Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of UCE Conditions on the TPC of Passion Fruit
2.2. Building Models and Analyzing Statistics
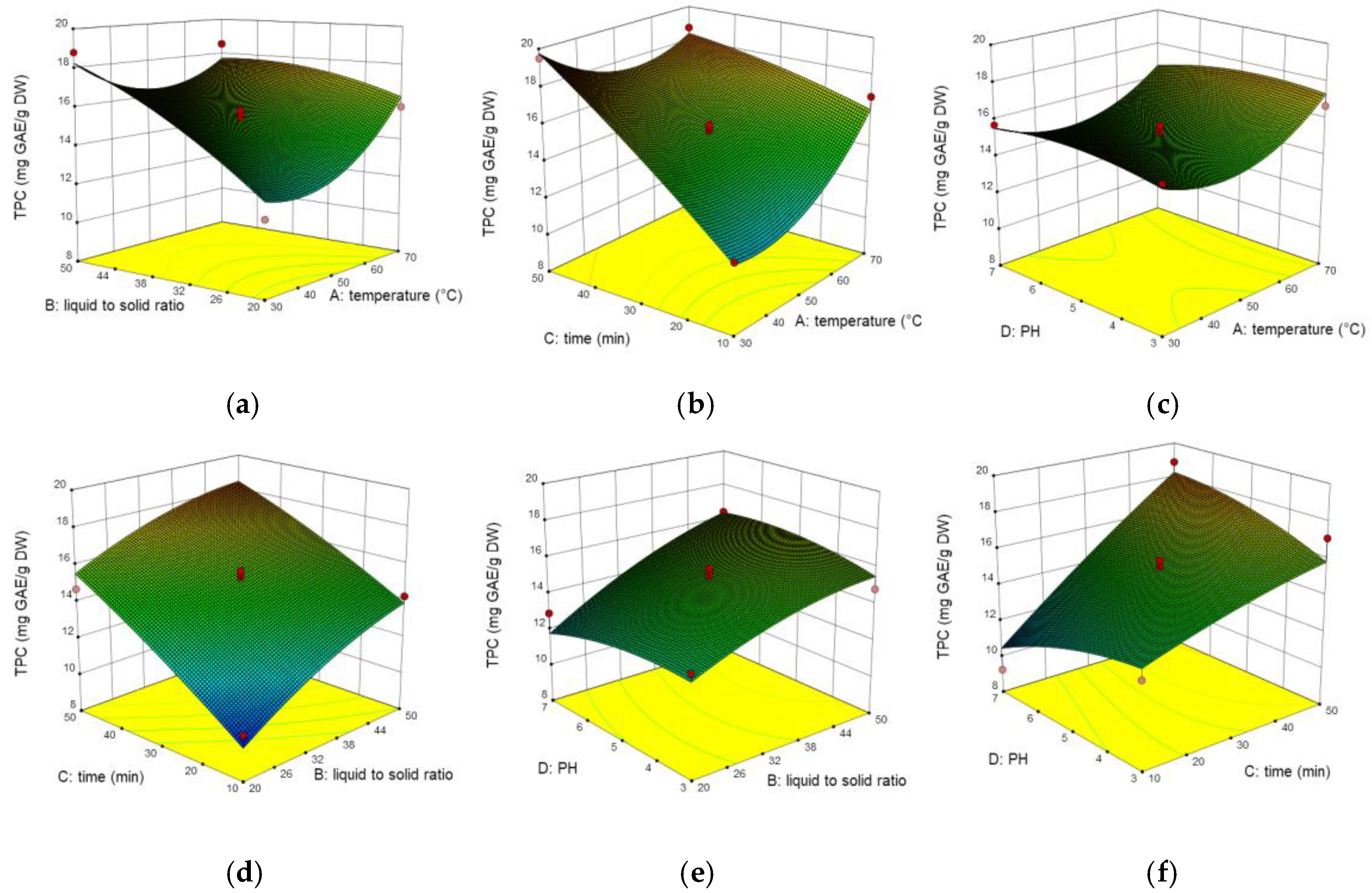
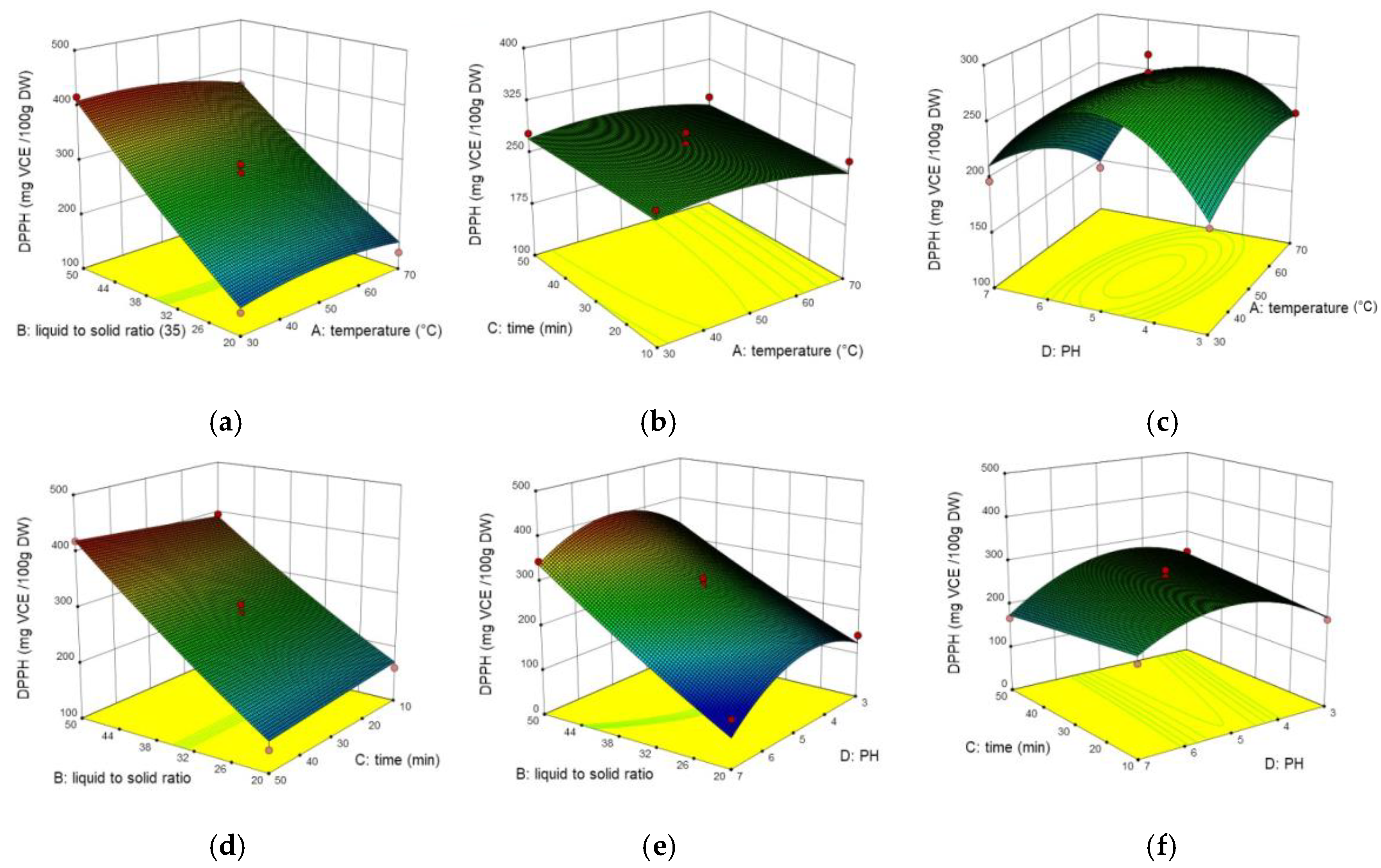
2.3. Interaction of Process Variables
2.4. Optimization and Verification of UCE
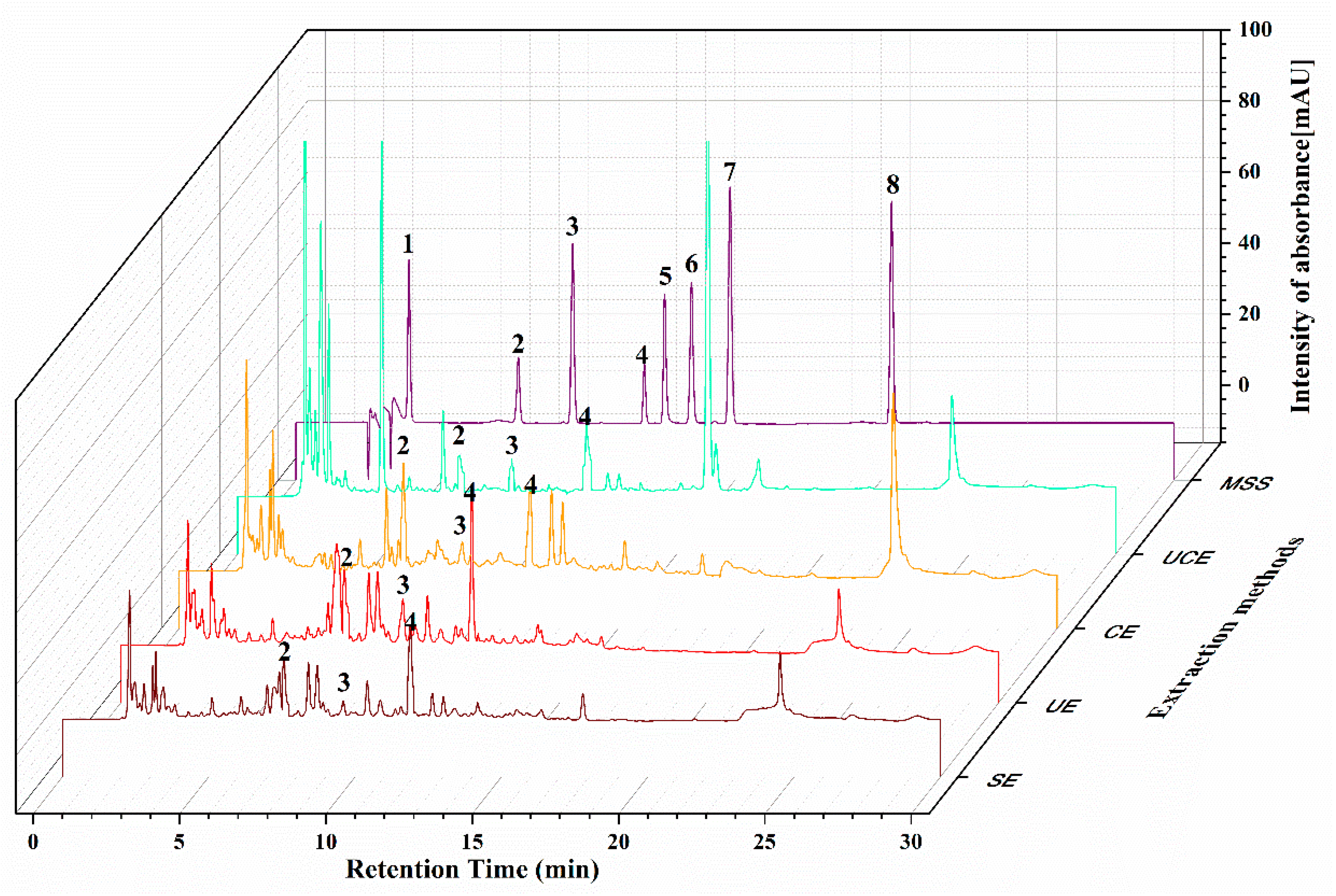
2.5. Comparison of UCE with Other Methods
2.6. HPLC Analysis
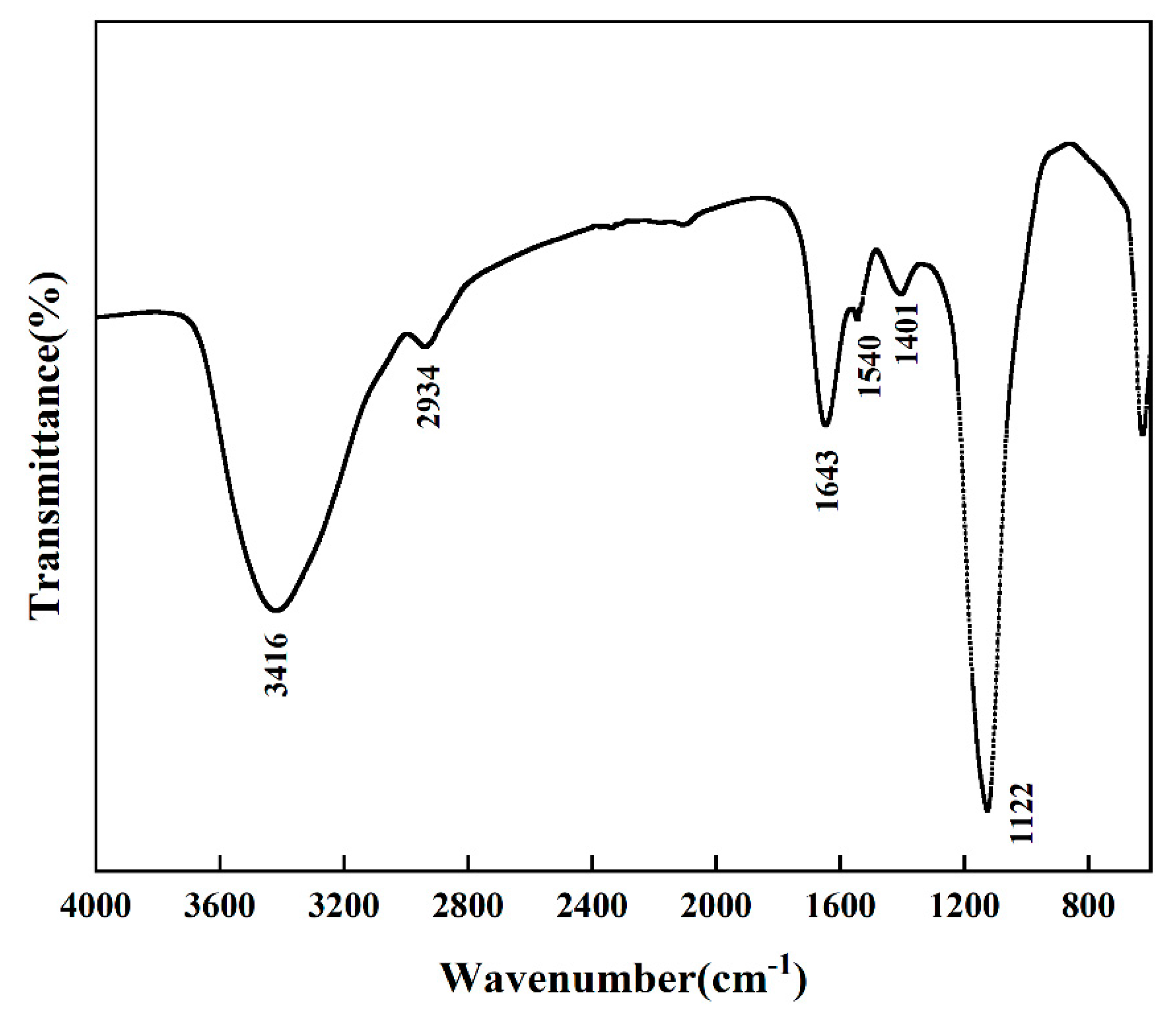
2.7. Infrared (IR) Spectroscopy
3. Materials and Methods
3.1. Experimental Materials
3.2. Chemical and Reagents
3.3. Extraction of Polyphenols from Passion Fruit
3.3.1. Cellulase-Assisted Extraction
3.3.2. Ultrasound Extraction
3.3.3. Soxhlet Extraction
3.3.4. Cellulase Extraction
3.4. Determination of Total Polyphenols Content
3.5. Determination of Antioxidant Capacity
3.5.1. DPPH Radical Scavenging Assay
3.5.2. ABTS Radical Scavenging Assay
3.5.3. Single-Factor Experiments
3.5.4. Response Surface Methodology Experiments
3.6. HPLC Analysis
3.7. Infrared Spectroscopy (IR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Da Silva, J.K.; Cazarin, C.B.B.; Colomeu, T.C.; Batista, Â.G.; Meletti, L.M.M.; Paschoal, J.A.R.; Bogusz Júnior, S.; Furlan, M.F.; Reyes, F.G.R.; Augusto, F.; et al. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: In vitro and in vivo study. Food Res. Int. 2013, 53, 882–890. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC–ESI(–)–MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Angonese, M.; Gomes, C.; Ferreira, S.R.S. Valorization of passion fruit (Passiflora edulis sp.) by-products: Sustainable recovery and biological activities. J. Supercrit. Fluids 2016, 111, 55–62. