Evaluation of Electrochemical Stability of Sulfonated Anthraquinone-Based Acidic Electrolyte for Redox Flow Battery Application
Abstract
1. Introduction
2. Results and Discussion
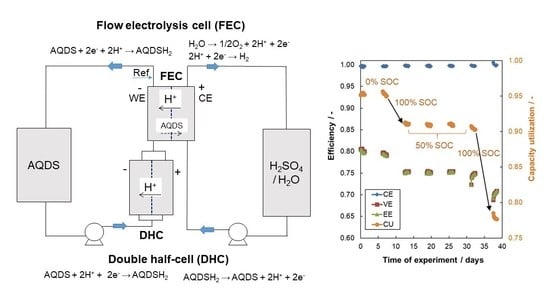
2.1. AQDS—Br Single-Cell Performance
2.2. Electrochemical Stability of AQDS Sulfonation Mixture
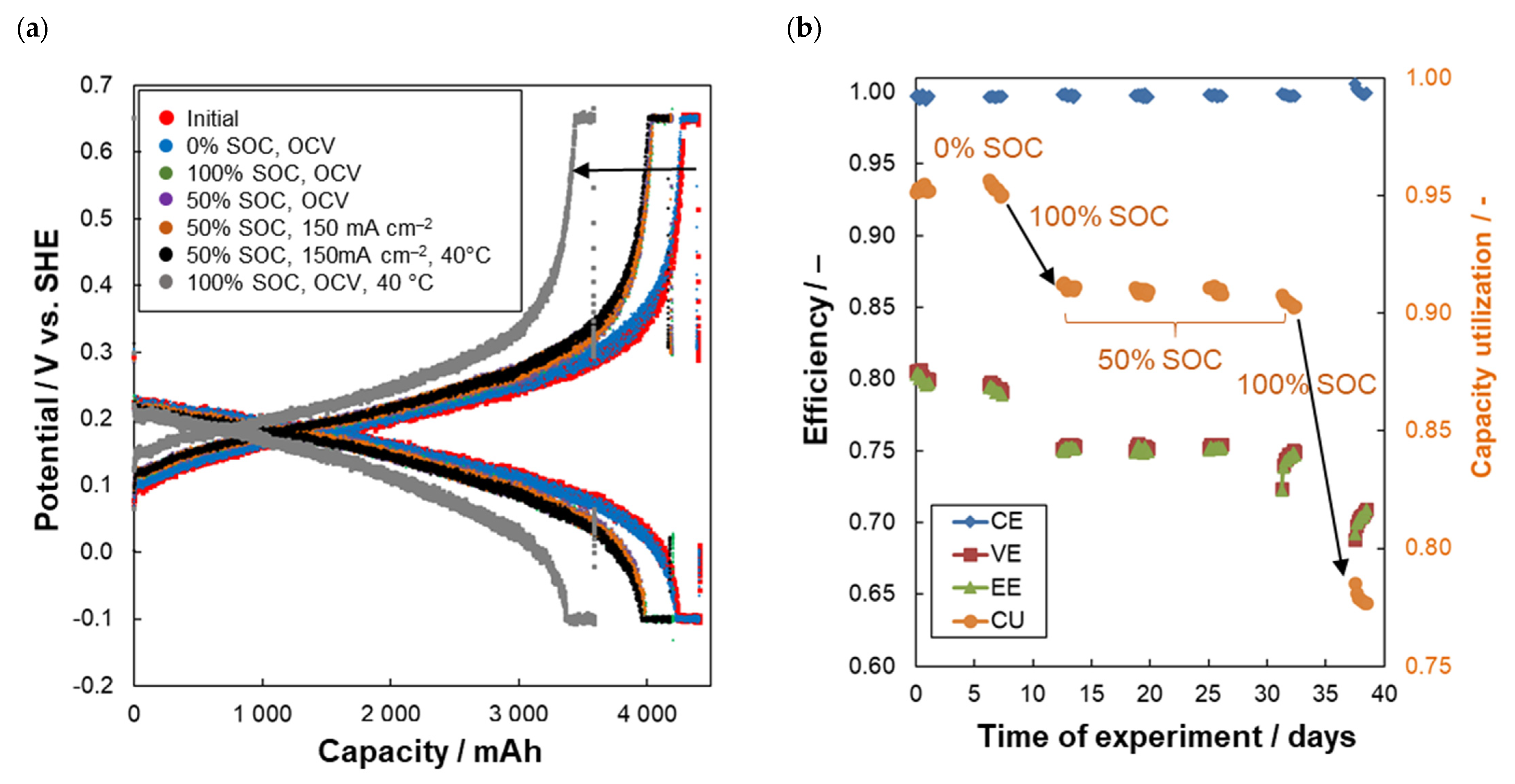
2.3. Chemical Degradation Tests
2.4. DHC Parameters Evolution
3. Materials and Methods
3.1. Preparation and Analysis of AQDS-Based Electrolytes
3.2. AQDS-Br RFB Single-Cell Tests
3.3. Evaluation of Electrochemical Stability of Sulfonation Mixture
- (a)
- Combined galvanostatic-potentiostatic (U-I) cycling of FEC: 10 reduction-oxidation cycles were performed in full SoC range of working electrolyte to assess the actual available capacity of the working electrolyte. Each cycle consisted of galvanostatic reduction/oxidation at 150 mA cm–2 until corresponding potential limit was reached (reduction limit of –0.1 V and oxidation limit of +0.65 V vs. SHE) with subsequent potentiostatic hold at the limiting potential until absolute current density decrease below 5 mA cm−2.
- (b)
- Hold for five days at required SoC of electrolyte (0, 50, or 100%) under either OCV conditions or current load of 150 mA cm−2 realized by DHC battery. Electrolyte was circulated through both cells during the hold.
- (c)
