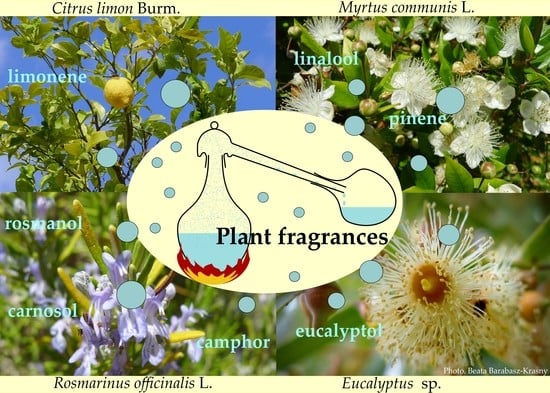
Fleeting Beauty—The World of Plant Fragrances and Their Application
Abstract
:1. Introduction
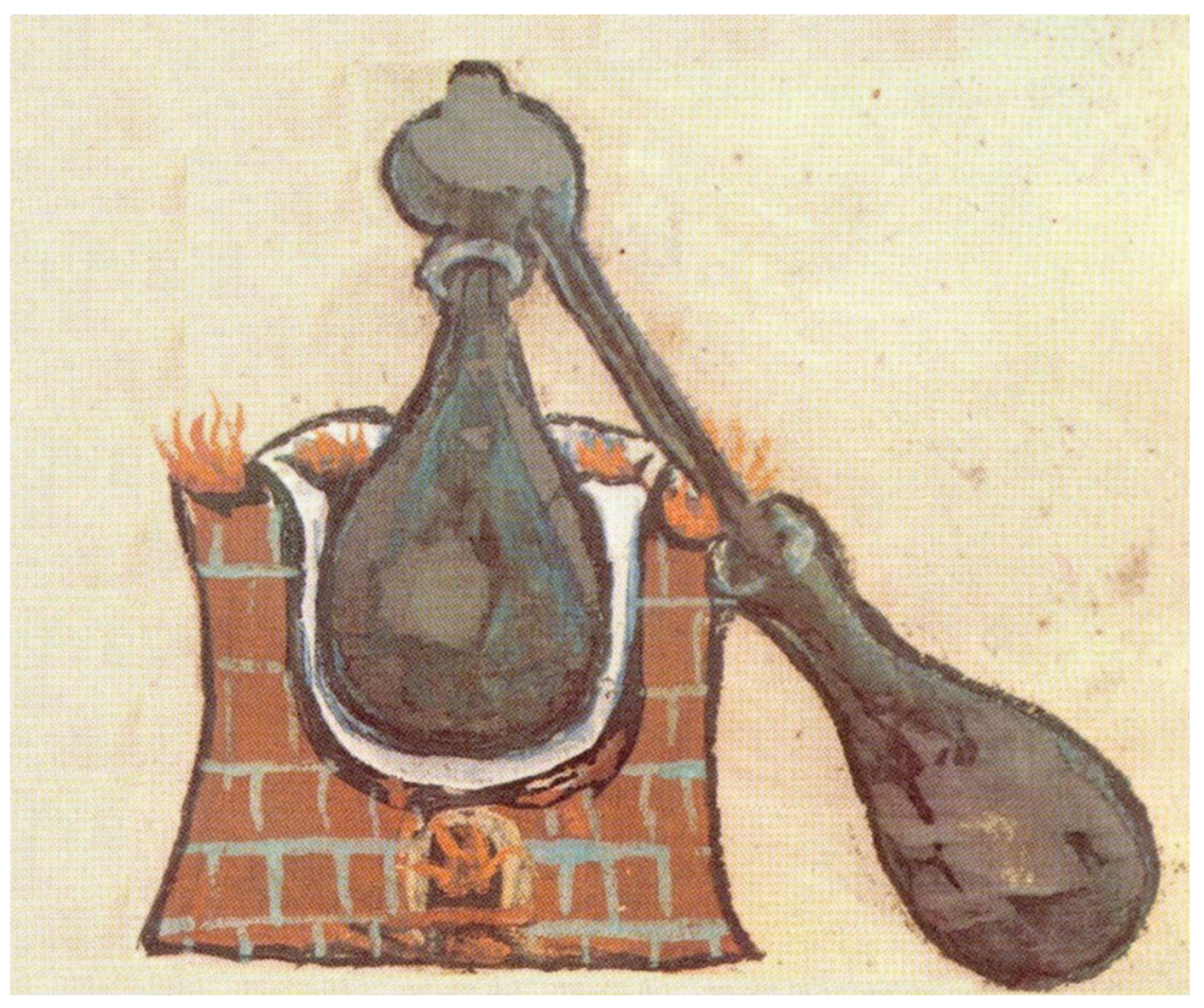
2. Preparation Techniques of Essential Oils
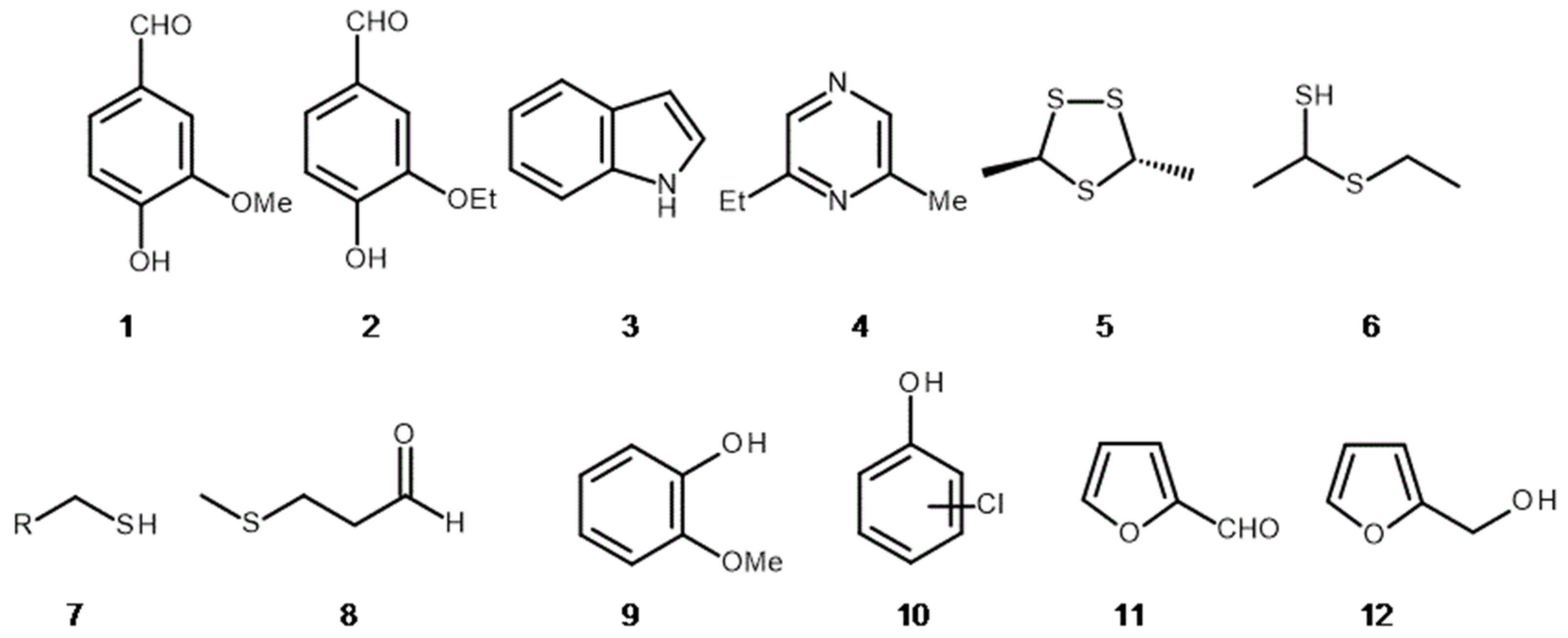
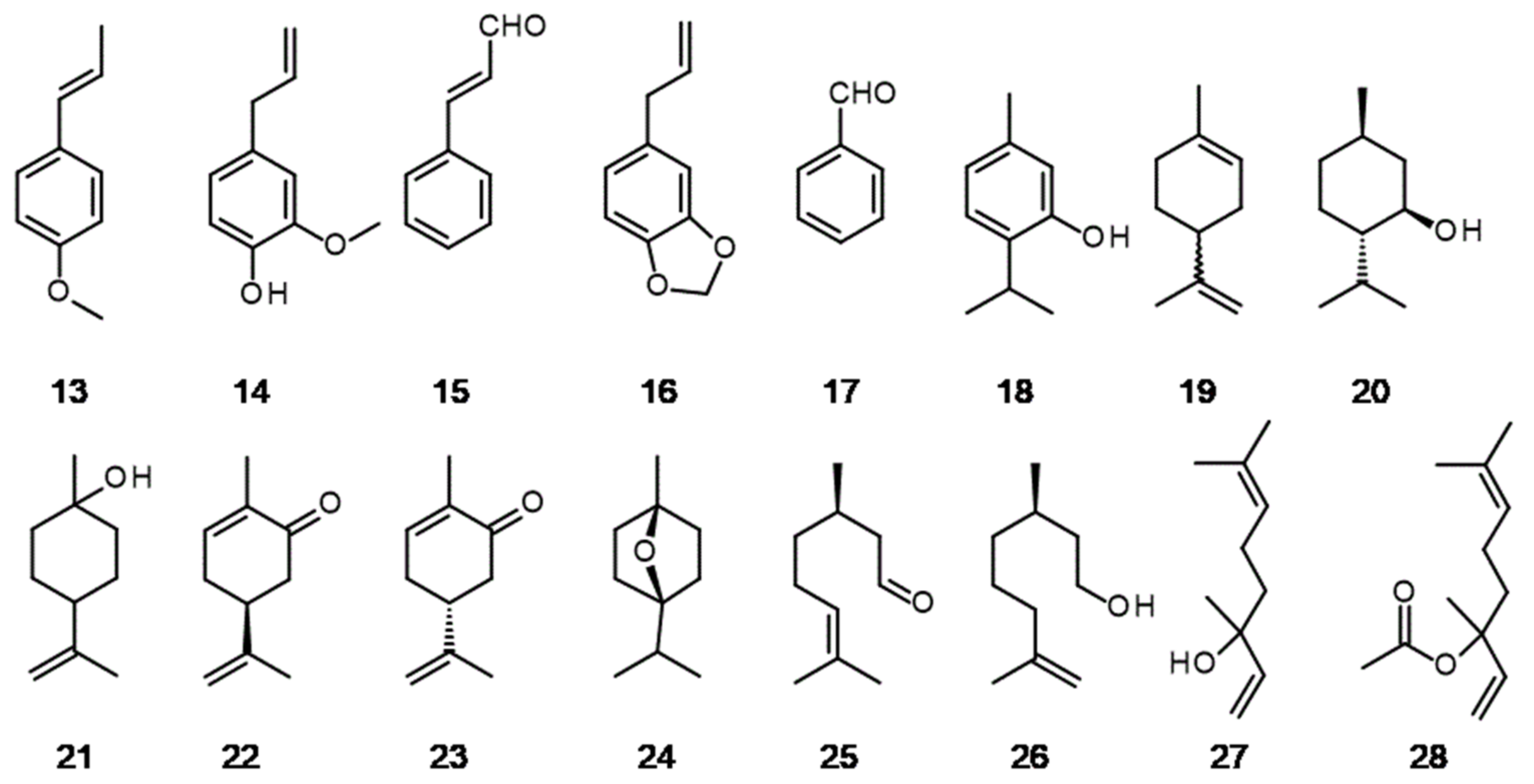
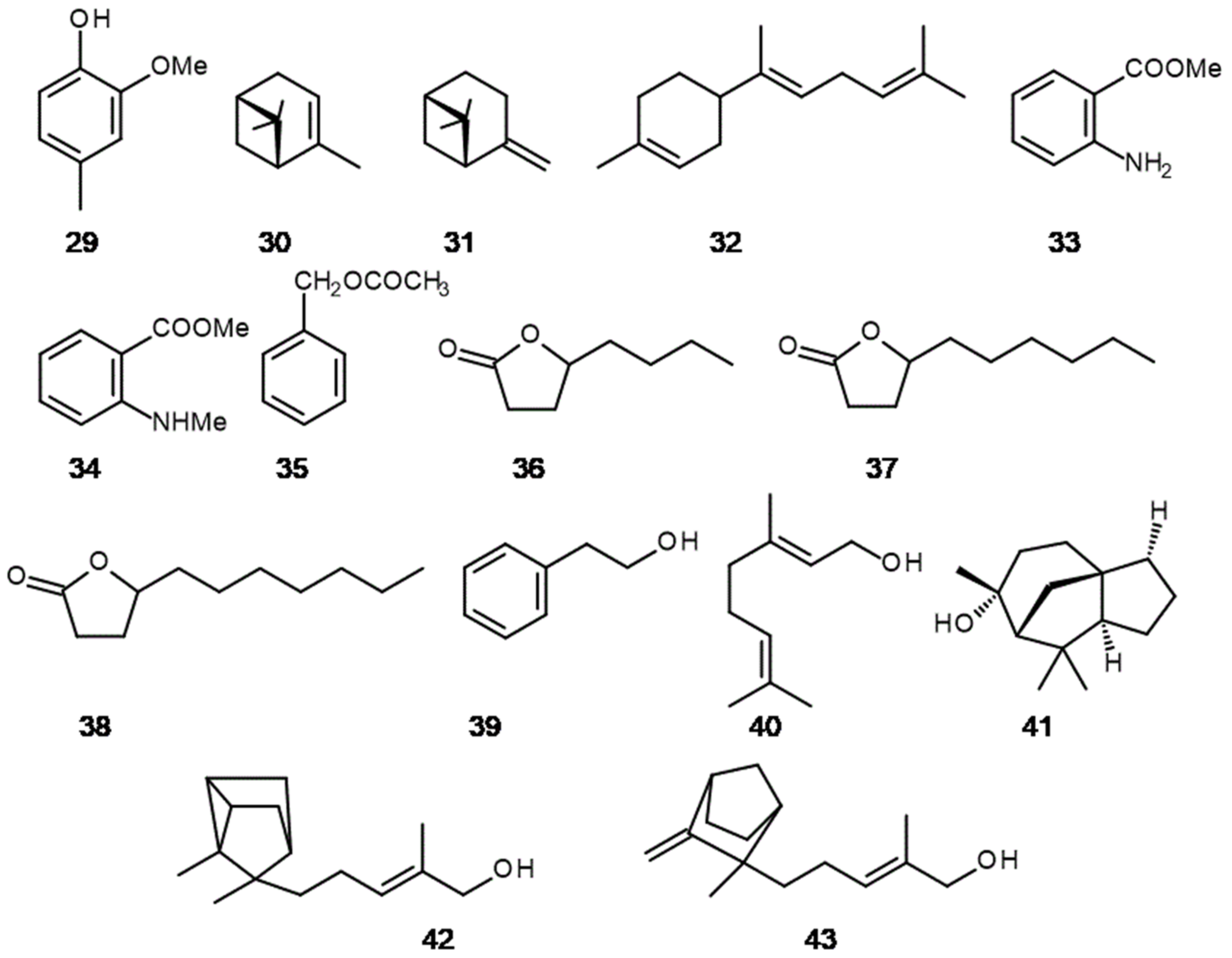
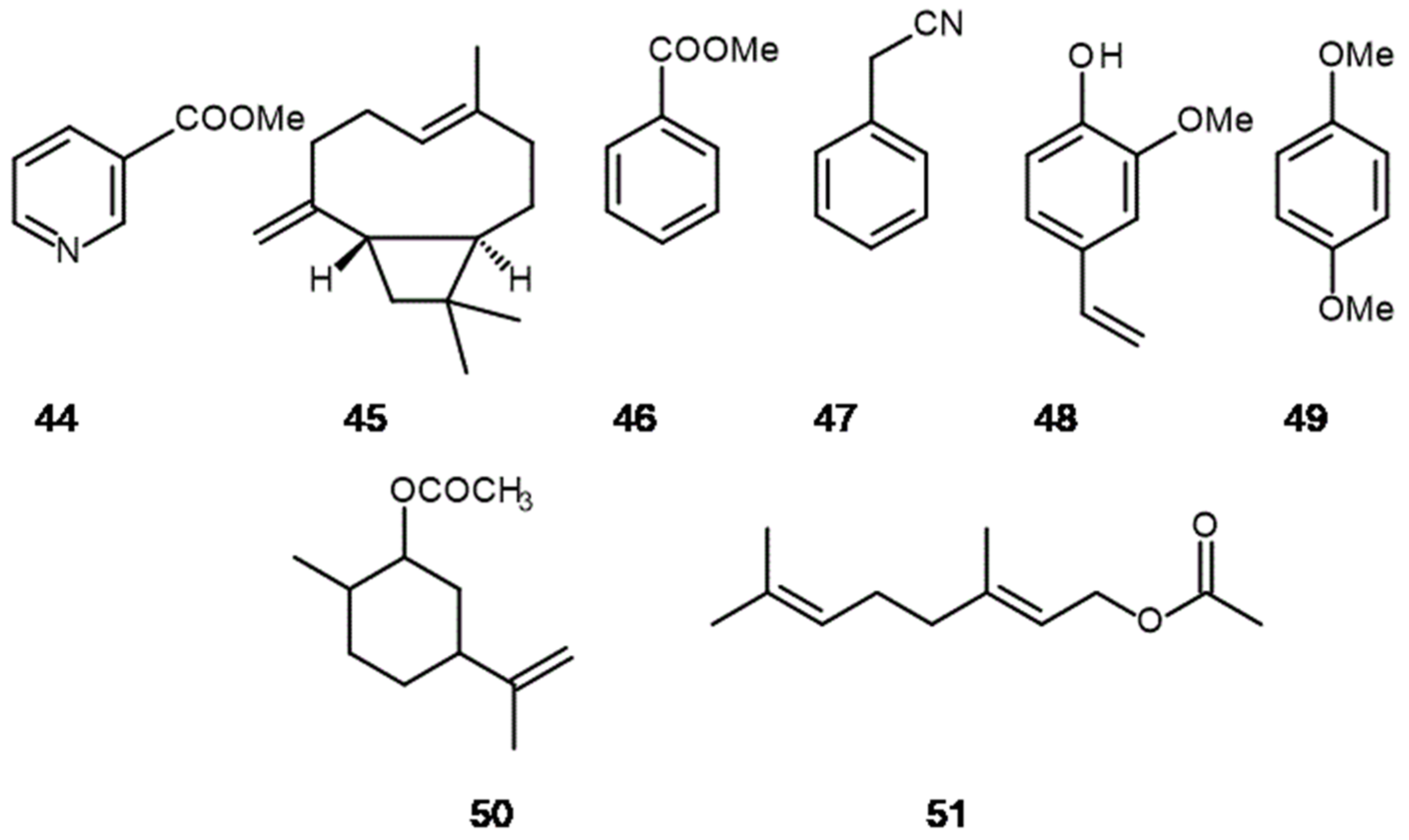
3. The Hidden Ingredients of EOS
4. The View from the Nature Side—Allelopathy
5. The Fragrant Danger—Examples of Biopesticides
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOS | Essential Oils |
| EU | European Union |
| EC | European Commission |
| GC-MS | Gas Chromatography—Mass Spectrometry |
| HMF | Hydroxymethylofurfural |
| MDMA | Methylenedioxy-methylamphetamine (CAS-42542-10-09) |
| ORNs | Olfactory receptor neurons |
| SFE | Supercritical fluid extraction |
| SFME | Solvent-free microwave extraction |
| VOCs | Volatile organic compounds |
References
- Bryson, L. Tasting Whiskey: An Insider’s Guide to the Unique Pleasures of the World’s Finest Spirits, 1st ed.; Storey Publishing: North Adams, MA, USA, 2014; pp. 41–46. [Google Scholar]
- Brieger, L. About the volatile constituents of human castings (Über die flüchtigen Bestandtheile der menschlichen Excremente). Ber. Deutsch. Chem. Ges. 1877, 10, 1027–1032. [Google Scholar] [CrossRef]
- Clark, G.S. Aroma chemical profile: Indole. Perfum. Flavorist 1995, 20, 21–31. Available online: https://www.perfumerflavorist.com/fragrance/application/multiuse/Indole-370125351.html (accessed on 14 March 2021).