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trends Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Reunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Do Socorro Fernandes Marques, S.; Libonati, R.M.F.; Sabaa-Srur, A.U.O.; Luo, R.; Shejwalkar, P.; Hara, K.; Dobbs, T.; Smith, R.E. Evaluation of the effects of passion fruit peel flour (Passiflora edulis fo. flavicarpa) on metabolic changes in HIV patients with lipodystrophy syndrome secondary to antiretroviral therapy. Rev. Bras. Farmacogn. 2016, 26, 420–426. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Chapter 28—Passion fruit seed: Its antioxidative extracts and potency in protection of skin aging. In Aging, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 283–288. [Google Scholar]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Sharmila, G.; Nikitha, V.S.; Ilaiyarasi, S.; Dhivya, K.; Rajasekar, V.; Kumar, N.M.; Muthukumaran, K.; Muthukumaran, C. Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2016, 84, 13–21. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, H.; Zhao, J.; Zhang, H.; Chen, S. Enzyme-assisted extraction of a cup plant (Silphium perfoliatum L.) Polysaccharide and its antioxidant and hypoglycemic activities. Process. Biochem. 2020, 92, 17–28. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Ultrasound assisted intensification of enzyme activity and its properties: A mini-review. World J. Microbiol. Biotechnol. 2017, 33, 170. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J. Mol. Catal. B Enzym. 