Wound Healing Activity of α-Pinene and α-Phellandrene
Abstract
:1. Introduction
2. Results
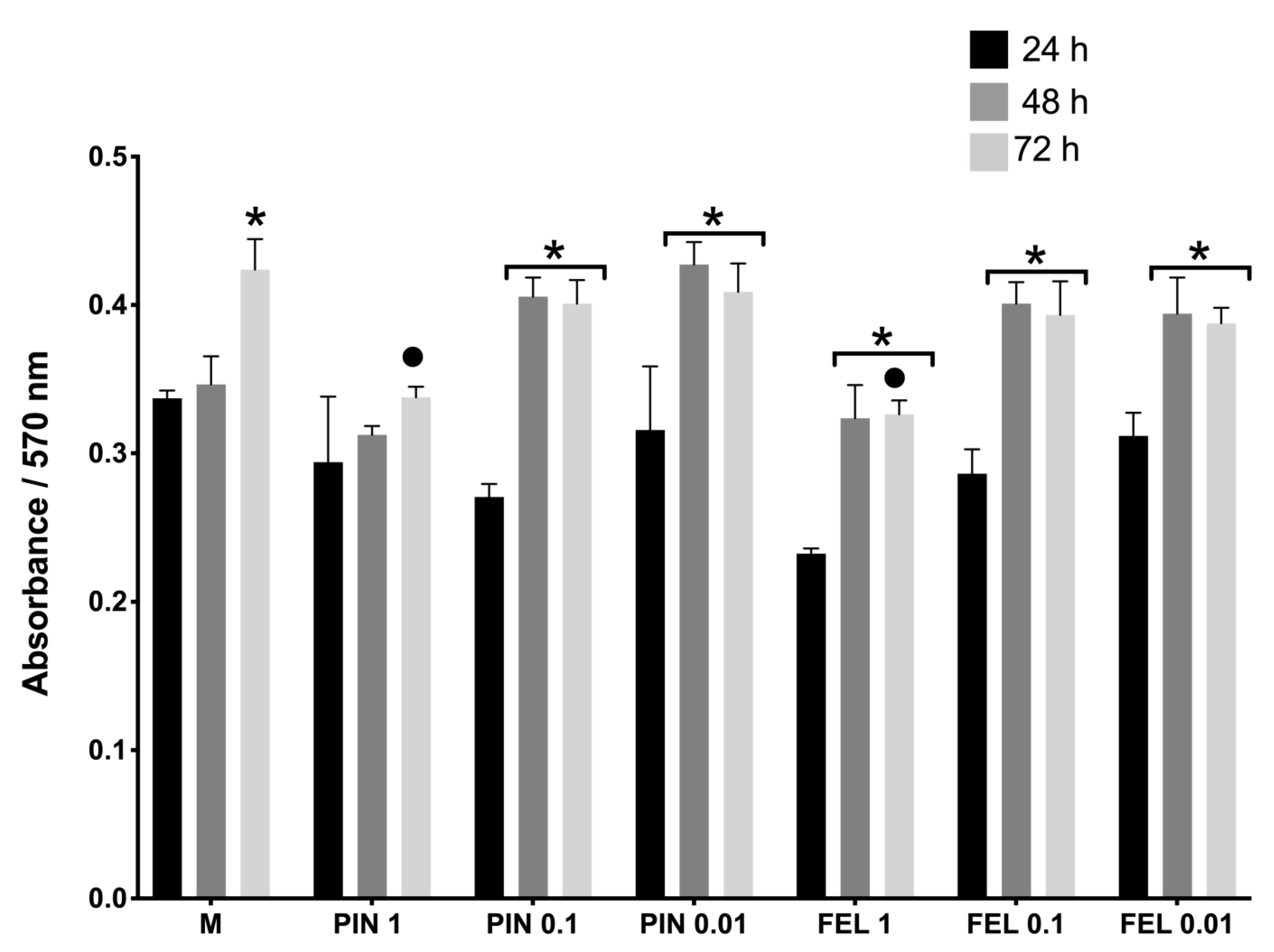
2.1. Cell Viability and Cell Proliferation Assays
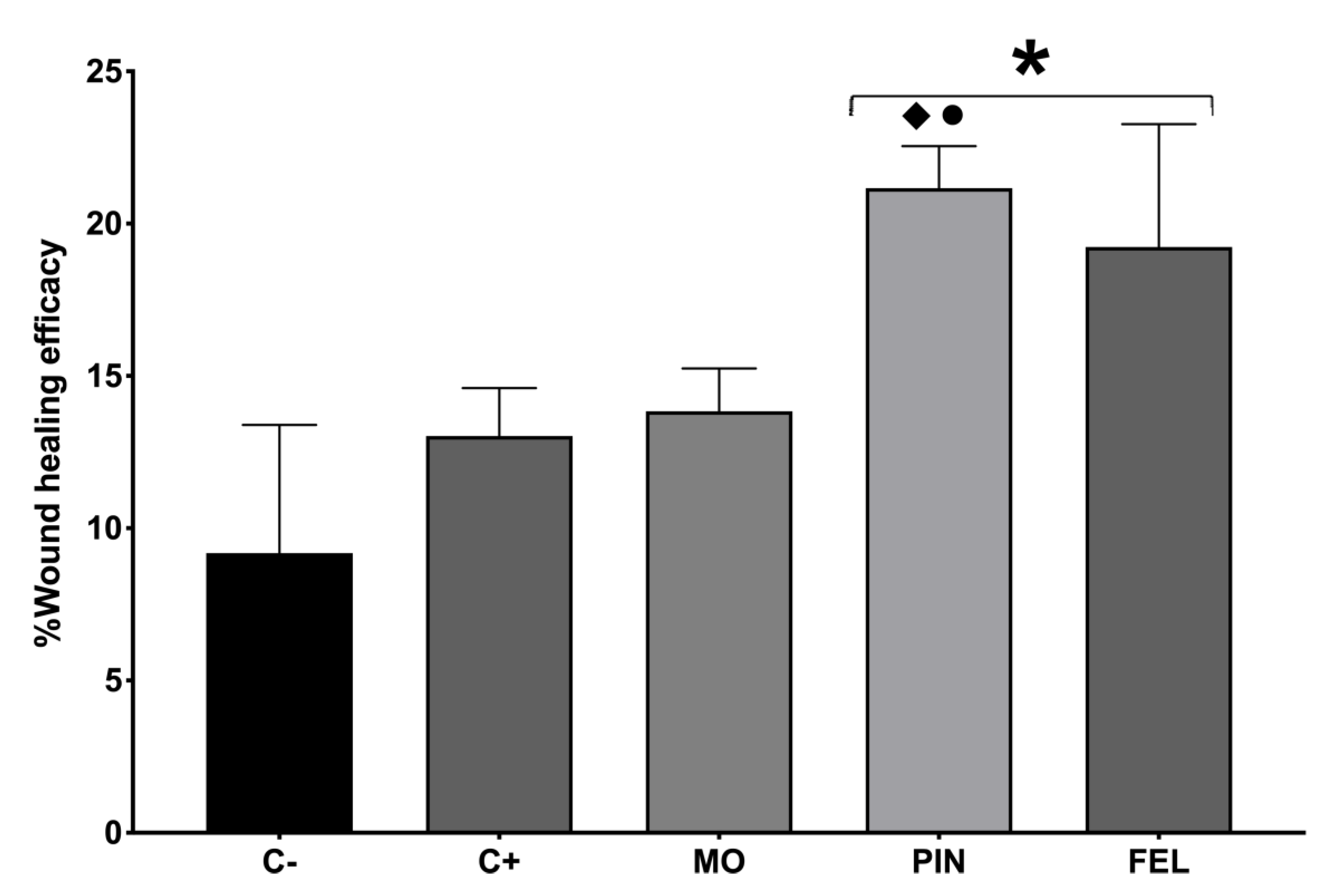
2.2. Wound Healing Efficacy (%WHE)
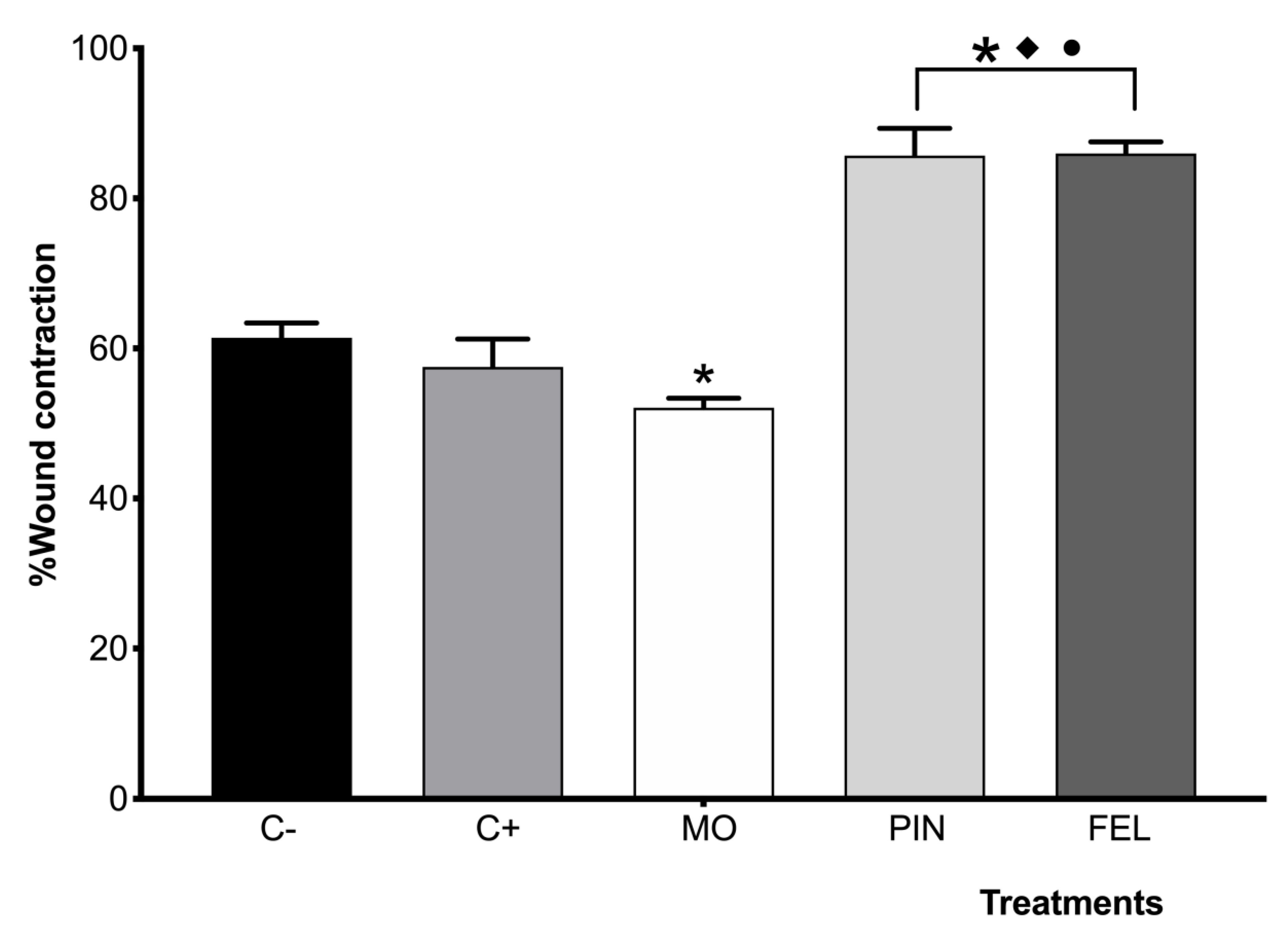
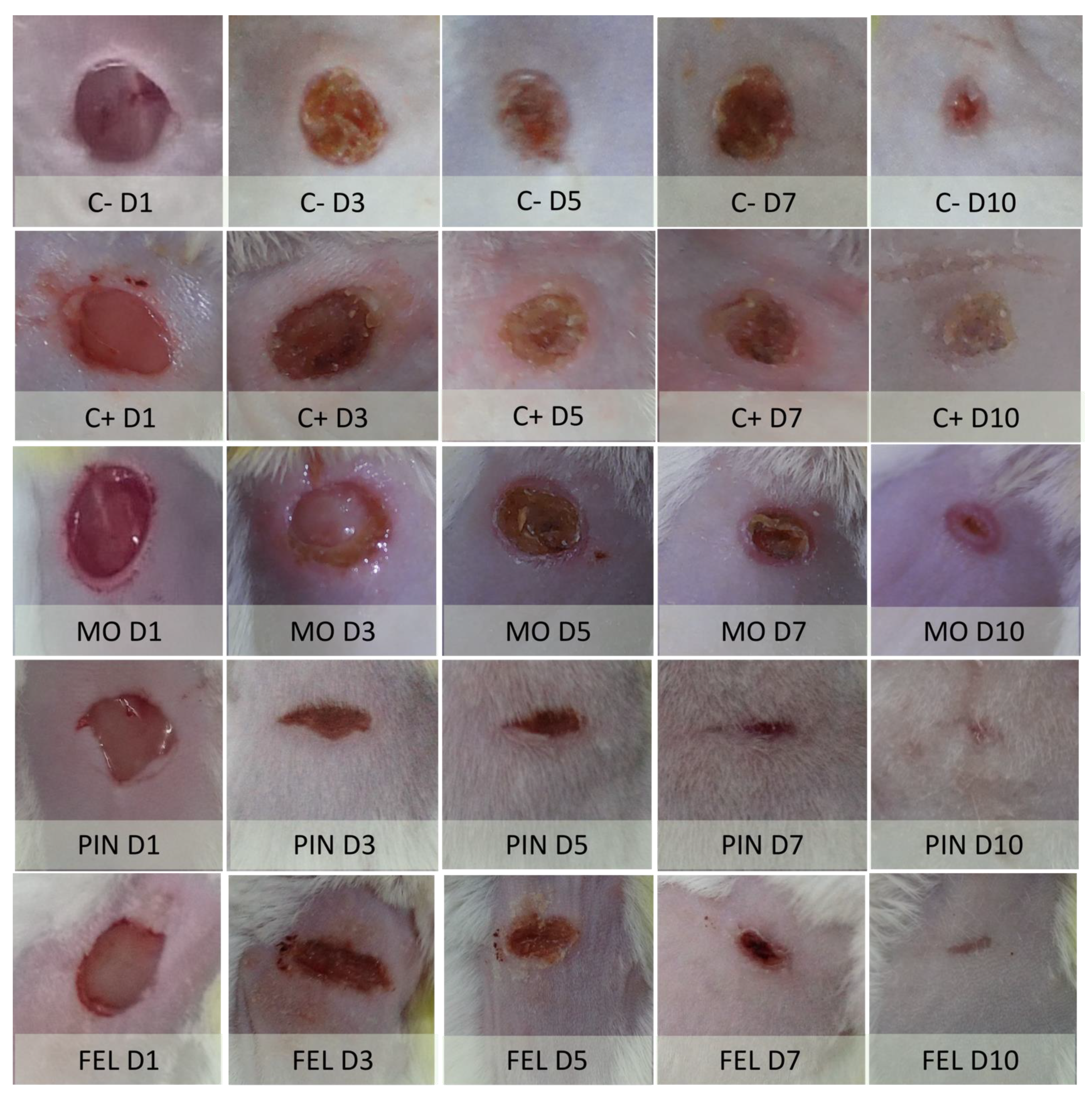
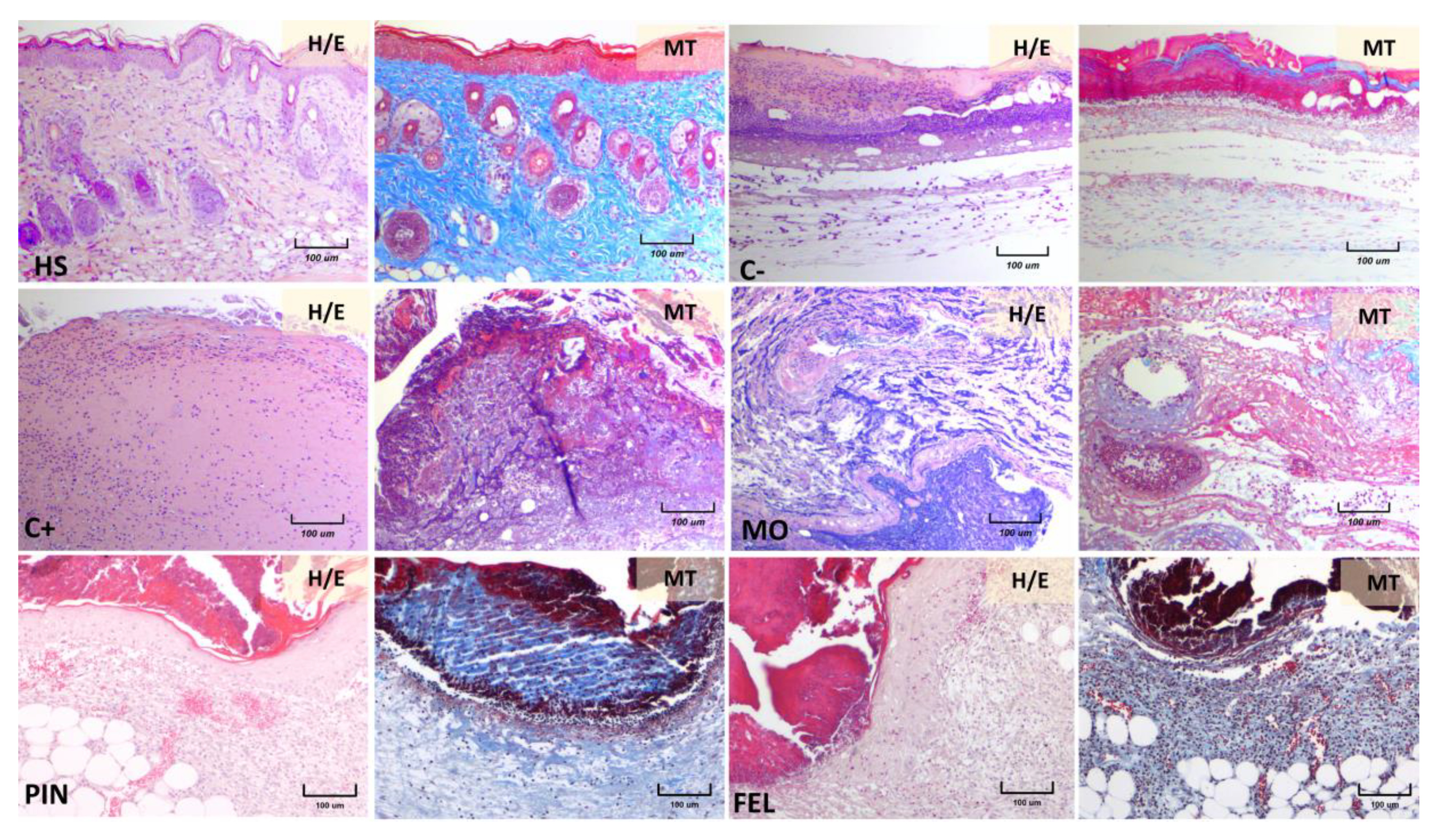
2.3. Incision Wound Model
3. Discussion
4. Materials and Methods
4.1. Isolation of Fibroblasts
4.2. Cell Viability and Proliferation
4.3. Animals
4.4. Wound Healing Efficacy
4.5. Wound Contraction Model
4.6. Histopathological Observation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Guan, X.; Ge, D.; Li, S.; Huang, K.; Liu, J.; Li, F. Chemical Composition and Antimicrobial Activities of Artemisia argyi Levl. et Vant Essential Oils Extracted by Simultaneous Distillation-Extraction, Subcritical Extraction and Hydrodistillation. Molecules 2019, 24, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.L.; Garlet, Q.I.; Benovit, S.C.; Dolci, G.; Mallmann, C.A.; Bürger, M.E.; Baldisserotto, B.; Longhi, S.J.; Heinzmann, B.M. Sedative and anesthetic activities of the essential oils of Hyptis mutabilis (Rich.) Briq. and their isolated components in