- Liu, Y.; Miao, Z.; Guan, W.; Sun, B. Analysis of organic volatile flavor compounds in fermented stinky tofu using SPME with different fiber coatings. Molecules 2012, 17, 3708–3722. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, E.; Ito, T.; Odagiri, S.; Kubota, K.; Kobayashi, A. Comparison of compositions of odor components of natto and cooked soybeans. Agric. Biol. Chem. 1985, 49, 311–317. [Google Scholar]
- Fox, P.F.; McSweenery, P.L.H.; Cogan, T.M.; Guinee, T.P. Cheese: Chemistry, Physics and Microbiology, 3rd ed.; Elsevier Academic Press: London, UK, 2004; Volume 1, pp. 459–461. [Google Scholar]
- Li, J.-X.; Schieberle, P.; Steinhaus, M. Insights into the key compounds of durian (Durio zibethinus L. ‘Monthong’) pulp odor by odorant quantitation and aroma simulation experiments. J. Agric. Food Chem. 2017, 65, 639–647. [Google Scholar] [CrossRef]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, T.; Snitz, K.; Yablonka, A.; Khan, R.M.; Gafsou, D.; Schneidman, E.; Sobel, N. Perceptual convergence of multi-component mixtures in olfaction implies an olfactory white. Proc. Natl. Acad. Sci. USA 2012, 109, 19959–19964. [Google Scholar] [CrossRef] [Green Version]
- Staszak, J.F. Towards the geography of scents. Białostockie Studia Lit. 2013, 4, 41–51. [Google Scholar] [CrossRef]
- Henshaw, V. Urban Smellscapes: Understanding and Designing City Smell Environments, 1st ed.; Routledge & CRC Press: London, UK, 2014; pp. 9–41. [Google Scholar]
- Henshaw, V.; McLean, K.; Medway, D.; Perkins, C.; Warnaby, G. Designing with Smell: Practices, Techniques and Challenges, 1st ed.; Routledge & CRC Press: London, UK, 2017. [Google Scholar]
- McLean, K. Smellmap: Amsterdam–Olfactory Art and Smell Visualization. Leonardo. 2016. Available online: https://repository.canterbury.ac.uk/download/7390b55d1b533f6251128f4a71bbd9c985038d57ae493cda4f9a5058215366ca/462783/13775.pdf (accessed on 14 March 2021).
- Reed, M.A.; Richey, L.C.; Cooke, J.S.; Schnell, P.G. Hard Coated Chewing Gum with Improved Shelf Life, with Xylitol and Polyol Coatings. U.S. Patent US5376389A, 27 December 1994. [Google Scholar]
- Sokołowska, B. Alicyclobacillus–thermophilic acidophilic spore-forming bacteria–profile and prevalence (Alicyclobacillus–termofilne kwasolubne bakterie przetrwalnikujące–charakterystyka i występowanie). Żywność Nauka Technol. Jakość 2014, 4, 5–17. [Google Scholar] [CrossRef]
- Macura, R.; Michalczyk, M.; Bana, J. Effect of essential oils of coriander (Coriandrum sativum L.) and lemon balm (Melissa officinalis L.) on quality of stored ground veal. Żywność Nauka Technol. Jakość 2011, 18, 127–137. [Google Scholar] [CrossRef]
- Waszkiewicz-Robak, B.; Karwowska, W. The influence of bioflavonoids on the quality of food products. Part I. The impact of grapefruit bioflavonoids on sensoric value, selected physico-chemical indicators and microbiological constancy of salads prepared on the basis of mayonnaise (Wpływ bioflawonoidów na jakość produktów spożywczych. Część I–Wpływ bioflawonoidów z grejpfruta na jakość sensoryczną, wybrane mierniki fizyko-chemiczne i trwałość mikrobiologiczną sałatek otrzymanych na bazie majonezu). Postępy Tech. Przetw. Spoż. 2004, 14, 24–28. [Google Scholar]
- Kolarzyk, E. Selected Topics on Hygiene and Human Ecology. Available online: https://books.google.pl/books?id=xAeXtgAACAAJ (accessed on 14 March 2021).
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Skarżyńska, K. Application of osmotic dehydration in the production of little processed food (Zastosowanie odwadniania osmotycznego w produkcji żywności mało przetworzonej). Postępy Tech. Przetw. Spoż. 2016, 1, 87–99. [Google Scholar]
- Piekut, J. Evaluation of selected spice plants extracts under their controls of antimicrobial properties and content of phenolic acids (Ocena wybranych ekstraktów roślin przyprawowych pod względem ich właściwości przeciwdrobnoustrojowych oraz zawartości fenolokwasów). Zesz. Probl. Postępów Nauk Rol. 2017, 588, 103–111. [Google Scholar] [CrossRef]
- Pierce, J.; Halpern, B.P. Orthonasal and retronasal odorant identification based upon vapor phase input from common substances. Chem. Senses 1996, 21, 529–543. [Google Scholar] [CrossRef] [Green Version]
- Spence, C. Gastrophysics: The New Science of Eating, 1st ed.; Penguin Random House: London, UK, 2017; pp. 22–23. [Google Scholar]
- Arn, H.; Acree, T.E. Flavornet: A database of aroma compounds based on odor potency in natural products. In Developments in Food Science; Contis, E.T., Ho, C.-T., Mussinan, C.J., Parliament, T.H., Shahidi, F., Spanier, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 40, p. 27. [Google Scholar] [CrossRef]
- Hoover, K.C. The geography of smell. Cartographica 2009, 44, 237–239. [Google Scholar] [CrossRef] [Green Version]