2014, 101, 108–114. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M. Optimization study on extraction of antioxidants from plum seeds (Prunus domestica L.). Optim. Eng. 2020, 22, 141–158. [Google Scholar] [CrossRef]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M. Optimization of ultrasound-assisted extraction of polyphenols from wheatgrass (Triticum aestivum L.). J. Food Sci. Technol. 2020, 57, 2809–2818. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization ultrasound-assisted deep eutectic solvent extraction of anthocyanins from raspberry using response surface methodology coupled with genetic algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef]
- Lihuan, Y.; Jiangyan, D. Optimization of Enzyme-assisted Extraction Technology for Tartary Buckwheat Shell Procyanidins with Response Surface Methodology. Agric. Sci. Technol. 2017, 18, 1196–1201. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J.; Wang, Y.; Li, X.; Zhou, H.; Zhu, L. Optimization of ultrasonic-assisted extraction of wedelolactone and antioxidant polyphenols from Eclipta prostrate L using response surface methodology. Sep. Purif. Technol. 2014, 138, 55–64. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, X.; Li, P.; Zhang, J.; Wang, S.; Ma, H. Effects of low intensity ultrasound on cellulase pretreatment. Bioresour. Technol. 2012, 117, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Şener, N.; Kılıç Apar, D.; Özbek, B. A modelling study on milk lactose hydrolysis and β-galactosidase stability under sonication. Process. Biochem. 2006, 41, 1493–1500. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Curko, N.; Tomasevic, M.; Kovacevic Ganic, K.; Radojcic Redovnikovic, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Vigano, J.; Aguiar, A.C.; Moraes, D.R.; Jara, J.L.P.; Eberlin, M.N.; Cazarin, C.B.B.; Marostica, M.R.J.; Martinez, J. Sequential high pressure extractions applied to recover piceatannol and scirpusin B from passion fruit bagasse. Food Res. Int. 2016, 85, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Laura, G.; Andree, Á.; Elizabeth, M.; Carlos, G.; Jonh, M. Potential Uses of the Peel and Seed of Passiflora Edulis Sims F. Edulis (Gulupa) from Its Chemical Characterization, Antioxidant, and Antihypertensive Functionalities. Asian J. Pharm. Clin. Res. 2019, 104–112. [Google Scholar] [CrossRef]
- Zeraik, M.L.; Serteyn, D.; Deby-Dupont, G.; Wauters, J.-N.; Tits, M.; Yariwake, J.H.; Angenot, L.; Franck, T. Evaluation of the antioxidant activity of passion fruit (Passiflora edulis and Passiflora alata) extracts on stimulated neutrophils and myeloperoxidase activity assays. Food Chem. 2011, 128, 259–265. [Google Scholar] [CrossRef]
- Costa, G.M.; Cárdenas, P.A.; Gazola, A.C.; Aragón, D.M.; Castellanos, L.; Reginatto, F.H.; Ramos, F.A.; Schenkel, E.P. Isolation of C-glycosylflavonoids with α-glucosidase inhibitory activity from Passiflora bogotensis Benth by gradient high-speed counter-current chromatography. J. Chromatogr. B 2015, 990, 104–110. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Silva, T.O.M.; Barros, R.G.C.; Oliveira, C.S.d.; Cunha, G.C.; Gualberto, N.C.; Rajan, M.; Narain, N. Enzymatic and ultrasonic-assisted pretreatment in the extraction of bioactive compounds from Monguba (Pachira aquatic Aubl) leaf, bark and seed. Food Res. Int. 2021, 140, 109869. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; De Luca, M.P.; Ribeiro, T.G.; Castilho, R.O.; Moreira, A.N.; Santos, V.R.; Faraco, A.A.G. Propolis-based chitosan varnish: Drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complem. Altern. Med. 2014, 14, 478. [Google Scholar] [CrossRef]
- Sharaf, S.; Higazy, A.; Hebeish, A. Propolis induced antibacterial activity and other technical properties of cotton textiles. Int. J. Biol. Macromol. 2013, 59, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.D.; Passos, T.M.; Quilty, B.; Thiré, R.M.d.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Felhi, S.; Hajlaoui, H.; Ncir, M.; Bakari, S.; Ktari, N.; Saoudi, M.; Gharsallah, N.; Kadri, A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016, 36, 646–655. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1664. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]


| Run | X1 (Temperature, °C) | X2 (Liquid-to-Solid Ratio, mL/g) | X3 (Ultrasound Time, Min) | X4 (pH) | Y1 (TPC Value, mg GAE/g DW) | DPPH (mg VCE/100 g DW) | ABTS (mg VCE/100 g DW) |
|---|---|---|---|---|---|---|---|
| 1 | 70 | 35 | 10 | 5 | 16.85 | 269.65 | 285.85 |
| 2 | 50 | 50 | 30 | 3 | 14.89 | 326.20 | 380.78 |
| 3 | 50 | 20 | 30 | 3 | 13.43 | 146.38 | 155.37 |
| 4 | 50 | 35 | 10 | 3 | 12.68 | 198.83 | 208.63 |
| 5 | 50 | 50 | 10 | 5 | 14.31 | 397.26 | 446.66 |
| 6 | 50 | 50 | 50 | 5 | 17.37 | 420.14 | 450.09 |
