Redox and Antioxidant Modulation of Circadian Rhythms: Effects of Nitroxyl, N-Acetylcysteine and Glutathione
Abstract
:1. Introduction
2. Results
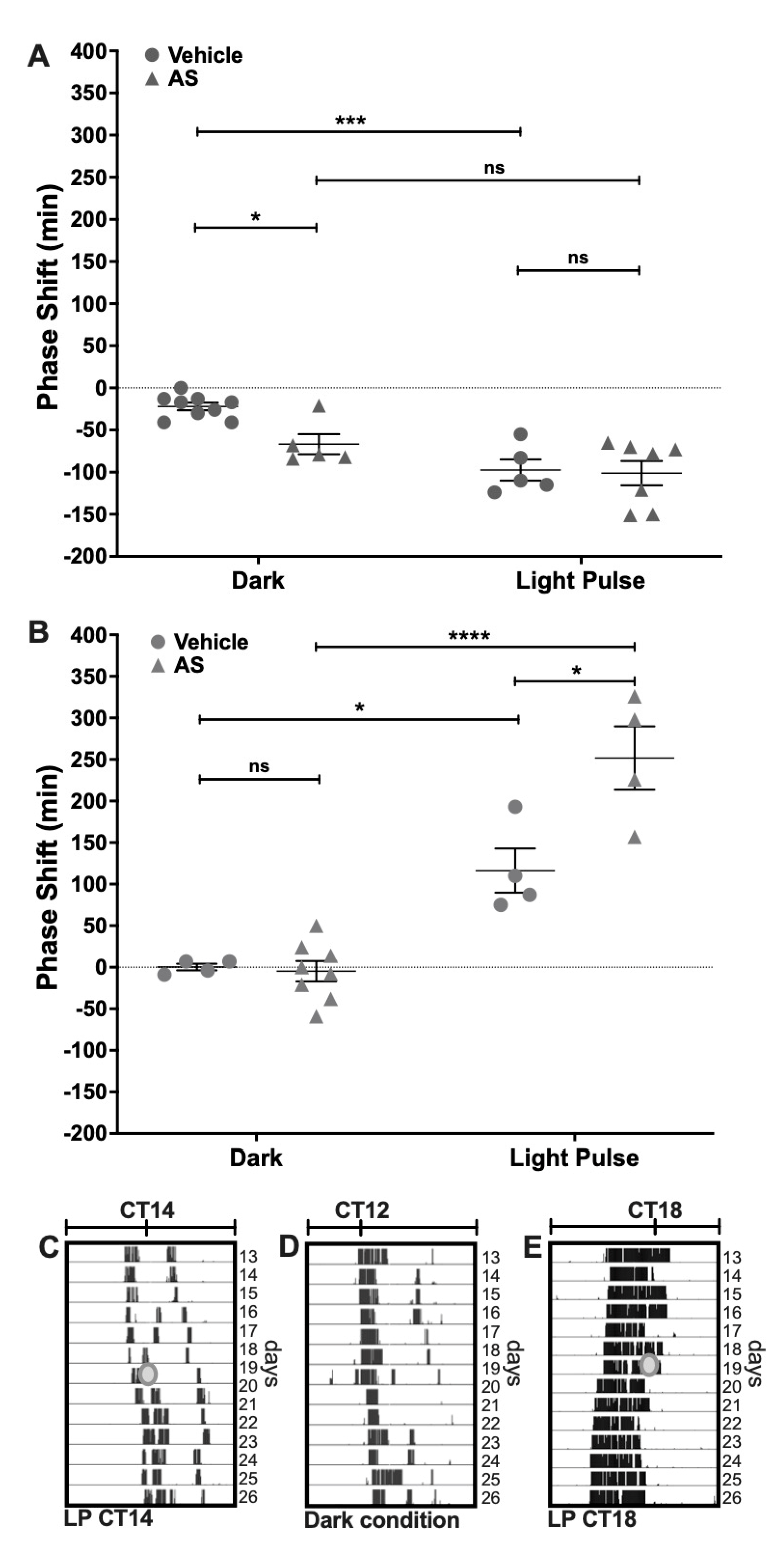
2.1. Effect of the Nitroxyl Donor Angeli’s Salt on Phase-Shifts of Locomotor Rhythms
2.2. Electrochemical Detection of Nitroxyl (HNO) In Vivo at the SCN
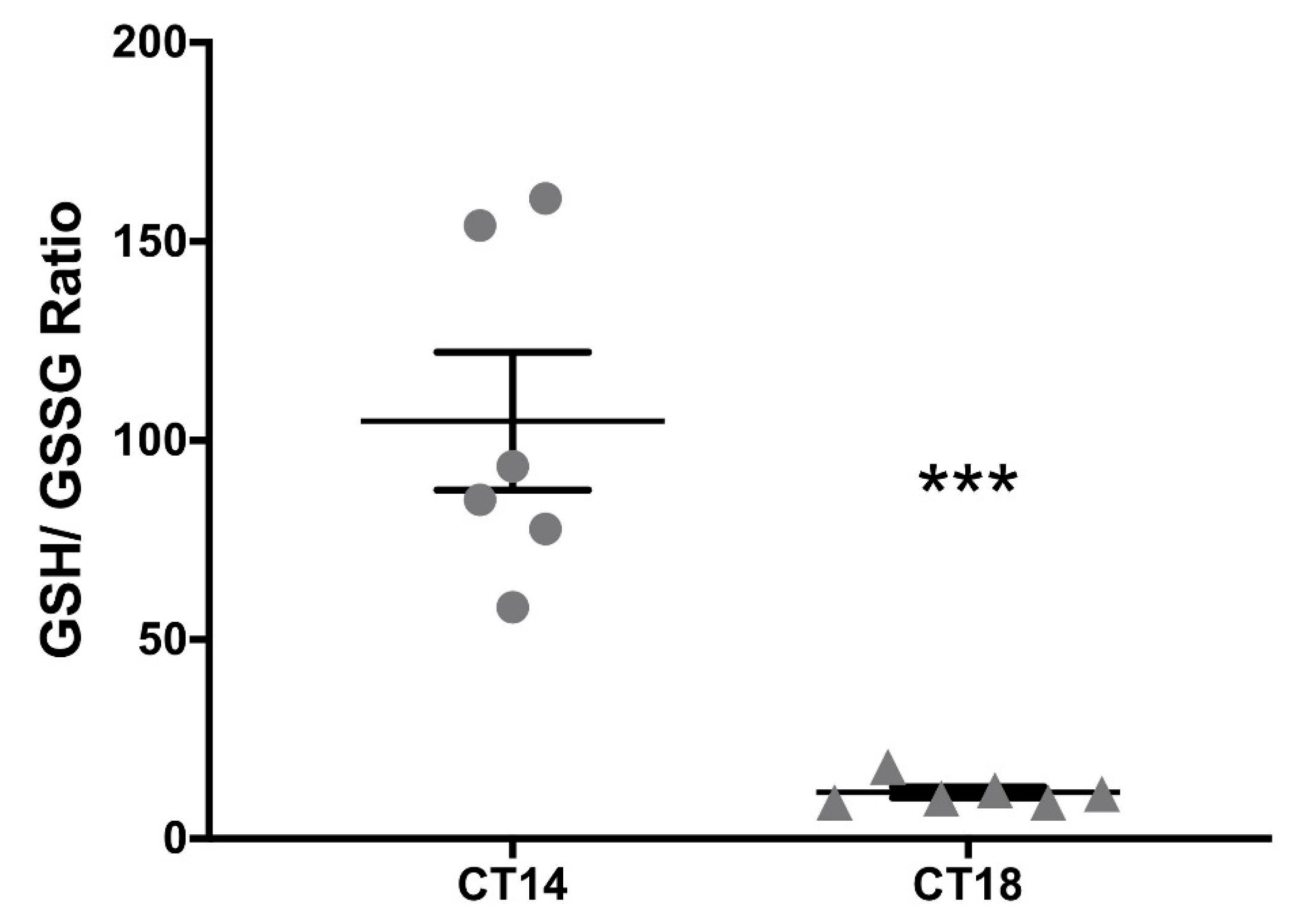
2.3. Measurement of the Redox Pair GSH–GSSG at the SCN
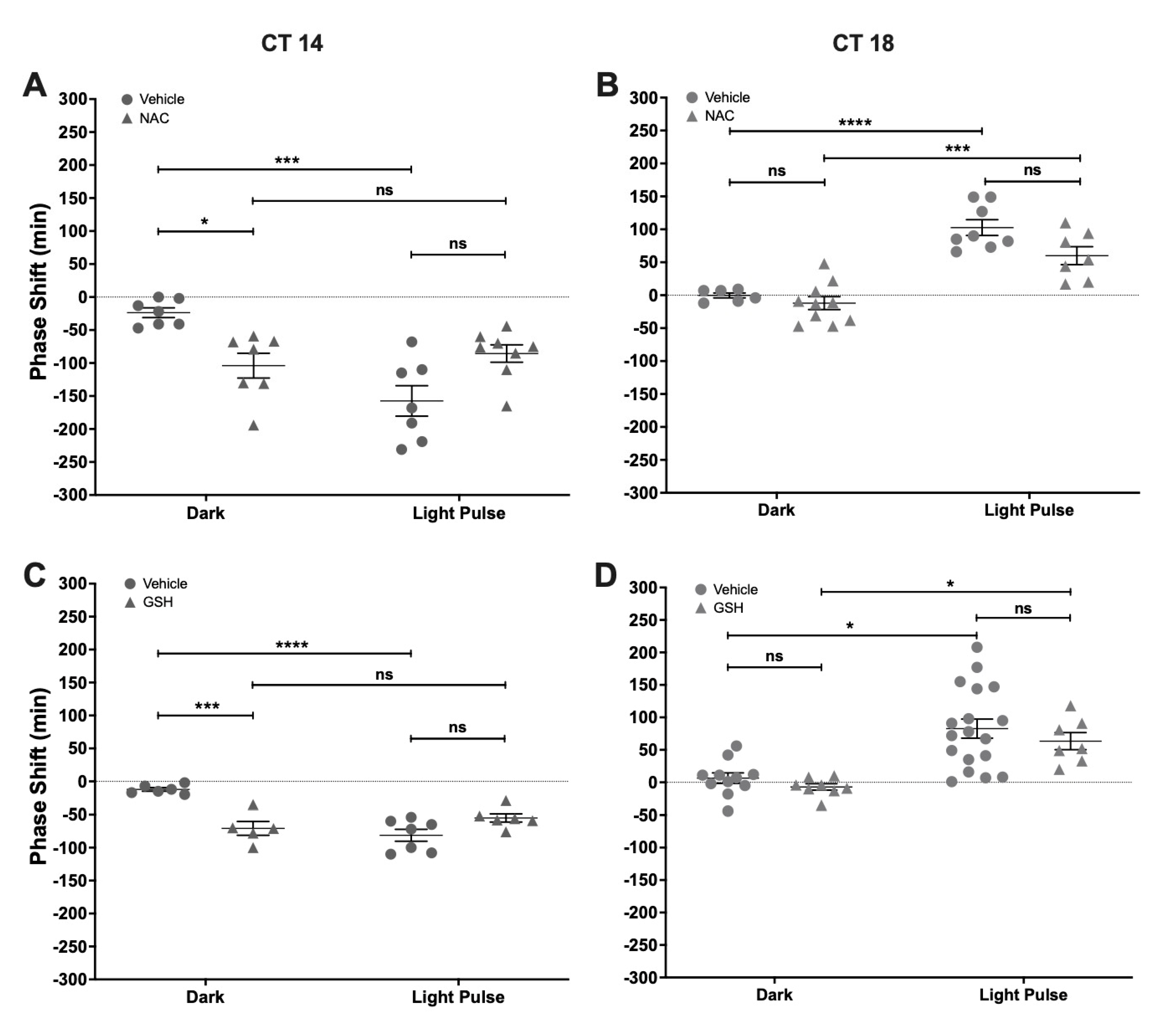
2.4. Effect of NAC and GSH Administration on Light-Induced Phase-Shifts
3. Discussion
4. Materials and Methods
4.1. Pharmacological Modulation of Photic Entrainment
4.1.1. Animals and Housing
4.1.2. Drugs
4.1.3. Surgeries and Intracerebroventricular Microinjections
4.1.4. Behavioral Experiments
4.1.5. Electrochemical Detection of Nitroxyl In Vivo at the SCN
4.1.6. Measurement of the Redox Pair GSH/GSSG at the SCN
