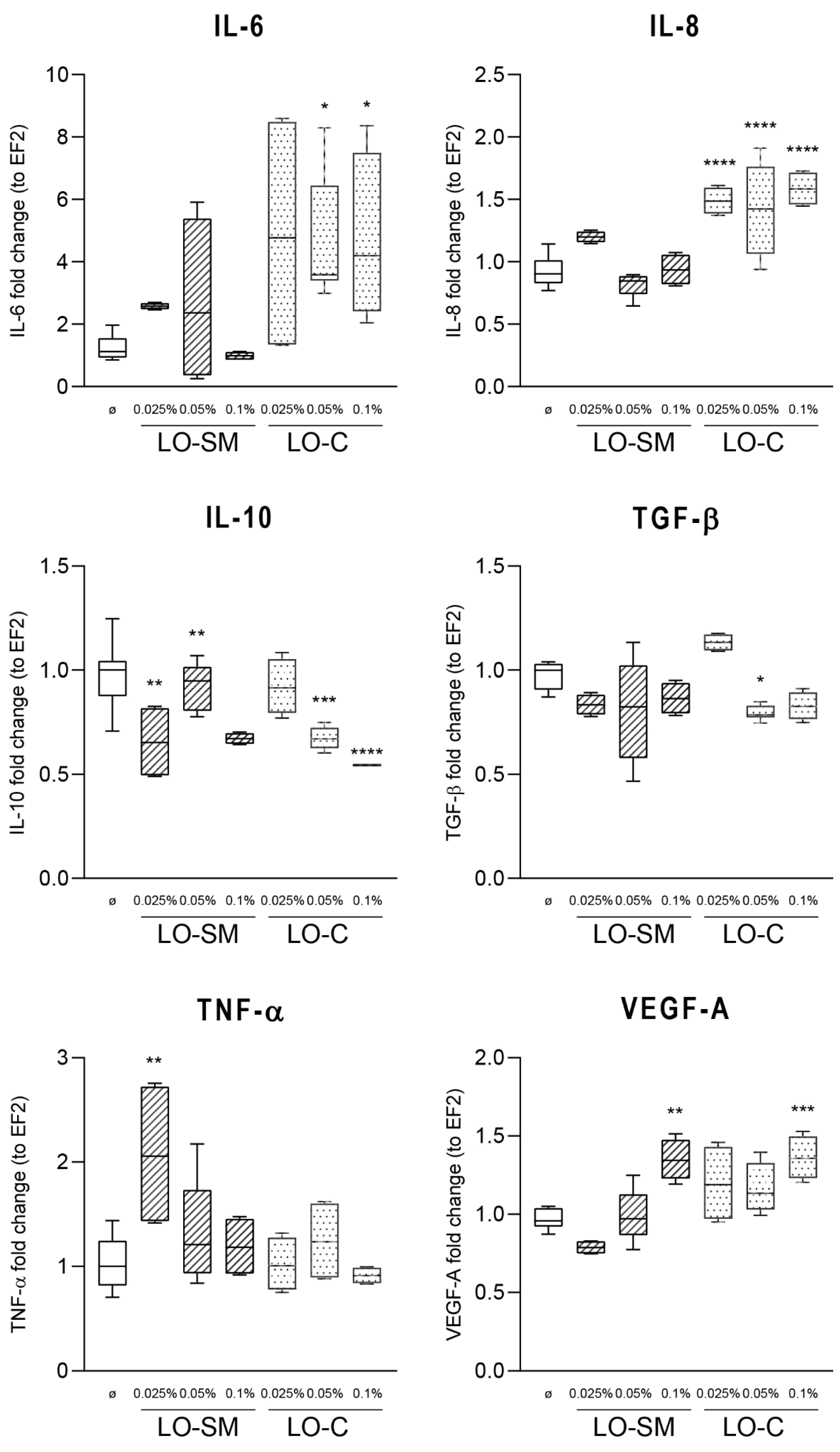
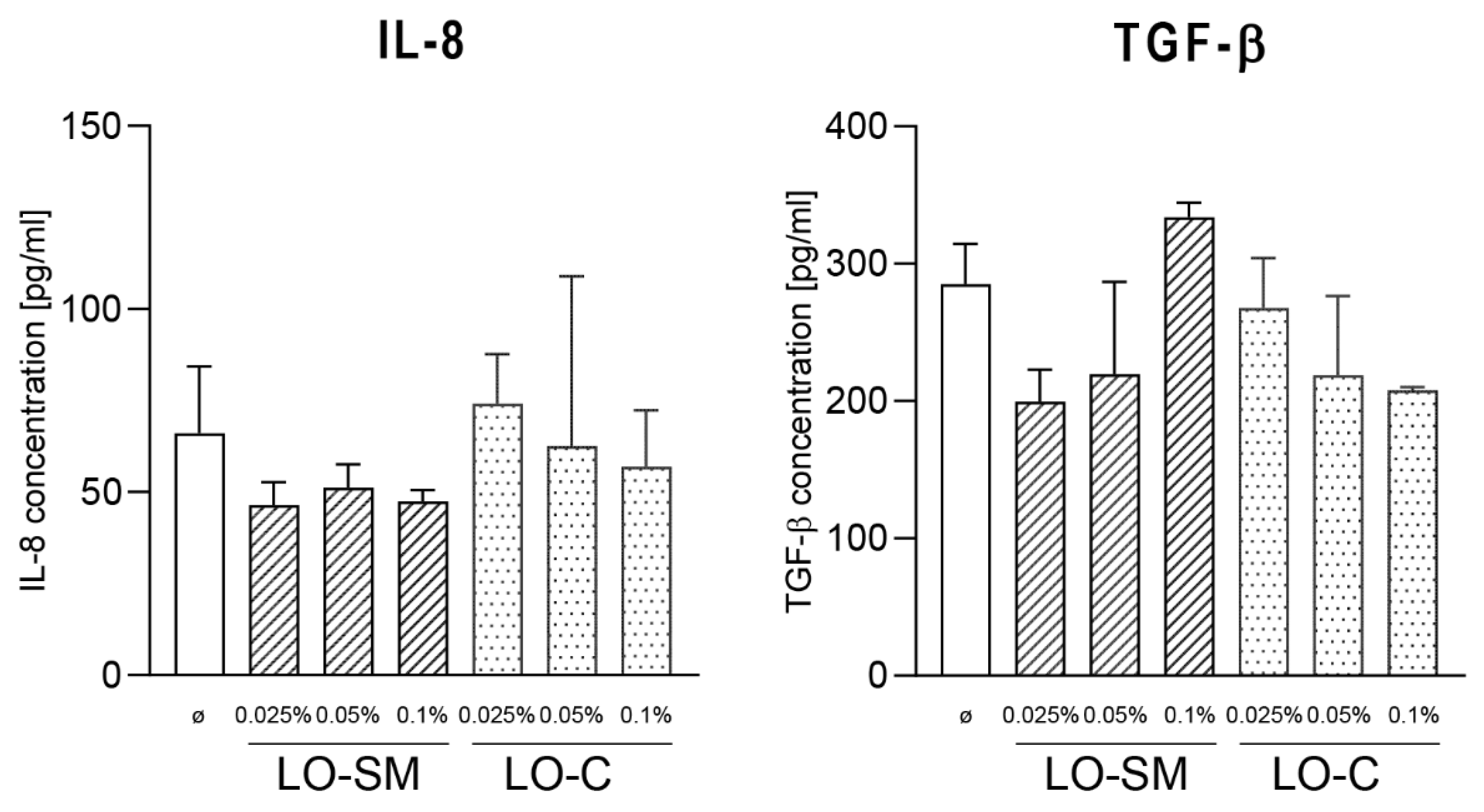
2.3. Effect of Lavender Oil Preparations on the Cytokine Cellular Response
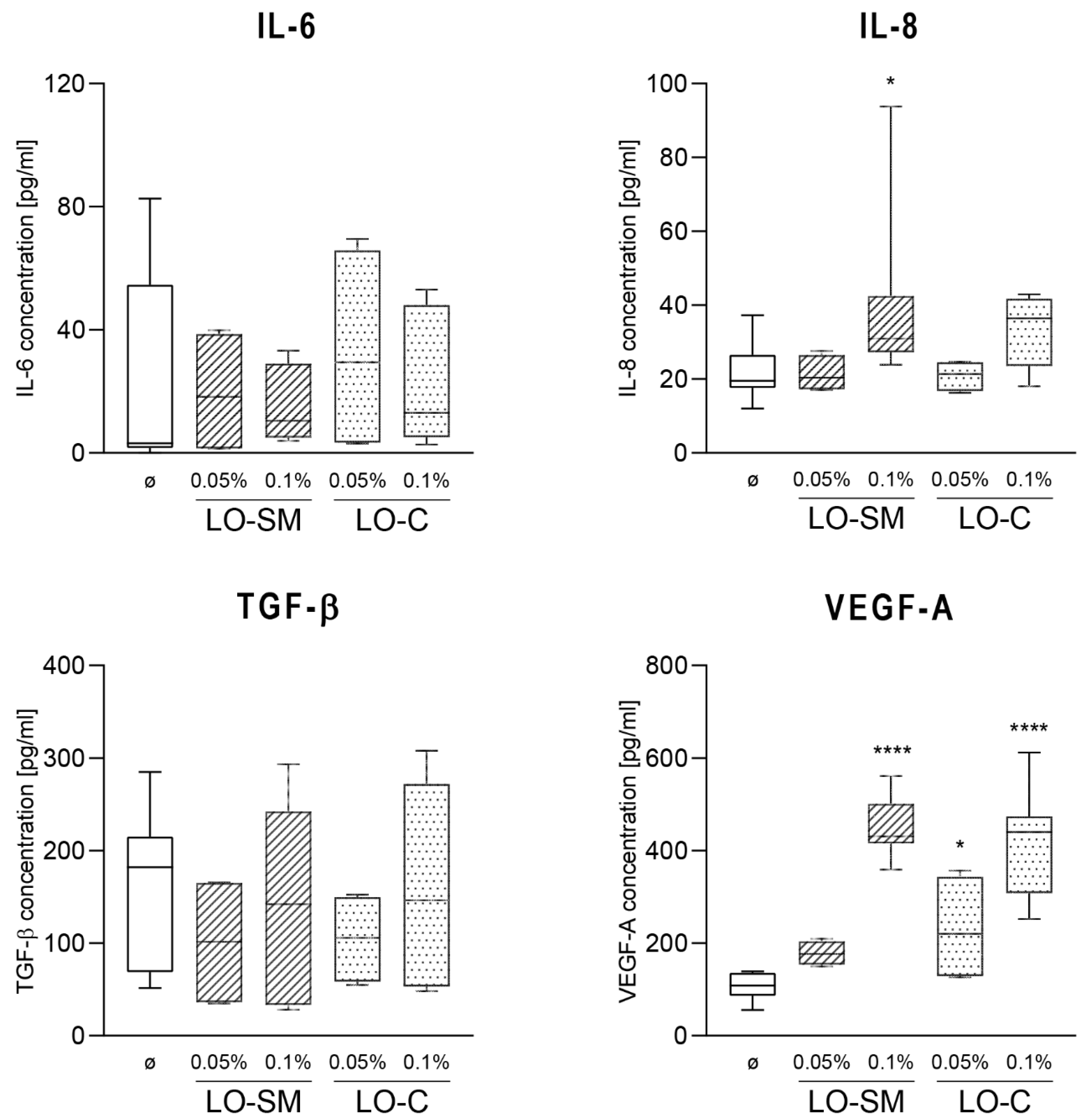
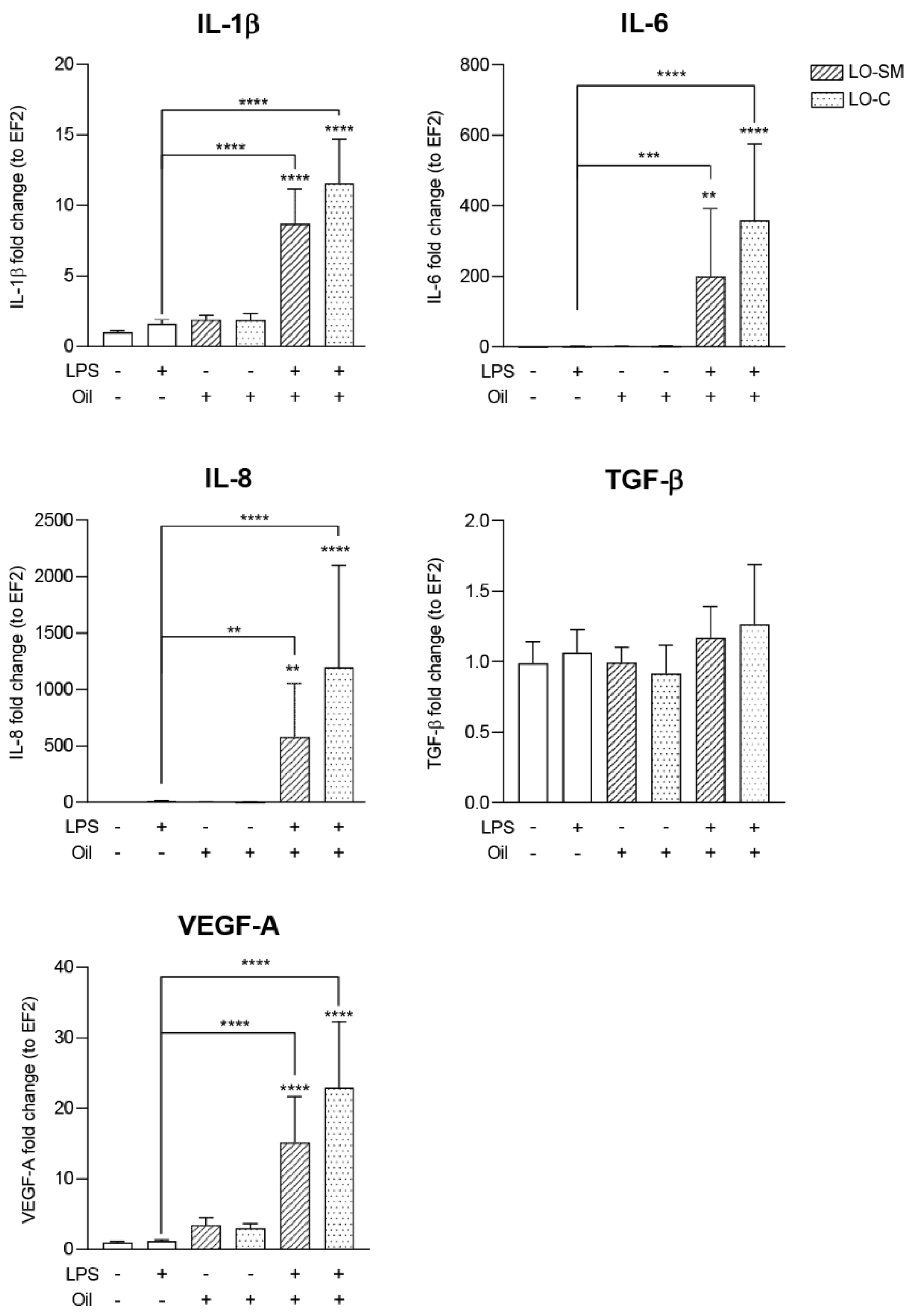
2.4. Effect of the Lavender Oil on LPS Stimulated HaCaT Keratinocytes
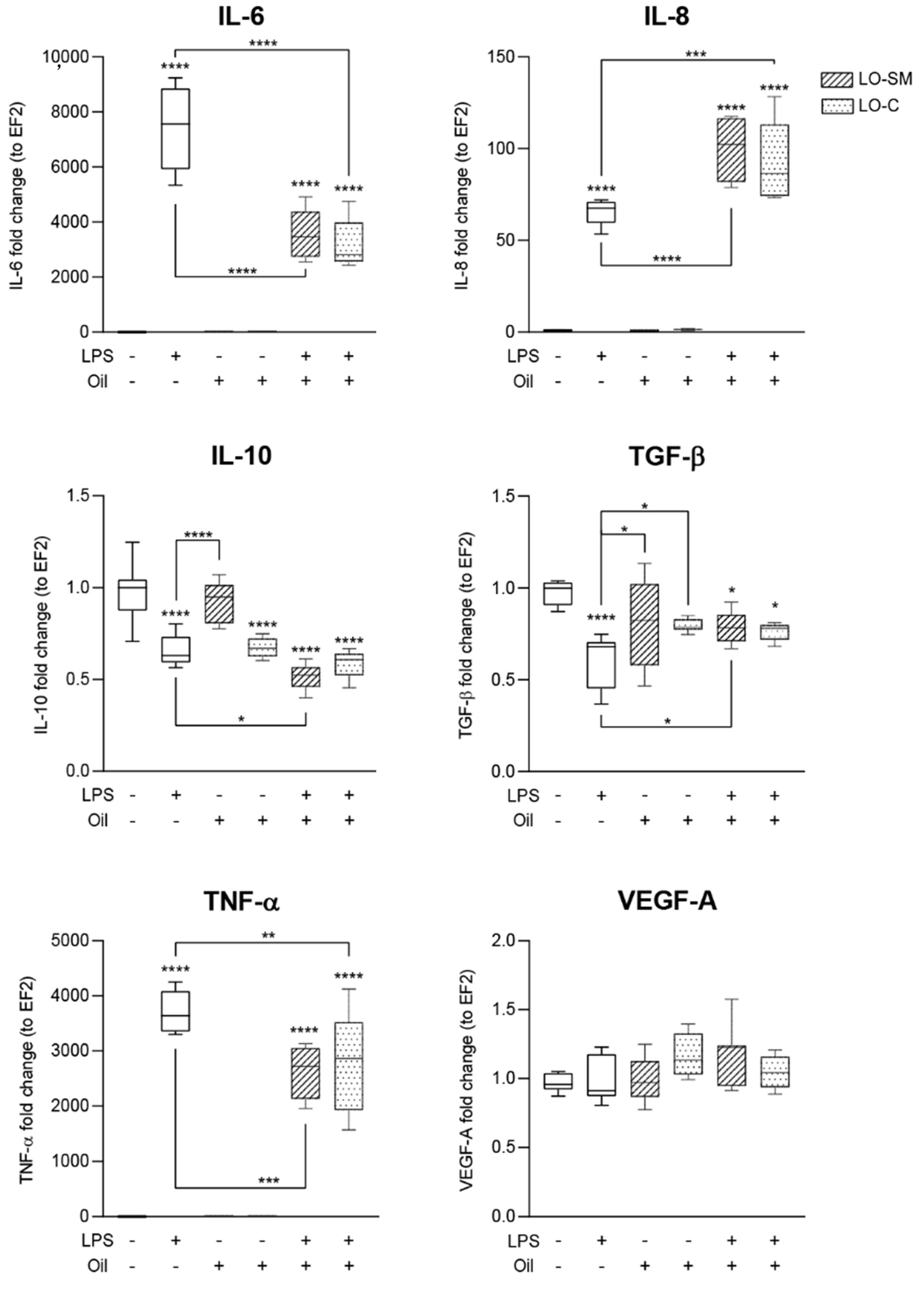
2.5. Effects of Lavender Oil on LPS-Prestimulated hMDMs
3. Discussion