| 7 | 50 | 20 | 30 | 7 | 12.99 | 105.62 | 142.26 |
| 8 | 50 | 50 | 30 | 7 | 16.44 | 347.28 | 441.23 |
| 9 | 30 | 35 | 30 | 7 | 15.79 | 198.84 | 375.39 |
| 10 | 50 | 35 | 50 | 7 | 18.90 | 170.17 | 240.24 |
| 11 | 30 | 35 | 30 | 3 | 15.59 | 192.73 | 232.13 |
| 12 | 50 | 35 | 30 | 5 | 15.17 | 279.43 | 293.03 |
| 13 | 50 | 35 | 30 | 5 | 15.73 | 279.86 | 291.96 |
| 14 | 70 | 35 | 30 | 7 | 16.06 | 144.10 | 217.17 |
| 15 | 70 | 35 | 50 | 5 | 18.78 | 270.27 | 205.42 |
| 16 | 50 | 35 | 30 | 5 | 15.55 | 296.01 | 348.90 |
| 17 | 30 | 35 | 50 | 5 | 19.51 | 281.17 | 294.26 |
| 18 | 50 | 35 | 50 | 3 | 17.16 | 253.69 | 286.53 |
| 19 | 30 | 20 | 30 | 5 | 11.77 | 140.17 | 187.27 |
| 20 | 70 | 35 | 30 | 3 | 16.74 | 228.39 | 256.69 |
| 21 | 70 | 50 | 30 | 5 | 18.57 | 371.62 | 387.55 |
| 22 | 30 | 50 | 30 | 5 | 18.80 | 417.74 | 433.85 |
| 23 | 30 | 35 | 10 | 5 | 11.78 | 281.93 | 294.80 |
| 24 | 50 | 20 | 10 | 5 | 10.37 | 162.70 | 191.89 |
| 25 | 50 | 20 | 50 | 5 | 14.77 | 139.45 | 157.33 |
| 26 | 70 | 20 | 30 | 5 | 15.78 | 130.87 | 143.73 |
| 27 | 50 | 35 | 30 | 5 | 14.22 | 262.98 | 298.08 |
| 28 | 50 | 35 | 30 | 5 | 15.37 | 276.85 | 302.22 |
| 29 | 50 | 35 | 10 | 7 | 9.28 | 198.63 | 359.82 |
| TPC | DPPH | ABTS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient Estimate | F Value | p Value | Coefficient Estimate | F Value | p Value | Coefficient Estimate | F Value | p Value | |
| intercept | |||||||||
| X0 | 15.21 | 279.03 | 306.84 | ||||||
| linear | |||||||||
| X1 | 0.80 | 8.01 | 0.0133 * | −8.14 | 2.33 | 0.1494 | −26.77 | 24.65 | 0.0002 *** |