- UV–VIS characterization of counter electrolyte before and after the hold was done to assess capacity losses due to permeation of AQDS through the membrane in electrolysis cell.
- (d)
- EIS and LC characterization of FEC and DHC in 50% SoC of AQDS electrolyte was done to monitor the performance evolution of both cells during the experiment. EIS was measured under OCV conditions with 5 mV amplitude in frequency range of 10 kHz–20 mHz. Load curve was measured using current scan from 0 to 500 mA cm−2 with 5 mA cm−2 s−1 scan rate.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Acronyms | |
| AQ | Anthraquinone |
| 1-AQS | 9,10-Anhraquinone-1-sulfonic acid |
| 2- AQS | 9,10-Anhraquinone-2-sulfonic acid |
| 1,6-AQDS | 9,10-Anhraquinone-1,6-disulfonic acid |
| 1,7-AQDS | 9,10-Anhraquinone-1,7-disulfonic acid |
| 2,6-AQDS | 9,10-Anhraquinone-2,6-disulfonic acid |
| 2,7-AQDS | 9,10-Anhraquinone-2,7-disulfonic acid |
| 2,6-DHAH | 2,6-Dihydroxidy-9,10-anhraquinone |
| OCP | Open circuit potential |
| OCV | Open circuit voltage |
| CE | Coulombic efficiency |
| VE | Voltage efficiency |
| EE | Energy efficiency |
| Qdis. | Discharge capacity |
| Qtheor. | Theoretical capacity |
| CU | Capacity utilization |
| CDrel. | Relative capacity decay per cycle |
| ΔQrel. | Relative capacity decay after the hold |
| ΔQperm. | Relative capacity decay due to AQDS permeation |
| RΩ | Ohmic resistance |
| RCT | Charge transfer resistance |