- Jedlińska, A.; Witrowa-Rajchert, D. Variety of food aromas, their chemical composition and labelling. Vistula Sci. Q. 2017, 2, 300–309. [Google Scholar]
- Farahani, M.A.; Afsargharehbagh, R.; Marandi, F.; Moradi, M.; Hashemi, S.-M.; Moghadam, M.P.; Balouchi, A. Effect of aromatherapy on cancer complications: A systematic review. Complementary Ther. Med. 2019, 47, 102169. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press Taylor&Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
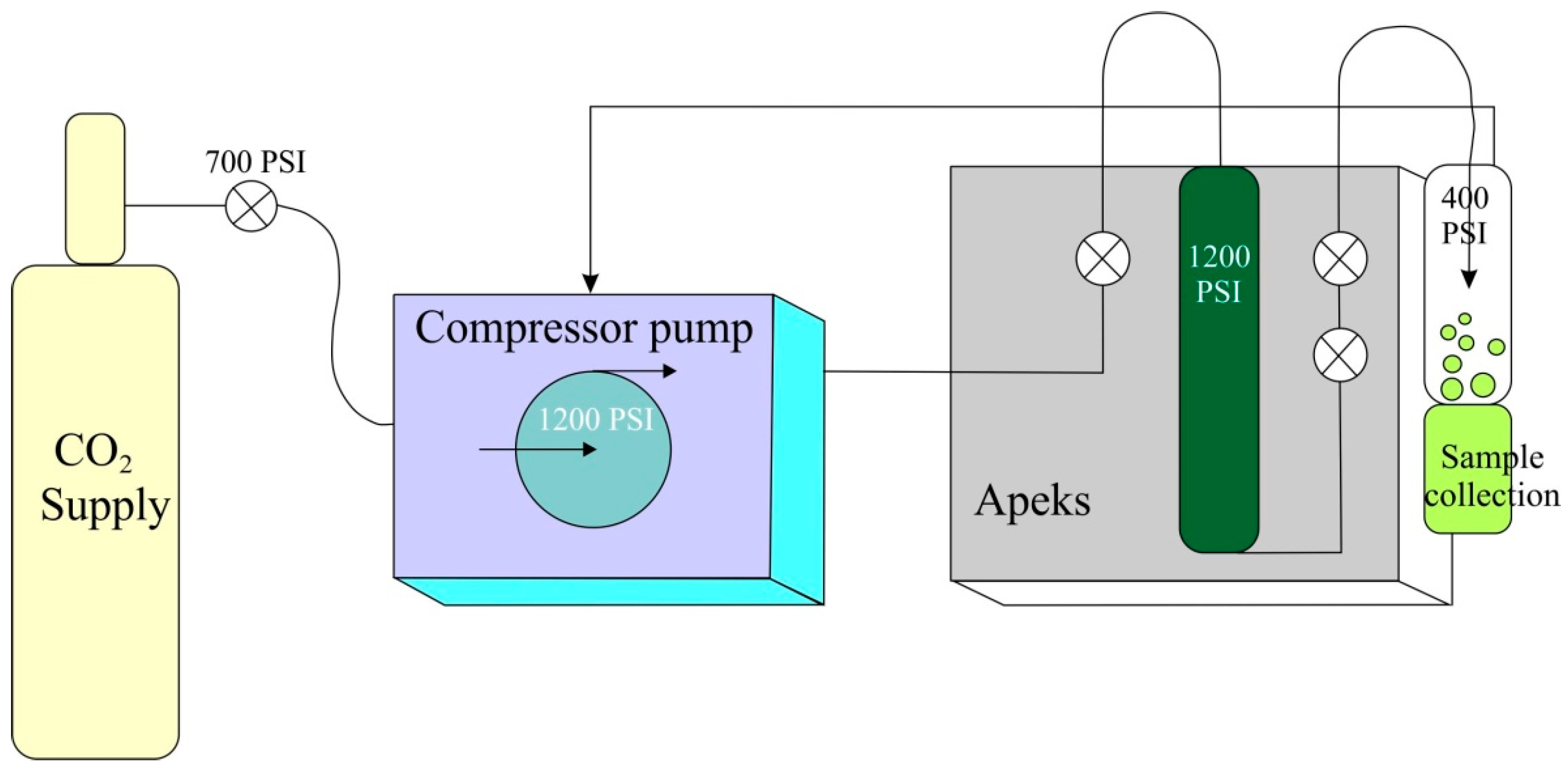
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Analyt. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Shahsavarpour, M.; Lashkarbolooki, M.; Eftekhari, M.J.; Esmaeilzadeh, F. Extraction of essential oils from Mentha spicata L. (Labiatae) via optimized supercritical carbon dioxide process. J. Supercrit. Fluids 2017, 130, 253–260. [Google Scholar] [CrossRef]
- Elgndi, M.A.; Filip, S.; Pavlić, B.; Vladić, J.; Stanojković, T.; Žižak, Ž.; Zeković, Z. Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 128–137. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; de Lampasona, M.P.; Catalan, C.A.N. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. FCT 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmad, A.; Bushra, R. Supercritical carbon dioxide extraction of essential oils from leaves of Eucalyptus globulus L., their analysis and application. Anal. Methods 2016, 8, 1339–1350. [Google Scholar] [CrossRef]
- Levey, M. Chemistry and chemical technologies in ancient Mesopotamia. J. Chem. Educ. 1960, 37, A436. [Google Scholar]
- Wikipedia T Original Uploader Was M at G. Deutsch: Alambic in Einer Mittelalterlichen Handschrift 2006. Available online: https://commons.wikimedia.org/wiki/File:Alambik1.jpg (accessed on 14 March 2021).
- Chemat, F.; Lucchesi, M.E.; Smadja, J.; Favretto, L.; Colnaghi, G.; Visinoni, F. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Anal. Chim. Acta. 2006, 555, 157–160. [Google Scholar] [CrossRef]
- Moradi, S.; Fazlali, A.; Hamedi, H. Microwave-assisted hydro-distillation of essential oil from rosemary: Comparison with traditional distillation. Avicenna J. Med. Biotechnol. 2018, 10, 22–28. [Google Scholar] [PubMed]
- Sahraoui, N.; Vian, M.A.; Bornard, I.; Boutekedjiret, C.; Chemat, F. Improved microwave steam distillation apparatus for isolation of essential oils: Comparison with conventional steam distillation. J. Chromatogr. A 2008, 1210, 229–233. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction: An innovative tool for rapid extraction of essential oil from aromatic herbs and spices. JMPEE 2004, 39, 135–139. [Google Scholar] [CrossRef]
- Capelo-Martínez, J.-L. Ultrasound in Chemistry: Analytical Applications, 1st ed.; WILEY—VCH Verlag GmbH&Co, KGaA: Weiheim, Germany, 2009. [Google Scholar] [CrossRef]
- Cravotto, G.; Delattre, F.; Leveque, J.-M.; Cintas, P. Organic Sonochemistry: Challenges and Perspectives for the 21st Century; Springer International Publishing: Cham, Swizerland, 2018. [Google Scholar] [CrossRef]
- Paniwnyk, L.; Cai, H.; Albu, S.; Mason, T.J.; Cole, R. The enhancement and scale up of the extraction of anti-oxidants from Rosmarinus officinalis using ultrasound. Ultrason. Sonochem. 2009, 16, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Condon-Abanto, S.; Condon, S.; Alvarez, I.; Raso, J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep. Purif. Technol. 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Danel, A.; Puła, J. Plants as a treasury of fragrant substances for food industry and perfumery. AUPC Studia Nat. 2019, 4, 149–160. [Google Scholar] [CrossRef]
- Rutkowski, A.; Gwiazda, S.; Dąbrowski, K. Kompendium Dodatków do Żywności; Hortimex: Konin, Poland, 2003; pp. 362–363. [Google Scholar]
- Kołodziejczyk, A. Natural Organic Compounds (Naturalne Związki Organiczne), 1st ed.; PWN: Warszawa, Poland, 2013; pp. 649–651. [Google Scholar]
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH&Co KGaA: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Economic analysis of rosemary, fennel and anise essential oils obtained by supercritical fluid extraction. Flavour Fragr. J. 2007, 22, 407–413. [Google Scholar] [CrossRef]
- Rodrigues, V.M.; Rosa, P.T.V.; Marques, M.O.M.; Petenate, A.J.; Meireles, M.A.A. Supercritical extraction of essential oil from aniseed (Pimpinella anisum L.) using CO2: solubility, kinetics, and composition data. J. Agric. Food. Chem. 2003, 51, 1518–1523. [Google Scholar] [CrossRef]
- Ashurst, P.R. Food Flavorings, 3rd ed.; Aspen Publishers Inc.: Graithersburg, MD, USA, 1999. [Google Scholar]
- Wright, J. Flavor Bites: Anethole. Perfum. Flavorist 2019. Available online: https://www.perfumerflavorist.com/flavor/application/beverage/Flavor-Bites-Anethole-564621761.html (accessed on 14 March 2021).