3.1. Effect of Lavender Oil Origin on the Chemical Composition
3.2. The Lavender Oil Origin and the Proinflammatory and Proregenerative Cytokine Cellular Response
4. Materials and Methods
4.1. Plant Material
4.2. Isolation of Lavandula Angustifolia Essential Oil
4.3. GC/MS Analysis
4.3.1. Chemicals and Reagents
4.3.2. Preparation of Standards and Samples
4.3.3. Apparatus and Chromatographic Conditions
4.3.4. Method Validation
4.4. Biological Activity of Lavender Oils
4.4.1. Cell Culture
4.4.2. MTT
4.4.3. Isolation of Monocyte-Derived Macrophages from Human Peripheral Blood
4.4.4. Effect of Lavender Oils on Eukaryotic Cells (HaCaT and hMDM)
4.4.5. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
4.4.6. ELISA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Swarcewicz, M. Chemical composition and biological activity of lavender. Wiadomości. Chem. 2014, 68, 11–12. [Google Scholar]
- Adaszyńska, M.; Swarcewicz, M.; Markowska-Szczupak, A. Comparison of the chemical composition and antimicrobial activity of essential oil obtained from various domestic varieties of narrow-leaved lavender (Lavandula angustifolia L.). Postępy Fitoter. 2013, 2, 90–96. [Google Scholar]
- Kraśniewska, K.; Gniewosz, M.; Kosakowska, O.; Pobiega, K. Assessment of chemical composition and antimicrobial properties of narrow-leaved lavender (Lavandula angustifolia L.) essential oil in a commercially available preparation. Postępy Fitoter. 2017, 18, 113–118. [Google Scholar]
- ISO 3515:2002/Cor 1:2004. Oil of Lavender (Lavandula angustifolia Mill.)—Technical Corrigendum 1; ISO: Geneva, Switzerland, 2004; Available online: https://www.iso.org/standard/39888.html (accessed on 15 March 2021).