| Rdis | Discharging resistance |
| SoC | State of charge |
References
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium Redox Flow Batteries: A Comprehensive Review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem. Int. Ed. 2017, 56, 686–711. [Google Scholar] [CrossRef]
- Kwabi, D.G.; Lin, K.; Ji, Y.; Kerr, E.F.; Goulet, M.-A.; De Porcellinis, D.; Tabor, D.P.; Pollack, D.A.; Aspuru-Guzik, A.; Gordon, R.G.; et al. Alkaline Quinone Flow Battery with Long Lifetime at pH 12. Joule 2018, 2, 1894–1906. [Google Scholar] [CrossRef]
- Lin, K.; Chen, Q.; Gerhardt, M.R.; Tong, L.; Kim, S.B.; Eisenach, L.; Valle, A.W.; Hardee, D.; Gordon, R.G.; Aziz, M.J.; et al. Alkaline Quinone Flow Battery. Science 2015, 349, 1529–1532. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.S.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A Metal-Free Organic–Inorganic Aqueous Flow Battery. Nat. Cell Biol. 2014, 505, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Eisenach, L.; Aziz, M.J. Cycling Analysis of a Quinone-Bromide Redox Flow Battery. J. Electrochem. Soc. 2015, 163, A5057–A5063. [Google Scholar] [CrossRef]
- Chen, Q.; Gerhardt, M.R.; Hartle, L.; Aziz, M.J. A Quinone-Bromide Flow Battery with 1 W/cm2 Power Density. J. Electrochem. Soc. 2015, 163, A5010–A5013. [Google Scholar] [CrossRef]