| X2 | 1.77 | 39.83 | <0.0001 *** | 121.25 | 516.20 | <0.0001 *** | 130.19 | 582.74 | <0.0001 *** |
| X3 | 2.60 | 85.82 | <0.0001 *** | 2.16 | 0.16 | 0.6927 | −12.82 | 5.65 | 0.0323 * |
| X4 | −0.086 | 0.7644 | −15.13 | −15.13 | 8.04 | 0.0132 * | 21.33 | 15.64 | 0.0014 ** |
| Quadratic | |||||||||
| X12 | 1.54 | 16.25 | 0.0012 ** | −14.99 | 4.26 | 0.058 | −21.44 | 8.55 | 0.0111 * |
| X22 | −0.53 | 1.9 | 0.1897 | 7.26 | 1.00 | 0.3343 | 4.04 | 0.30 | 0.5901 |
| X32 | −0.24 | 0.4 | 0.5354 | −0.44 | 3.56 × 10−3 | 0.9533 | −8.02 | 1.20 | 0.2928 |
| X42 | −0.47 | 1.51 | 0.2398 | −67.07 | 85.36 | <0.0001 *** | −23.68 | 10.42 | 0.0061 ** |
| Crossproduct | |||||||||
| X12 | −1.06 | 4.75 | 0.0469 * | −9.21 | 0.99 | 0.3362 | −0.69 | 5.45 × 10−3 | 0.9422 |
| X13 | −1.45 | 8.89 | 0.0099 ** | 0.34 | 1.39 × 10−3 | 0.9708 | −19.97 | 4.57 | 0.0506 |
| X14 | −0.22 | 0.2 | 0.658 | −22.60 | 5.98 | 0.0283 * | −45.7 | 23.93 | 0.0002 *** |
| X23 | −0.34 | 0.47 | 0.5023 | 11.53 | 1.55 | 0.2330 | 9.5 | 1.03 | 0.3265 |
| X24 | 0.50 | 1.05 | 0.3238 | 15.46 | 2.80 | 0.1167 | 18.39 | 3.88 | 0.0691 |
| X34 | 1.28 | 6.98 | 0.0193 * | −20.83 | 5.08 | 0.0408 * | −49.37 | 27.93 | 0.0001 *** |
| model | 9.26 | <0.001 *** | 45.64 | <0.001 *** | 50.61 | <0.001 *** | |||
| lack of fit | 3.4 | 0.1245 | 3.07 | 0.1454 | 0.46 | 0.856 | |||
| R2 | 0.928 | 0.979 | 0.981 | ||||||
| Extracting Method | Temperature (°C) | Liquid-to-Solid Ratio (mL/g) | Time (Min) | The Total Polyphenols Content (mg GAE/g DW) | DPPH (mg VCE/100 g DW) | ABTS (mg VCE/100 g DW) |
|---|---|---|---|---|---|---|
| UCE | 30 | 50 | 47 | 21.03 | 420.50 | 453.83 |