- Marinov, V.; Valcheva-Kuzmanova, S. Review on the pharmacological activities of anethole. Scr. Sci. Pharm. 2015, 2, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Sulistyoningrum, A.S.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Amelia, B.; Haib, J. Chemical profiling of clove bud oil (Syzygium aromaticum) from Toli-Toli and Bali by GC-MS analysis. AIP Conf. Proc. 2017, 1862, 030089. [Google Scholar] [CrossRef] [Green Version]
- Gowder, S.; Devaraj, H. A review of the nephrotoxicity of the food flavor cinnamaldehyde. Curr. Bioact. Compd. 2010, 6, 106–117. [Google Scholar] [CrossRef]
- Kemprai, P.; Mahanta, B.P.; Sut, D.; Barman, R.; Banik, D.; Lal, M.; Saikia, S.P.; Haldar, S. Review on safrole: Identity shift of the ‘candy shop’ aroma to a carcinogen and deforester. Flavour Fragr. J. 2020, 35, 5–23. [Google Scholar] [CrossRef]
- Burdock, G.A.; Fenaroli, G. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Townsend, G.F. Benzaldehyde: A new repellent for driving bees. Bee World 1963, 44, 146–149. [Google Scholar] [CrossRef]
- Nieto, G. A review on applications and uses of Thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Lanham, C. Aromatic Molecules: Terpenes Are Newest Flavoring Agent on the Local Market. Available online: https://www.newtimesslo.com/sanluisobispo/aromatic-molecules-the-newest-flavoring-agent-on-the-local-market-starts-with-cannabis-even-though-its-not-derived-from-cannabis/Content?oid=8326281 (accessed on 14 March 2021).
- Sell, C.S. Terpenoids. In Kirk-Othmer Chemical Technology of Cosmetics, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 247–375. [Google Scholar]
- Villanti, A.C.; Collins, L.K.; Niaura, R.S.; Gagosian, S.Y.; Abrams, D.B. Menthol cigarettes and the public health standard: A systematic review. BMC Public Health 2017, 17, 983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.-S.; Guo, W.-F.; Lu, Y.; Jiang, Y.-X. Flavor characteristics of lapsang souchong and smoked lapsang souchong, a special Chinese black tea with pine smoking process. J. Agric. Food. Chem. 2005, 53, 8688–8693. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; de Oliveira Felipe, L.; Bicas, J.L. Production, properties, and applications of α-terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Ray, A.M.; Hanks, L.M.; Millar, J.G. The common natural products (S)-α-terpineol and (E)-2-hexenol are important pheromone components of Megacyllene antennata (Coleoptera: Cerambycidae). Environ. Entomol. 2018, 47, 1547–1552. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 5th ed.; WILEY-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Boelens, M.H. Sensory and chemical evaluation of tropical grass oils. Perfum. Flavorist 1994, 19, 29–45. [Google Scholar]
- Drapeau, J.; Rossano, M.; Touraud, D.; Obermayr, U.; Geier, M.; Rose, A.; Kunz, W. Green synthesis of para-Menthane-3,8-diol from Eucalyptus citriodora: Application for repellent products. C. R. Chim. 2011, 7, 629–635. [Google Scholar] [CrossRef]
- Hussein, K.N.; Friedrich, L.; Pinter, R.; Németh, C.; Kiskó, G.; Dalmadi, I. Effect of linalool and piperine on chicken meat quality during refrigerated conditions. Acta Aliment. 2019, 48, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Kraśniewska, K.; Gniewosz, M.; Kosakowska, O.; Pobiega, K. Chemical composition and antimicrobial properties of essential oil from lavender (Lavandula angustifolia L.) in commercial available preparation. Adv. Phytother. 2017, 2, 113–118. [Google Scholar] [CrossRef]
- Renard, A. Pine Tar. J. Chem. Soc. 1895, 68, 294. [Google Scholar]
- Alpha-Pinene. Available online: http://www.thegoodscentscompany.com/data/rw1006351.html (accessed on 4 March 2021).
- Podlaski, S.; Chołuj, D.; Wiśniewski, G. Production of biomass from energy crops. Adv. Agric. Res. 2010, 2, 163–174. [Google Scholar]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. Bisabolene, a sesquiterpene from the Essential oil extract of opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2015, 9, 418–425. [Google Scholar] [CrossRef]
- Kusuhara, M.; Maruyama, K.; Ishii, H.; Masuda, Y.; Sakurai, K.; Tamai, E.; Urakami, K. A fragrant environment containing α-pinene suppresses tumor growth in mice by modulating the hypothalamus/sympathetic nerve/leptin axis and immune system. Integr. Cancer Ther. 2019, 18, 53473541984513. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex–32009L0128–EN–EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 14 March 2021).
- Luo, Z.W.; Cho, J.S.; Lee, S.Y. Microbial production of methyl anthranilate, a grape flavor compound. Proc. Natl. Acad. Sci. USA 2019, 116, 10749–10756. [Google Scholar] [CrossRef] [Green Version]
- Page, G.V.; Scire, B.; Farbood, M.I. A Method for the Preparation of “Natural” Methyl Anthranilate. PCT Patent WO1989000203A1, 12 January 1989. [Google Scholar]
- Benzyl Acetate Natural Isolate (Ylang Oil Fractions) Hermitage Essential Oils. Available online: https://hermitageoils.com/product/benzyl-acetate-natural-isolate-ylang-fractions/ (accessed on 14 March 2021).
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.-M.; Rabenhorst, J. Applied biocatalysis for the synthesis of natural flavour compounds–current industrial processes and future prospects. Biotechnol. Lett. 2004, 26, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Belo, I. Biotechnological production of γ-decalactone, a peach like aroma, by Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2016, 32, 169. [Google Scholar] [CrossRef] [Green Version]
- Gamma Octalactone Natural Isolate from hermitageoils.com. Hermitage Essential Oils. Available online: https://hermitageoils.com/product/gamma-octalactone-natural-isolate/ (accessed on 14 March 2021).