silver catfish (Rhamdia quelen). Braz. J. Med. Biol Res. 2013, 46, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Lenardão, E.J.; Savegnago, L.; Jacob, R.G.; Victoria, F.N.; Martinez, D.M. Antinociceptive Effect of Essential Oils and Their Constituents: An Update Review. J. Braz. Chem. Soc. 2016, 27, 435–474. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid. Based Complementary Altern. Med. eCAM 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, F.; Curini, M.; Di Sano, C.; Zadra, C.; Gigliarelli, G.; Rascon-Valenzuela, L.A.; Robles Zepeda, R.E.; Marcotullio, M.C. Diterpenoids and Triterpenoids from the Resin of Bursera microphylla and Their Cytotoxic Activity. J. Nat. Prod. 2015, 78, 1184–1188. [Google Scholar] [CrossRef]
- Becerra, J.X. Evolution of Mexican Bursera (Burseraceae) inferred from ITS, ETS, and 5S nuclear ribosomal DNA sequences. Mol. Phylogenet Evol. 2003, 26, 300–309. [Google Scholar] [CrossRef]
- Becerra, J.X.; Venable, D.L.; Evans, P.H.; Bowers, W.S. Interactions between Chemical and Mechanical Defenses in the Plant Genus Bursera and Their Implications for Herbivores1. Am. Zool. 2001, 41, 865–876. [Google Scholar] [CrossRef]