- Gerhardt, M.R.; Rafael, T.; Gómez, B.; Chen, Q.; P, M.; Cooper, M.; Galvin, J.; Aspuru-Guzik, A.; G, R.; J, G.M. Aziz Anthraquinone Derivatives in Aqueous Flow Batteries. Adv. Energy Mater. 2017, 7, 1601488. [Google Scholar] [CrossRef]
- Khataee, A.; Wedege, K.; Dražević, E.; Bentien, A. Differential pH as a Method for Increasing Cell Potential in Organic Aqueous Flow Batteries. J. Mater. Chem. A 2017, 5, 21875–21882. [Google Scholar] [CrossRef]
- Hu, B.; Luo, J.; Hu, M.; Yuan, B.; Liu, T.L. A pH-Neutral, Metal-Free Aqueous Organic Redox Flow Battery Employing an Ammonium Anthraquinone Anolyte. Angew. Chem. 2019, 58, 16629–16636. [Google Scholar] [CrossRef]
- Lee, W.; Permatasari, A.; Kwon, Y. Neutral pH Aqueous redox Flow Batteries Using an Anthraquinone-Ferrocyanide Redox Couple. J. Mater. Chem. C 2020, 8, 5727–5731. [Google Scholar] [CrossRef]
- Yang, B.; Murali, A.; Nirmalchandar, A.; Jayathilake, B.; Prakash, G.K.S.; Narayanan, S.R. A Durable, Inexpensive and Scalable Redox Flow Battery Based on Iron Sulfate and Anthraquinone Disulfonic Acid. J. Electrochem. Soc. 2020, 167, 060520. [Google Scholar] [CrossRef]
- Hoober-Burkhardt, L.; Krishnamoorthy, S.; Yang, B.; Murali, A.; Nirmalchandar, A.; Prakash, G.K.S.; Narayanan, S.R. A New Michael-Reaction-Resistant Benzoquinone for Aqueous Organic Redox Flow Batteries. J. Electrochem. Soc. 2017, 164, A600–A607. [Google Scholar] [CrossRef]