- Kucharska, M.; Szymańska, J.A.; Wesołowski, W.; Frydrych, B.; Bruchajze, E. Analysis of volatile ingredients of selected essential oils with a relaxing effect. Med. Pract. 2019, 70, 229–247. [Google Scholar]
- Dezici, S. Promising anticancer activity of lavender (Lavandula angustifolia Mill.) essential oil through induction of both apoptosis and necrosis. Ann. Phytomed. 2018, 7, 38–45. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sabara, D.; Kunicka-Styczyńska, A. Lavender oil—Flavouring or active cosmetic ingredient? Food Chem. Biotechnol. 2009, 73, 33–41. [Google Scholar]
- Kunicka-Styczyńska, A.; Sikora, M.; Kalemba, D. Antimicrobial activity of lavender, tea tree and lemon oils in cosmetic preservative systems. J. Appl. Microbiol. 2009, 107, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Kıvrak, Ş. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crop. Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Khayyat, S. Thermal, photo-oxidation and antimicrobial studies of linalyl acetate as a major ingredient of lavender essential oil. Arab. J. Chem. 2020, 13, 1575–1581. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şerban, E.; Ionescu, M.; Matinca, D.; Maier, C.; Bojiţă, M. Screening of the antibacterial and antifungal activity of eight volatile essential oils. Farmacia 2011, 59, 440–446. [Google Scholar]
- Soković, M.; Griensvbeni, J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Verma, R.; Rahman, L.; Chanotiya, C.; Verma, R.K.; Chauhan, A.; Sing, A.; Yadav, A. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- ISO 8902:2009 Specifies Certain Characteristics of the Essential Oil of Lavandin Grosso [Lavandula angustifolia Mill. × Lavandula latifolia Medik.] French Type, Intended for Facilitating the Assessment of Its Quality. Available online: https://www.iso.org/standard/45332.html (accessed on 15 March 2021).
- Boelens, M.H. Chemical and sensory evaluation of Lavandula oils. Perfum. Flavorist 1995, 20, 23–51. [Google Scholar]
- Cong, Y.; Abulizi, P.; Zhi, L.; Wang, X. Chemical composition of the essential oil from Lavandula angustifolia from Xinjiang, China. Chem. Nat. Comp. 2008, 44, 810–815. [Google Scholar] [CrossRef]
- Śmigielski, K.; Raj, A.; Krosowiak, K.; Gruska, R. Chemical Composition of the Essential Oil of Lavandula angustifolia Cultivated in Poland. J. Essent. Oil Bear. Plants 2013, 12, 338–347. [Google Scholar] [CrossRef]
- Lavandulae Aetheroleum. Polish Pharmacopoeia, XII; COGNO MEDICAL Sp. z o.o.: Warsaw, Poland, 2020. [Google Scholar]
- D’Arpa, P.; Leung, K.P. Toll-Like Receptor Signaling in Burn Wound Healing and Scarring. Adv. Wound Care 2017, 6, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.Z.; Stevenson, A.W.; Prele, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S.; Min, K.; Sehgal, P.; Mizutani, H.; Birchall, N.; Ray, A.; May, L. Production of IL-6 by keratinocytes. Implications for epidermal inflammation and immunity. Ann. N. Y. Acad. Sci. 1989, 557, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Derocq, J.M.; Segui, M.; Poinot-Chazel, C.; Minty, A.; Caput, D.; Ferrara, P.; Casellas, P. Interleukin13 stimulates interleukin-6 production by human keratinocytes. Similarity with interleukin-4. FEBS Lett. 1994, 343, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef]