- Evans, P.H.; Becerra, J.X.; Venable, D.L.; Bowers, W.S. Chemical Analysis of Squirt-gun Defense in Bursera and Counterdefense by Chrysomelid Beetles. J. Chem. Ecol. 2000, 26, 745–754. [Google Scholar] [CrossRef]
- Marcotullio, M.C.; Curini, M.; Becerra, J.X. An Ethnopharmacological, Phytochemical and Pharmacological Review on Lignans from Mexican Bursera spp. Molecules 2018, 23, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigliarelli, G.; Becerra, J.X.; Curini, M.; Marcotullio, M.C. Chemical Composition and Biological Activities of Fragrant Mexican Copal (Bursera spp.). Molecules 2015, 20, 22383–22394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zúñiga, B.; Guevara-Fefer, P.; Herrera, J.; Contreras, J.L.; Velasco, L.; Pérez, F.J.; Esquivel, B. Chemical composition and anti-inflammatory activity of the volatile fractions from the bark of eight Mexican Bursera species. Planta Med. 2005, 71, 825–828. [Google Scholar] [CrossRef]
- Carrera-Martínez, C.A.; Rosas-López, R.; Rodríguez-Monroy, M.A.; Canales-Martínez, M.M.; Román-Guerrero, A.; Jiménez-Alvarado, R. Chemical Composition and In vivo Anti-inflammatory Activity of Bursera morelensis Ramírez Essential Oil. J. Essent. Oil Bear. Plants 2014, 17, 758–768. [Google Scholar]