| UE | 30 | 50 | 47 | 15.11 | 386.75 | 362.63 |
| CE | 30 | 50 | 47 | 10.08 | 250.85 | 225.86 |
| SE | 95 | 50 | 180 | 8.02 | 196.51 | 188.29 |
| Polyphenol Compound | Retention Time | Regression Equation | R2 | Extraction Method (μg/g; Dry Material) | |||
|---|---|---|---|---|---|---|---|
| UCE | UE | CE | SE | ||||
| Chlorogenic acid | 7.60 | y = 27.208x − 53.724 | 0.9991 | 150.2 | 164.7 | 175.6 | 152.7 |
| Caffeic Acid | 9.46 | y = 60.199x − 9.9358 | 0.9998 | 9.8 | 12.2 | 14.2 | 10.4 |
| Rutin | 11.95 | y = 9.0995x + 6.5236 | 0.9997 | 187.5 | 1.4 | 49.1 | 68.1 |
| Levels | Temperature | Liquid-to-Solid Ratio | Ultrasound Time | pH |
|---|---|---|---|---|
| −1 | 30 | 20 | 10 | 3 |
| 0 | 50 | 35 | 30 | 5 |
| 1 | 70 | 50 | 50 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Gao, Y.-T.; Wei, J.-W.; Chen, Y.-F.; Liu, Q.-L.; Liu, H.-M. Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit. Molecules 2021, 26, 2494. https://doi.org/10.3390/molecules26092494
Wang W, Gao Y-T, Wei J-W, Chen Y-F, Liu Q-L, Liu H-M. Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit. Molecules. 2021; 26(9):2494. https://doi.org/10.3390/molecules26092494
Chicago/Turabian StyleWang, Wei, Yu-Ting Gao, Ji-Wen Wei, Yin-Feng Chen, Qing-Lei Liu, and Hui-Min Liu. 2021. "Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit" Molecules 26, no. 9: 2494. https://doi.org/10.3390/molecules26092494
APA StyleWang, W., Gao, Y.-T., Wei, J.-W., Chen, Y.-F., Liu, Q.-L., & Liu, H.-M. (2021). Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit. Molecules, 26(9), 2494. https://doi.org/10.3390/molecules26092494