- Tong, L.; Chen, Q.; Wong, A.A.; Gómez-Bombarelli, R.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. UV–VIS Spectrophotometry of Quinone flow Battery Electrolyte for in Situ Monitoring and Improved Electrochemical Modeling of Potential and Quinhydrone Formation. Phys. Chem. Chem. Phys. 2017, 19, 31684–31691. [Google Scholar] [CrossRef] [PubMed]
- Carney, T.J.; Steven, J.; Collins, J.; Moore, S.; Fikile, R. Brushett Concentration-Dependent Dimerization of Anthraquinone Disulfonic Acid and Its Impact on Charge Storage. Chem. Mater. 2017, 29, 4801–4810. [Google Scholar] [CrossRef]
- Wiberg, C.; Carney, T.J.; Brushett, F.; Ahlberg, E.; Wang, E. Dimerization of 9,10-anthraquinone-2,7-Disulfonic acid (AQDS). Electrochim. Acta 2019, 317, 478–485. [Google Scholar] [CrossRef]
- Wermeckes, B.; Beck, F. Acid Catalyzed Disproportionation of Anthrahydroquinone to Anthraquinone and Anthrone. Denki Kagaku Oyobi Kogyo Butsuri Kagaku 1994, 62, 1202–1205. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Tong, L.; Pollack, D.A.; Tabor, D.P.; Odom, S.A.; Aspuru-Guzik, A.; Kwan, E.E.; Gordon, R.G.; Aziz, M.J. Extending the Lifetime of Organic Flow Batteries via Redox State Management. J. Am. Chem. Soc. 2019, 141, 8014–8019. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Aziz, M.J. Flow Battery Molecular Reactant Stability Determined by Symmetric Cell Cycling Methods. J. Electrochem. Soc. 2018, 165, A1466–A1477. [Google Scholar] [CrossRef]