- Gallucci, R.M.; Sugawara, T.; Yucesoy, B.; Berryann, K.; Simeonova, P.P.; Matheson, J.M.; Luster, M.I. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J. Interferon Cytokine Res. 2001, 21, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 26, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, S.; Fujiwara, H.; Yoneda, M.; Furutani, A.; Todo, M.; Ikai, A.; Tada, H.; Okamura, H.; Umehara, S.; Shiozaki, A.; et al. Overexpression of IL-6 by gene transfer stimulates IL-8-mediated invasiveness of KYSE170 esophageal carcinoma cells. Anticancer Res. 2013, 33, 1483–1489. [Google Scholar]
- Lederle, W.; Depner, S.; Schnur, S.; Obermueller, E.; Catone, N.; Just, A.; Fusenig, N.E.; Mueller, M.M. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int. J. Cancer 2011, 128, 2803–2814. [Google Scholar] [CrossRef]
- Fee, D.; Grzybicki, D.; Dobbs, M.; Ihyer, S.; Clotfelter, J.; Macvilay, S.; Hart, M.N.; Sandor, M.; Fabry, Z. Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine 2000, 12, 655–665. [Google Scholar] [CrossRef]
- Rennekampff, H.O.; Hansbrough, J.F.; Kiessig, V.; Dore, C.; Sticherling, M.; Schroder, J.M. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J. Surg. Res. 2000, 93, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.N.; Vachon, D.J.; Gould, L.J. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009, 17, 832–839. [Google Scholar] [CrossRef]
- Fivenson, D.P.; Faria, D.T.; Nickoloff, B.J.; Poverini, P.J.; Kunkel, S.; Burdick, M.; Strieter, R.M. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen. 1997, 5, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Rossiter, H.; Barresi, C.; Pammer, J.; Rendl, M.; Haigh, J.; Wagner, E.F.; Tschachler, E. Loss of vascular endothelial growth factor a activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res. 2004, 64, 3508–3516. [Google Scholar] [CrossRef] [Green Version]
- Frank, S.; Hubner, G.; Breier, G.; Longaker, M.T.; Greenhalgh, D.G.; Werner, S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 1995, 270, 12607–12613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef] [Green Version]
- Galeano, M.; Deodato, B.; Altavilla, D.; Cucinotta, D.; Arsic, N.; Marini, H.; Torre, V.; Giacca, M.; Squadrito, F. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia 2003, 46, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilgus, T.A.; Matthies, A.M.; Radek, K.A.; Dovi, J.V.; Burns, A.L.; Shankar, R.; DiPietro, L.A. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am. J. Pathol. 2005, 167, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Brem, H.; Kodra, A.; Golinko, M.S.; Entero, H.; Stojadinovic, O.; Wang, V.M.; Sheahan, C.M.; Weinberg, A.D.; Woo, S.L.; Ehrlich, H.P.; et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J. Investig. Dermatol. 2009, 129, 2275–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, B.; Driessens, G.; Goossens, S.; Youssef, K.K.; Kuchnio, A.; Caauwe, A.; Sotiropoulou, P.A.; Loges, S.; Lapouge, G.; Candi, A.; et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011, 478, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, B.M.; Tan, P.K.; Niederleithner, H.; Ferrara, N.; Petzelbauer, P.; Sibilia, M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010, 140, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Riese, A.; Eilert, Y.; Meyer, Y.; Arin, M.; Baron, J.M.; Eming, S.; Krieg, T.; Kurschat, P. Epidermal expression of neuropilin 1 protects murine keratinocytes from UVB-induced apoptosis. PLoS ONE 2012, 7, e50944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.W.; Wu, X.J.; Luo, D.; Lu, Z.F.; Cai, S.Q.; Zheng, M. Activation of VEGFR-2 signaling in response to moderate dose of ultraviolet B promotes survival of normal human keratinocytes. Int. J. Biochem. Cell Biol. 2012, 44, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, R.; Koh, T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef]