- Canales-Martinez, M.; Rivera-Yañez, C.R.; Salas-Oropeza, J.; Lopez, H.R.; Jimenez-Estrada, M.; Rosas-Lopez, R.; Duran, D.A.; Flores, C.; Hernandez, L.B.; Rodriguez-Monroy, M.A. Antimicrobial activity of Bursera morelensis Ramírez essential oil. Afr. J. Tradit. Complementary Altern. Med. AJTCAM 2017, 14, 74–82. [Google Scholar]
- Rivera-Yanez, C.R.; Terrazas, L.I.; Jimenez-Estrada, M.; Campos, J.E.; Flores-Ortiz, C.M.; Hernandez, L.B.; Cruz-Sanchez, T.; Garrido-Farina, G.I.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, alpha-Pinene and gamma-Terpinene-An In Vitro Study. Molecules 2017, 22, 2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canales, M.; Hernández, T.; Caballero, J.; Romo de Vivar, A.; Avila, G.; Duran, A.; Lira, R. Informant consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlán, Puebla, México. J. Ethnopharmacol. 2005, 97, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Wound healing activity of the essential oil of Bursera morelensis, in mice. Molecules 2020, 25, 1795. [Google Scholar] [CrossRef] [Green Version]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-caryophyllene enhances wound healing through multiple routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H.; et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Bae, G.