- Gerhardt, M.R.; Beh, E.S.; Tong, L.; Gordon, R.G.; Aziz, M.J. Comparison of Capacity Retention Rates During Cycling of Quinone-Bromide Flow Batteries. MRS Adv. 2017, 2, 431–438. [Google Scholar] [CrossRef]
- Kwabi, D.G.; Wong, A.A.; Aziz, M.J. Rational Evaluation and Cycle Life Improvement of Quinone-Based Aqueous Flow Batteries Guided by In-Line Optical Spectrophotometry. J. Electrochem. Soc. 2018, 165, A1770–A1776. [Google Scholar] [CrossRef]
- Charvát, J.; Mazúra, P.; Dundáleka, J.; Pocedič, J.; Vrána, J.; Mrlík, J.; Kosek, J.; Dinter, S. Performance Enhancement of Vanadium Redox flow Battery by Optimized Electrode Compression and Operational Conditions. J. Energy Storage 2020, 30, 101468. [Google Scholar] [CrossRef]
- Derr, I.; Bruns, M.; Langner, J.; Fetyan, A.; Melke, J.; Roth, C. Degradation of all-Vanadium Redox flow Batteries (VRFB) Investigated by Electrochemical Impedance and X-ray Photoe-Lectron Spectroscopy: Part 2 Electrochemical Degradation. J. Power Sources 2016, 325, 351–359. [Google Scholar] [CrossRef]
- Derr, I.; Przyrembel, D.; Schweer, J.; Fetyan, A.; Langner, J.; Melke, J.; Weinelt, M.; Roth, C. Electroless Chemical Aging of Carbon Felt Electrodes for the All-Vanadium Redox Flow Battery (VRFB) Investigated by Electrochemical Impedance and X-Ray Photoelectron Spectroscopy. Electrochim. Acta 2017, 246, 783–793. [Google Scholar] [CrossRef]