- Bryzek, D.; Ciaston, I.; Dobosz, E.; Gasiorek, A.; Makarska, A.; Sarna, M.; Eick, S.; Puklo, M.; Lech, M.; Potempa, B.; et al. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019, 15, e1007773. [Google Scholar] [CrossRef]


| No | Substance | Range (µg/mL) | Linear Regression | r | Precision (%) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|---|
| 1 | Linalool | 487 ÷ 3893 | y = 1321.3x + 876850 | 0.9997 | 1.3 | 1.18 | 3.92 |
| 2 | Linalyl acetate | 513 ÷ 4108 | y = 1789.9x + 830995 | 0.9996 | 0.9 | 0.99 | 3.30 |
| 3 | Lavandulyl acetate | 107 ÷ 1074 | y = 838.52x + 42043 | 0.9997 | 2.6 | 1.54 | 5.12 |
| 4 | (–)-Bornylu acetate | 49 ÷ 487 | y = 3751.4x + 68284 | 0.9995 | 1.8 | 0.69 | 2.30 |
| 5 | (–)-trans-Caryophyllene | 50 ÷ 501 | y = 2597.3x + 8671.5 | 0.9996 | 1.2 | 1.01 | 3.36 |
| 6 | (–)-Geraniol | 49 ÷ 491 | y = 3062x + 8924.9 | 0.9996 | 0.6 | 0.79 | 2.62 |
| No | Substance | Percentage (%) | |
|---|---|---|---|
| LO-C | LO-SM | ||
| 1 | Linalool | 40.2 | 23.2 |
| 2 | Linalyl acetate | 44.0 | 40.6 |
| 3 | Lavandulyl acetate | 5.5 | 23.2 |
| 4 | (–)-trans-Caryophyllene | 1.8 | 2.3 |
| 5 | (–)-Geraniol | 0.6 | 1.1 |
| Gene | Forward (5′ » 3′) | Reverse (5′ » 3′) |
|---|---|---|
| EF2 | GACATCACCAAGGGTGTGCAG | TTCAGCACACTGGCATAGAGGC |
| EGF | AATAGTGACTCTGAATGTCC | GCGCAGTTCCCACCA |
| TGFβ1 | CACCCGCGTGCTAATGG | ATGCTGTGTGTACTCTGCTTGAACT |
| VEGF-A | CGGTGTCTGTCTGTGTGTC | AAGAGGAAAGAGGTAGCAAGAG |
| IL1β | CCACAGACCTTCCAGGAGAATG | GTGCAGTTCAGTGATCGTACAGG |
| IL6 | CAGGAGCCCAGCTATGAACT | GAAGGCAGCAGGCAACAC |
| IL8 | AGACAGCAGAGCACACAAGC | AGGAAGGCTGCCAAGAGAG |
| IL10 | TCCTTGCTGGAGGACTTTAAGGGT | TGTCTGGGTCTTGGTTCTCAGCTT |
| TNFα | GTCAGATCATCTTCTCGAACCCCGA | CAGGGCAATGATCCCAAAGTAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miastkowska, M.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Kucharska, M.; Kilanowicz, A.; Cudzik, D.; Cudzik, K. Enhanced Biological Activity of a Novel Preparation of Lavandula angustifolia Essential Oil. Molecules 2021, 26, 2458. https://doi.org/10.3390/molecules26092458
Miastkowska M, Kantyka T, Bielecka E, Kałucka U, Kamińska M, Kucharska M, Kilanowicz A, Cudzik D, Cudzik K. Enhanced Biological Activity of a Novel Preparation of Lavandula angustifolia Essential Oil. Molecules. 2021; 26(9):2458. https://doi.org/10.3390/molecules26092458
Chicago/Turabian StyleMiastkowska, Małgorzata, Tomasz Kantyka, Ewa Bielecka, Urszula Kałucka, Marta Kamińska, Małgorzata Kucharska, Anna Kilanowicz, Dariusz Cudzik, and Krzysztof Cudzik. 2021. "Enhanced Biological Activity of a Novel Preparation of Lavandula angustifolia Essential Oil" Molecules 26, no. 9: 2458. https://doi.org/10.3390/molecules26092458
APA StyleMiastkowska, M., Kantyka, T., Bielecka, E., Kałucka, U., Kamińska, M., Kucharska, M., Kilanowicz, A., Cudzik, D., & Cudzik, K. (2021). Enhanced Biological Activity of a Novel Preparation of Lavandula angustifolia Essential Oil. Molecules, 26(9), 2458. https://doi.org/10.3390/molecules26092458