-S.; Park, K.-C.; Choi, S.B.; Jo, I.-J.; Choi, M.-O.; Hong, S.-H.; Song, K.; Song, H.-J.; Park, S.-J. Protective effects of alpha-pinene in mice with cerulein-induced acute pancreatitis. Life Sci. 2012, 91, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, H.D.A.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Cândido, A.G.F.; Wong, D.V.T.; et al. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-pinene regulates miR-221 and induces G(2)/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 38, BSR20180980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, D.F.; Brandão, M.S.; Moura, J.B.; Leitão, J.M.R.S.; Carvalho, F.A.A.; Miúra, L.M.C.V.; Leite, J.R.S.A.; Sousa, D.P.; Almeida, F.R.C. Antinociceptive activity of the monoterpene α-phellandrene in rodents: Possible mechanisms of action. J. Pharm Pharm. 2012, 64, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Hsu, S.-C.; Lu, K.-W.; Ma, Y.-S.; Wu, C.-C.; Lu, H.-F.; Chen, J.-C.; Lin, J.-G.; Wu, P.-P.; Chung, J.-G. Alpha-phellandrene-induced apoptosis in mice leukemia WEHI-3 cells in vitro. Environ. Toxicol. 2016, 31, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Stoddart, M.J. Cell viability assays: Introduction. Methods Mol. Biol 2011, 740, 1–6. [Google Scholar]