- Mazur, P.; Mrlik, J.; Pocedic, J.; Vrana, J.; Dundalek, J.; Kosek, J.; Bystron, T. Effect of Graphite Felt Properties on the Long-Term Durability of Negative Electrode in Vanadium Redox Flow Battery. J. Power Sources 2019, 414, 354–365. [Google Scholar] [CrossRef]
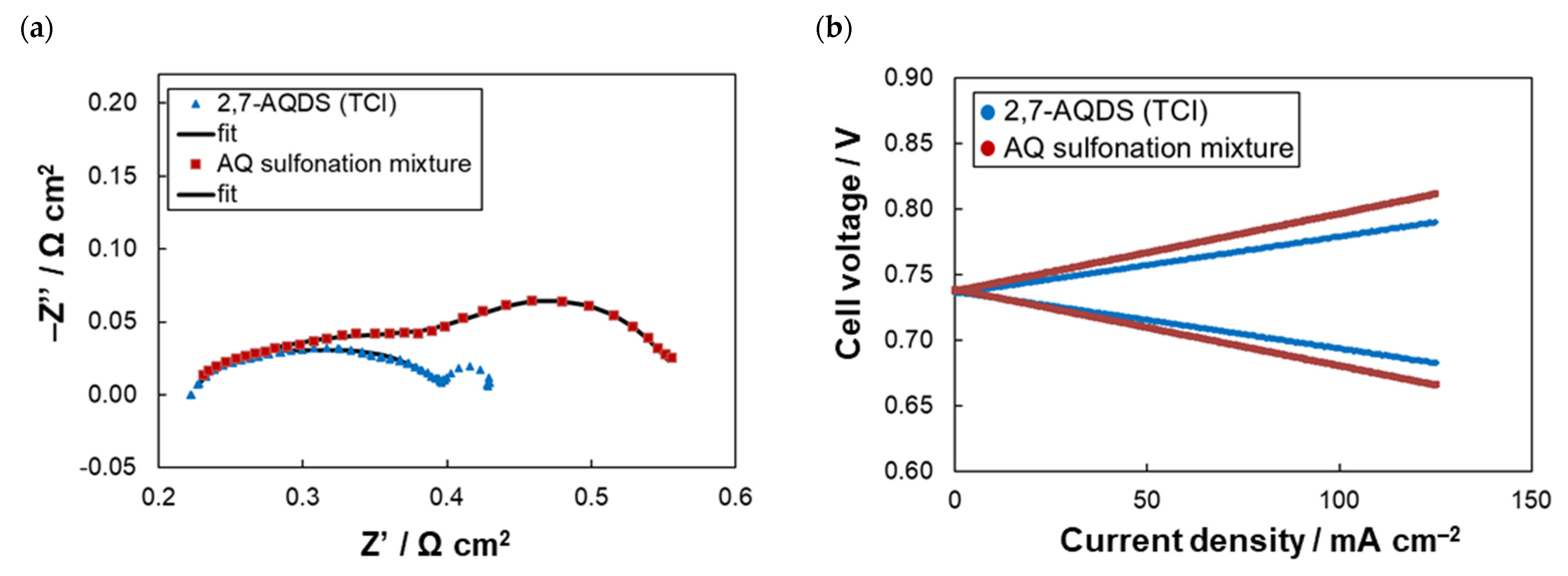
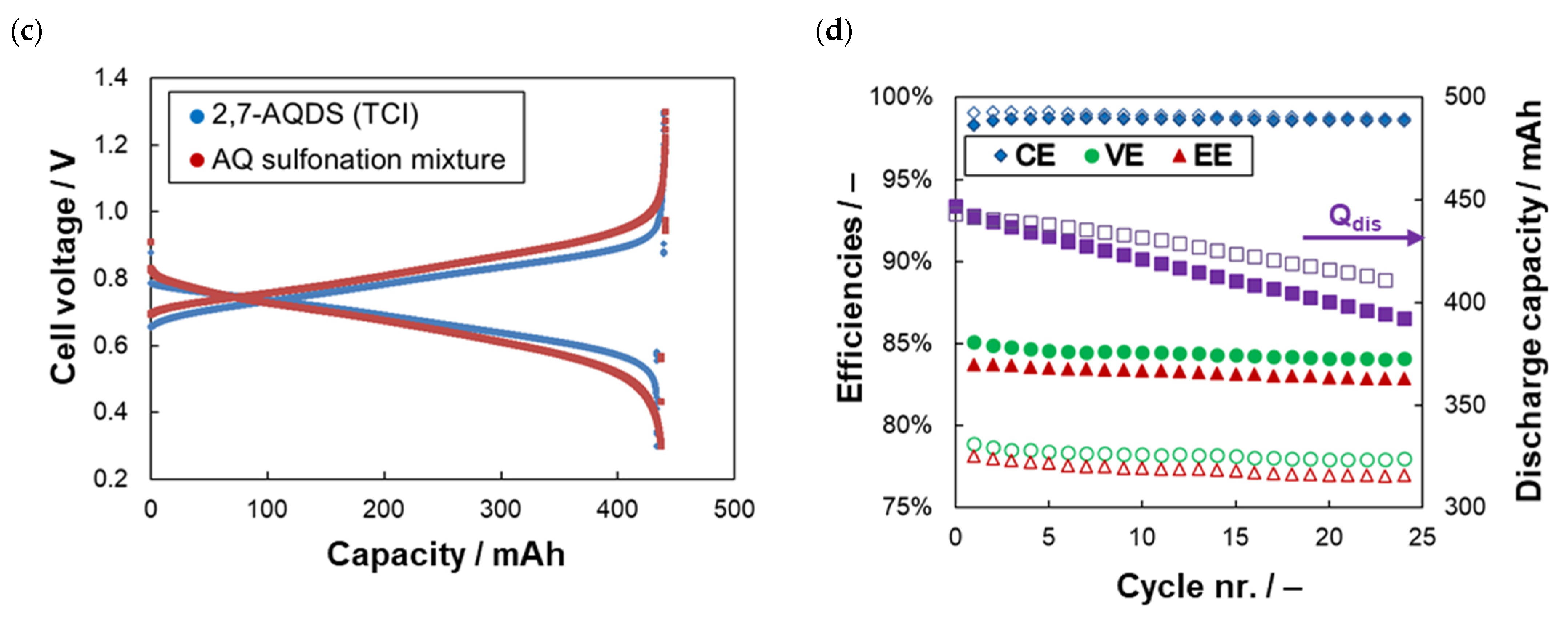


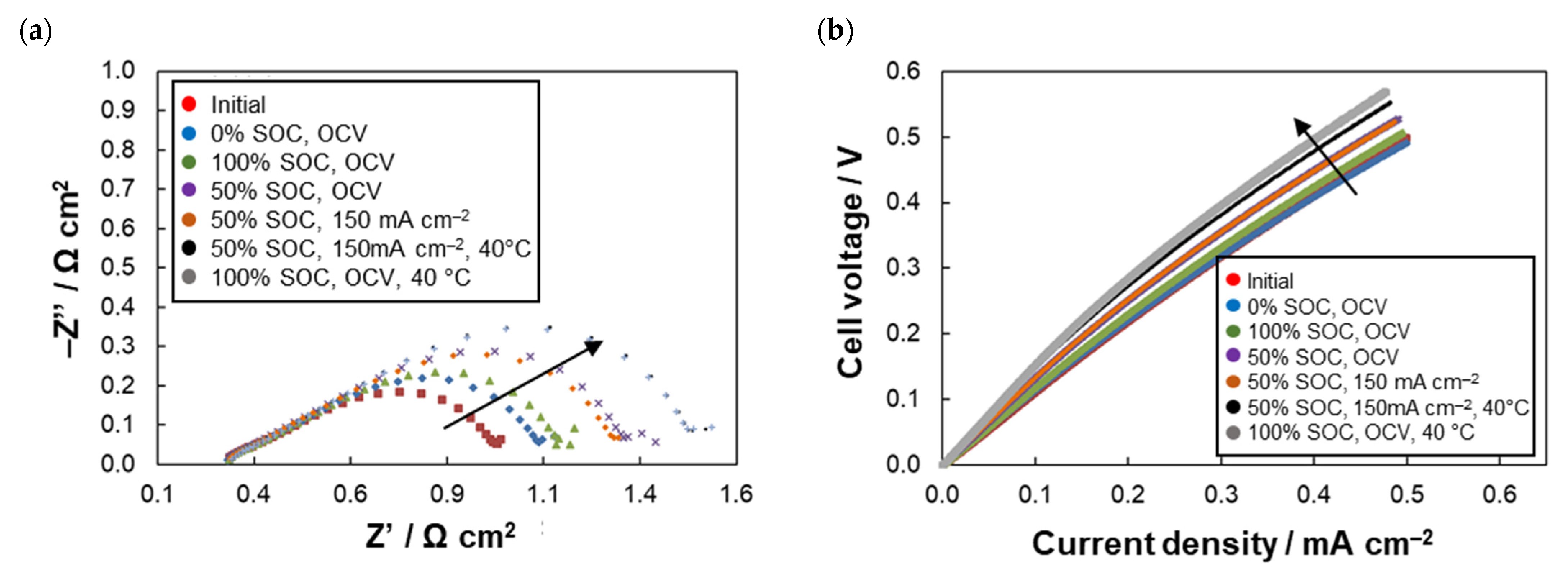
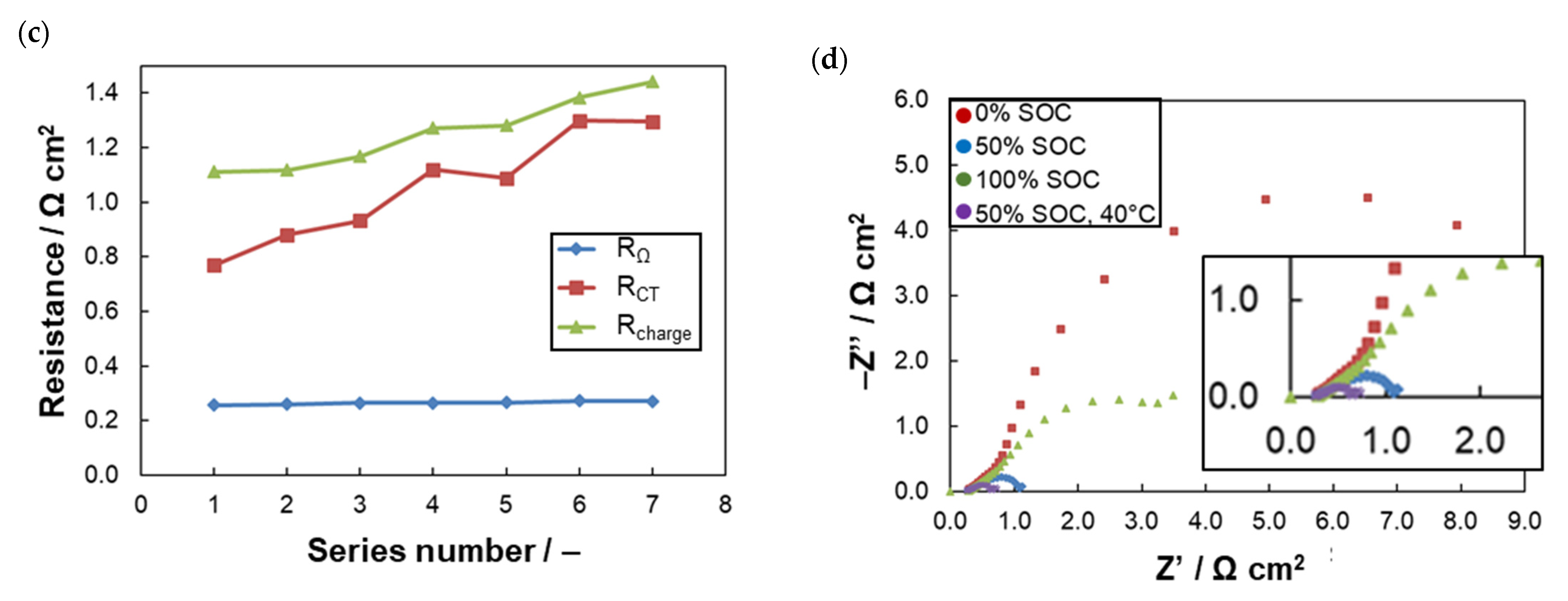
| Anolyte | RΩ(1) (Ω cm2) | RCT(1) (Ω cm2) | Rdis(2) (Ω cm2) | CE(3) (%) | VE(3) (%) | EE(3) (%) | CU(3) (%) | CD(3) (%Qtheor/Cycle) |
|---|---|---|---|---|---|---|---|---|
| 2,7-AQDS (TCI) | 0.22 | 0.18 | 0.44 | 98.6 | 84.3 | 83.2 | 97.0 | –0.45 |
| AQ sulfonation mixture | 0.20 | 0.38 | 0.58 | 98.8 | 78.1 | 77.2 | 96.1 | –0.34 |
| Series nr. | CE (%) | VE (%) | EE (%) | CU (%) | CD (%Qtheor/Cycle) | ΔQcap. (%Qtheor/hold) | ΔQperm. (%Qtheor/hold) | Conditions: |
|---|---|---|---|---|---|---|---|---|
| 1 | 99.7 | 80.0 | 79.8 | 95.5 | –0.08 | – | – | Initial |
| 2 | 99.7 | 79.3 | 79.0 | 95.7 | –0.08 | 0.17 | 0.004 | After five days at 0% SoC, OCV |
| 3 | 99.7 | 75.4 | 75.2 | 91.3 | 0.01 | –4.38 | 0.002 | After five days at 100% SoC, OCV |
| 4 | 99.7 | 75.2 | 75.0 | 91.1 | 0.00 | –0.21 | 0.002 | After five days at 50% SoC, OCV |
| 5 | 99.7 | 75.4 | 75.2 | 91.2 | 0.02 | 0.10 | 0.002 | After five days at 50% SoC, 150 mA cm–2 |
| 6 | 99.7 | 74.9 | 74.7 | 90.8 | –0.03 | –0.38 | 0.020 | After five days at 50% SoC, 150 mA cm–2, 40 °C |