- Simon, D.Z.; Beliveau, J.; Aube, C. Extraction by hydrodiffusion of the essential oil of Monarda fistulosa grown in the Province of Quebec: Assay of geraniol in the hydrodiffused oil. Int. J.Crude Drug Res. 1986, 24, 120–122. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol–A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Škrinjar, M.M.; Nemet, N.T. Antimicrobial effects of spices and herbs essential oils. Acta Period. Technol. 2009, 40, 195–209. [Google Scholar] [CrossRef]
- Wilkinson, S.M.; Love, S.B.; Westcombe, A.M.; Gambles, M.A.; Burgess, C.C.; Cargill, A.; Young, T.; Maher, E.J.; Ramirez, A.J. Effectiveness of aromatherapy massage in the management of anxiety and depression in patients with cancer: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Ozsavci, D.; Ozakpinar, O.B.; Cetin, M.; Aricioglu, F. Level of clinical evidence of herbal complementary therapies in psychiatric disorders. Psychiatr. Clin. Psychopharmacol. 2019, 29, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Aponso, M.; Patti, A.; Bennett, L.E. Dose-related effects of inhaled essential oils on behavioural measures of anxiety and depression and biomarkers of oxidative stress. J. Ethnopharmacol. 2020, 250, 112469. [Google Scholar] [CrossRef]
- Patel, S.; Gogna, P. Tapping botanicals for essential oils: Progress and hurdles in cancer mitigation. Ind. Crops Prod. 2015, 76, 1148–1163. [Google Scholar] [CrossRef]
- Izgu, N.; Ozdemir, L.; Bugdayci, B.F. Effect of aromatherapy massage on chemotherapy-induced peripheral neuropathic pain and fatigue in patients receiving oxaliplatin: An open label quasi–randomized controlled pilot study. Cancer Nurs. 2019, 42, 139–147. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombolà, L.; Morrone, L.A.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Tonin, P.; Corasaniti, M.T. Neuropharmacology of the neuropsychiatric symptoms of dementia and role of pain: Essential oil of bergamot as a novel therapeutic approach. Int. J. Mol. Sci. 2019, 20, 3327. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y.; Seo, H.J.; Min, S.S.; Park, M.; Seol, G.H. The effect of 1,8-cineole inhalation on preoperative anxiety: A randomized clinical trial. Evid. Based Complement. Alternat. Med. 2014, 820126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Malo, A.; Palou, E.; León-Cruz, R.; Alzamora, S.M. Mixtures of natural and synthetic antifungal agents. In Advances in Food Mycology. Advances in Experimental Medicine and Biology; Hocking, A.D., Pitt, J.I., Samson, R.A., Thrane, U., Eds.; Springer: Boston, MA, USA, 2006; Volume 571, pp. 261–286. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berstad, A.; Raa, J.; Valeur, J. Indole–the scent of a healthy ‘inner soil’. Microb. Ecol. Health Dis. 2015, 26, 27997. [Google Scholar] [CrossRef] [PubMed]
- Theis, K.R.; Schmidt, T.M.; Holekamp, K.E. Evidence for a bacterial mechanism for group-specific social odors among hyenas. Sci. Rep. 2012, 2, 615. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Zhang, H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015, 24, 774. [Google Scholar] [CrossRef] [PubMed]
- Sekutowski, T. Alleloherbicides and bioherbicides–myth or reality? J. Res. Appl. Agric. Eng. 2010, 55, 84–90. [Google Scholar]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Groom, N. The Perfume Handbook, 1st ed.; Springer: Dordrecht, The Netherlands, 1992. [Google Scholar] [CrossRef]
- Akter, S.; Islam, M.T.; Zulkefeli, M.; Khan, S.I. Agarwood production–a multidisciplinary field to be explored in Bangladesh. Int. J. Pharm. Life Sci. 2013, 2, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Sawer, J.C.; John, C. Chapter XIII: Lign-Aloes.-Patchouli.-Vetiver.-Camel grass.-Hennah. In Odorographia: A Natural History of Raw Materials and Drugs Used in the Perfume Industry: Intended to Serve Growers, Manufacturers and Consumers; Read Books Ltd.: Redditch, UK, 2013. [Google Scholar]
- Agarwood Prices–Oud Oil Prices. Available online: http://www.oudoiltrading.com/oud-oil/ (accessed on 14 March 2021).
- Bali, A.S.; Batish, D.R.; Singh, H.P. Allelopathic effect of aromatic plants: Role of volatile essential oils. J. Global. Biosci. 2016, 5, 4386–4395. [Google Scholar]
- Hazzoumi, Z.; Moustakime, Y.; Joutei, K.A. Chapter 6: Essential oil and glandular hairs: Diversity and roles. In Essential Oils; El-Shemy, H.-A., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–16. Available online: https://www.intechopen.com/books/essential-oils-oils-of-nature (accessed on 14 March 2021).
- Wang, G.; Tian, L.; Aziz, N.; Broun, P.; Dai, X.; He, J.; King, A.; Zhao, P.X.; Dixon, R.A. Terpene biosynthesis in glandular trichomes of hop plant physiology. ASPB Plant Physiol. 2008, 148, 1254–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffei, M.; Chialva, F.; Sacco, T. Glandular trichomes and essential oils in developing peppermint leaves. I. Variation of peltate trichome number and terpene distribution within leaves. New Phytol. 1989, 111, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.H.; Kasperbauer, M.J. Aroma content of fresh basil (Ocimum basilicum L.) leaves is affected by light reflected from colored mulches. J. Agric. Food Chem. 2003, 51, 2272–2276. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castelazo, C.; Rico-Gray, V.; Ortega, F.; Angeles, G. Morphological and secretory characterization of extrafloral nectaries in plants of coastal Veracruz, Mexico. Ann. Bot. 2005, 96, 1175–1189. [Google Scholar] [CrossRef] [Green Version]
- Kessler, D.; Diezel, C.; Clark, D.G.; Colquhoun, T.A.; Baldwin, I.T. Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecol. Lett. 2013, 16, 299–306. [Google Scholar] [CrossRef]
- Parachnowitsch, A.; Burdon, R.C.F.; Raguso, R.A.; Kessler, A. Natural selection on floral volatile production in Penstemon digitalis: Highlighting the role of linalool. Plant Signal. Behav. 2013, 8, e22704. [Google Scholar] [CrossRef] [Green Version]
- Junker, R.R.; Loewel, C.; Gross, R.; Dötterl, S.; Keller, A.; Blüthgen, N. Composition of epiphytic bacterial communities differs on petals and leaves: Bacterial communities on flowers and leaves. Plant Biol. 2011, 13, 918–924. [Google Scholar] [CrossRef]
- Guzicka, M.; Woźny, A. The types of plant cells. In Biology of Plant Cell Part I-Structure; Wojtaszek, P., Woźny, A., Ratajczak, L., Eds.; PWN: Warsaw, Poland, 2006; pp. 327–333. [Google Scholar]
- Tölke, E.D.; Bachelier, J.B.; Alves de Lima, E.; Ferreira, M.J.P.; Demarco, D.; Carmello-Guerreiro, S.M. Osmophores and floral fragrance in Anacardium humile and Mangifera indica (Anacardiaceae): An overlooked secretory structure in Sapindales. AoB Plants 2018, 10, ply062. [Google Scholar] [CrossRef] [Green Version]
- Burdon, R.C.; Junker, R.R.; Scofield, D.G.; Parachnowitsch, A.L. Bacteria colonising Penstemon digitalis show volatile and tissue-specific responses to a natural concentration range of the floral volatile linalool. Chemoecology 2018, 28, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Majetic, C.J.; Raguso, R.A.; Ashman, T.-L. Sources of floral scent variation: Can environment define floral scent phenotype? Plant Signal. Behav. 2009, 4, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.E.P.; Peñaflor, M.F.G.V.; Bento, J.M.S.; Salvador, M.J.; Sazima, M. The dilemma of being a fragrant flower: The major floral volatile attracts pollinators and florivores in the euglossine-pollinated orchid Dichaea pendula. Oecologia 2016, 182, 933–946. [Google Scholar] [CrossRef]