- Sawatdee, S.; Choochuay, K.; Chanthorn, W.; Srichana, T. Evaluation of the topical spray containing Centella asiatica extract and its efficacy on excision wounds in rats. Acta Pharm. (ZagrebCroat.) 2016, 66, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, L.G.; Phillips, M.N.; Nicholson, H.; Barnett, R.; Zhang, M. Skin ligaments: Regional distribution and variation in morphology. Clin. Anat. (N.Y.) 2004, 17, 287–293. [Google Scholar] [CrossRef]
- Diegelmann, R.; Evans, M. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, B.; Ghahary, A.; Scott, P.G.; Tredget, E.E. Control of wound contraction. Basic and clinical features. Hand Clin. 2000, 16, 289–302. [Google Scholar] [CrossRef]
- Heal, C.F.; Banks, J.L.; Lepper, P.D.; Kontopantelis, E.; van Driel, M.L. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst. Rev. 2016, 11, Cd011426. [Google Scholar] [CrossRef] [Green Version]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Guillard, O.; Masson, P.; Piriou, A.; Brugier, J.C.; Courtois, P. Comparison of the anti-inflammatory activity of sodium acexamate and zinc acexamate in healing skin wounds in rabbits. Pharmacology 1987, 34, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, B.; Jackson, J.; Thaker, N.H. Neomycin