| 7 | 99.9 | 70.7 | 70.6 | 78.5 | –0.10 | –12.3 | 0.023 | After five days at 100% SoC, OCV, 40 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazúr, P.; Charvát, J.; Mrlík, J.; Pocedič, J.; Akrman, J.; Kubáč, L.; Řeháková, B.; Kosek, J. Evaluation of Electrochemical Stability of Sulfonated Anthraquinone-Based Acidic Electrolyte for Redox Flow Battery Application. Molecules 2021, 26, 2484. https://doi.org/10.3390/molecules26092484
Mazúr P, Charvát J, Mrlík J, Pocedič J, Akrman J, Kubáč L, Řeháková B, Kosek J. Evaluation of Electrochemical Stability of Sulfonated Anthraquinone-Based Acidic Electrolyte for Redox Flow Battery Application. Molecules. 2021; 26(9):2484. https://doi.org/10.3390/molecules26092484
Chicago/Turabian StyleMazúr, Petr, Jiří Charvát, Jindřich Mrlík, Jaromír Pocedič, Jiří Akrman, Lubomír Kubáč, Barbora Řeháková, and Juraj Kosek. 2021. "Evaluation of Electrochemical Stability of Sulfonated Anthraquinone-Based Acidic Electrolyte for Redox Flow Battery Application" Molecules 26, no. 9: 2484. https://doi.org/10.3390/molecules26092484
APA StyleMazúr, P., Charvát, J., Mrlík, J., Pocedič, J., Akrman, J., Kubáč, L., Řeháková, B., & Kosek, J. (2021). Evaluation of Electrochemical Stability of Sulfonated Anthraquinone-Based Acidic Electrolyte for Redox Flow Battery Application. Molecules, 26(9), 2484. https://doi.org/10.3390/molecules26092484