4.1.7. Statistical and Chronobiological Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schibler, U.; Sassone-Corsi, P. A Web of Circadian Pacemakers. Cell 2002, 111, 919–922. [Google Scholar] [CrossRef] [Green Version]
- Hegazi, S.; Lowden, C.; Garcia, J.R.; Cheng, A.H.; Obrietan, K.; Levine, J.D.; Cheng, H.-Y.M. A Symphony of Signals: Intercellular and Intracellular Signaling Mechanisms Underlying Circadian Timekeeping in Mice and Flies. Int. J. Mol. Sci. 2019, 20, 2363. [Google Scholar] [CrossRef] [Green Version]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, A.; Braas, D.; Fu, Y.-H.; Ptáček, L.J. FAD Regulates CRYPTOCHROME Protein Stability and Circadian Clock in Mice. Cell Rep. 2017, 19, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.A.; Yu, Y.V.; Govindaiah, G.; Ye, X.; Artinian, L.; Coleman, T.P.; Sweedler, J.V.; Cox, C.L.; Gillette, M.U. Circadian Rhythm of Redox State Regulates Excitability in Suprachiasmatic Nucleus Neurons. Science 2012, 337, 839–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golombek, D.A.; Rosenstein, R.E. Physiology of Circadian Entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostino, P.V.; Ferreyra, G.A.; Murad, A.D.; Watanabe, Y.; Golombek, D.A. Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochem. Int. 2004, 44, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Golombek, D.A.; Agostino, P.V.; Plano, S.A.; Ferreyra, G.A. Signaling in the mammalian circadian clock: The NO/cGMP pathway. Neurochem. Int. 2004, 45, 929–936. [Google Scholar] [CrossRef]
- Plano, S.A.; Alessandro, M.S.; Trebucq, L.L.; Endo, S.; Golombek, D.A.; Chiesa, J.J. Role of G-Substrate in the NO/cGMP/PKG Signal Transduction Pathway for Photic Entrainment of the Hamster Circadian Clock. ASN Neuro 2021, 13, 1759091420984920. [Google Scholar] [CrossRef]