residues in kidneys of orally dosed non-ruminating calves determined by high-performance liquid chromatographic and microbiological assay methods. J. Vet. Pharmacol. Ther. 1995, 18, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Salas, J.; Tello, V.; Zavaleta, M.V.A.; Villegas, L.; Salas, M.; Fernández, I.; Vaisberg, A. Actividad cicatrizante del latex de Jatropha curcas (Angiospermae: Euforbiaceae). Rev. Biol. Trop. 1994, 42, 323–326. [Google Scholar] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488. https://doi.org/10.3390/molecules26092488
Salas-Oropeza J, Jimenez-Estrada M, Perez-Torres A, Castell-Rodriguez AE, Becerril-Millan R, Rodriguez-Monroy MA, Jarquin-Yañez K, Canales-Martinez MM. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules. 2021; 26(9):2488. https://doi.org/10.3390/molecules26092488
Chicago/Turabian StyleSalas-Oropeza, Judith, Manuel Jimenez-Estrada, Armando Perez-Torres, Andres Eliu Castell-Rodriguez, Rodolfo Becerril-Millan, Marco Aurelio Rodriguez-Monroy, Katia Jarquin-Yañez, and Maria Margarita Canales-Martinez. 2021. "Wound Healing Activity of α-Pinene and α-Phellandrene" Molecules 26, no. 9: 2488. https://doi.org/10.3390/molecules26092488