- Huang, Z.; Dostal, L.; Rosazza, J.P. Microbial transformations of ferulic acid by Saccharomyces cerevisiae and Pseudomonas fluorescens. Appl. Environ. Microbiol. 1993, 59, 2244–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theis, N.; Adler, L.S. Advertising to the enemy: Enhanced floral fragrance increases beetle attraction and reduces plant reproduction. Ecology 2012, 93, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sagae, M.; Oyama-Okubo, N.; Ando, T.; Marchesi, E.; Nakayama, M. Effect of temperature on the floral scent emission and endogenous volatile profile of Petunia axillaris. Biosci. Biotechnol. Biochem. 2008, 72, 110–115. [Google Scholar] [CrossRef] [Green Version]
- von Arx, M.; Goyret, J.; Davidowitz, G.; Raguso, R.A. Floral humidity as a reliable sensory cue for profitability assessment by nectar-foraging hawkmoths. Proc. Natl. Acad. Sci. USA 2012, 109, 9471–9476. [Google Scholar] [CrossRef] [Green Version]
- Campbell, D.R.; Sosenski, P.; Raguso, R.A. Phenotypic plasticity of floral volatiles in response to increasing drought stress. Ann. Bot. 2019, 123, 601–610. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Leng, P.; Zhao, J.; Wang, W.; Wang, S. The emission of floral scent from Lilium ‘siberia’ in response to light intensity and temperature. Acta Physiol. Plant. 2013, 35, 1691–1700. [Google Scholar] [CrossRef]
- Wang, T.N.; Clifford, M.R.; Martínez-Gómez, J.; Johnson, J.C.; Riffell, J.A.; Di Stilio, V.S. Scent matters: Differential contribution of scent to insect response in flowers with insect vs. wind pollination traits. Ann. Bot. 2019, 123, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Pareja, M.; Qvarfordt, E.; Webster, B.; Mayon, P.; Pickett, J.; Birkett, M.; Glinwood, R. Herbivory by a phloem-feeding insect inhibits floral volatile production. PLoS ONE 2012, 7, e31971. [Google Scholar] [CrossRef] [Green Version]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Puła, J.; Zandi, P.; Stachurska-Swakoń, A.; Barabasz-Krasny, B.; Możdżeń, K.; Wang, Y. Influence of alcoholic extracts from Helianthus annnus L. roots on the photosynthetic activity of Sinapis alba L. cv. Barka plants. Acta Agric. Scand. B Soil Plant Sci. 2020, 70, 8–13. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Zandi, P.; Puła, J. Effect of aqueous extracts of peppermint (Mentha ×piperita L.) on the germination and the growth of selected vegetable and cereal seeds. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009L0128&from=EN (accessed on 31 March 2021).
- Vieira, R.F.; Grayer, R.J.; Paton, A.; Simon, J.E. Genetic diversity of Ocimum gratissimum L. based on volatile oil constituents, flavonoids and RAPD markers. Biochem. Syst. Ecol. 2001, 29, 287–304. [Google Scholar] [CrossRef]
- Synowiec, A.; Smęda, A.; Adamiec, J.; Kalemba, D. The effect of microencapsulated essential oils on the initial growth of maize (Zea mays) and common weeds (Echinochloa crus-galli and Chenopodium album) (Wpływ mikrokapsułkowanych olejków eterycznych na początkowy wzrost kukurydzy (Zea mays) i chwastów (Echinochloa crus-galli i Chenopodium album). Prog. Plant. Protect. 2016, 56, 372–378. [Google Scholar]
- Stokłosa, A. Bioherbicides and alleloherbicides as weed control methods. Adv. Agric. Sci. 2006, 6, 41–52. [Google Scholar]
- Villaverde, J.J.; Sandín-España, P.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides from Natural products: Current development, legislative framework, and future trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef] [Green Version]
- Synowiec, A.; Halecki, W.; Wielgusz, K.; Byczyńska, M.; Czaplicki, S. Effect of fatty acid methyl esters on the herbicidal effect of essential oils on corn and weeds. Weed Technol. 2017, 31, 301–309. [Google Scholar] [CrossRef]
- Synowiec, A.; Możdżeń, K.; Krajewska, A.; Landi, M.; Araniti, F. Carum carvi L. essential oil: A promising candidate for botanical herbicide against Echinochloa crus-galli (L.) P. Beauv. in maize cultivation. Ind. Crop. Prod. 2019, 140, 111652. [Google Scholar] [CrossRef]
- Laosinwattana, C.; Wichittrakarn, P.; Teerarak, M. Chemical composition and herbicidal action of essential oil from Tagetes erecta L. leaves. Ind. Crop. Prod. 2018, 126, 129–134. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G. A Contemporary introduction to essential oils: Chemistry, bioactivity and prospects for Australian Agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.; Natal-da-Luz, T.; Sousa, J.P.; Gonçalves, M.J.; Salgueiro, L.; Canhoto, C. Effects of essential oils from Eucalyptus globulus leaves on soil organisms involved in leaf degradation. PLoS ONE 2013, 8, e61233. [Google Scholar] [CrossRef] [Green Version]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Dhima, K.V.; Vasilakoglou, I.B.; Gatsis, T.D.; Panou-Philotheou, E.; Eleftherohorinos, I.G. Effects of aromatic plants incorporated as green manure on weed and maize development. Field Crops Res. 2009, 110, 235–241. [Google Scholar] [CrossRef]
- Duryea, M.L.; English, R.J.; Hermansen, L.A. A comparison of landscape mulches: Chemical, allelopathic, and decomposition properties. J. Arboric. 1999, 25, 88–97. [Google Scholar]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crop. Prod. 2020, 154, 111979. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S. Effect of mulching on medicinal and aromatic plants in organic farm guest houses. Acta Hortic. 2006, 723, 405–410. [Google Scholar] [CrossRef]
- Wu, Y.; Xi, X.; Tang, X.; Luo, D.; Gu, B.; Lam, S.K.; Vitousek, P.M.; Chen, D. Policy distortions, farm size, and the overuse of agricultural chemicals in China. Proc. Natl. Acad. Sci. USA 2018, 115, 7010–7015. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a reduced reliance on conventional pesticides in european agriculture. Plant Dis. 2015, 100, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kliszcz, A.; Danel, A.; Puła, J.; Barabasz-Krasny, B.; Możdżeń, K. Fleeting Beauty—The World of Plant Fragrances and Their Application. Molecules 2021, 26, 2473. https://doi.org/10.3390/molecules26092473
Kliszcz A, Danel A, Puła J, Barabasz-Krasny B, Możdżeń K. Fleeting Beauty—The World of Plant Fragrances and Their Application. Molecules. 2021; 26(9):2473. https://doi.org/10.3390/molecules26092473
Chicago/Turabian StyleKliszcz, Angelika, Andrzej Danel, Joanna Puła, Beata Barabasz-Krasny, and Katarzyna Możdżeń. 2021. "Fleeting Beauty—The World of Plant Fragrances and Their Application" Molecules 26, no. 9: 2473. https://doi.org/10.3390/molecules26092473
APA StyleKliszcz, A., Danel, A., Puła, J., Barabasz-Krasny, B., & Możdżeń, K. (2021). Fleeting Beauty—The World of Plant Fragrances and Their Application. Molecules, 26(9), 2473. https://doi.org/10.3390/molecules26092473