- Ding, J.M.; Buchanan, G.F.; Tischkau, S.A.; Chen, D.; Kuriashkina, L.; Faiman, L.E.; Alster, J.M.; McPherson, P.S.; Campbell, K.P.; Gillette, M.U. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature 1998, 394, 381–384. [Google Scholar] [CrossRef]
- Gau, D.; Lemberger, T.; von Gall, C.; Kretz, O.; Le Minh, N.; Gass, P.; Schmid, W.; Schibler, U.; Korf, H.W.; Schütz, G. Phosphorylation of CREB Ser142 Regulates Light-Induced Phase Shifts of the Circadian Clock. Neuron 2002, 34, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.M.; Faiman, L.E.; Hurst, W.J.; Kuriashkina, L.R.; Gillette, M.U. Resetting the Biological Clock: Mediation of Nocturnal CREB Phosphorylation via Light, Glutamate, and Nitric Oxide. J. Neurosci. 1997, 17, 667–675. [Google Scholar] [CrossRef]
- Tischkau, S.A.; Gallman, E.A.; Buchanan, G.F.; Gillette, M.U. Differential cAMP Gating of Glutamatergic Signaling Regulates Long-Term State Changes in the Suprachiasmatic Circadian Clock. J. Neurosci. 2000, 20, 7830–7837. [Google Scholar] [CrossRef]
- Melo, L.; Golombek, D.A.; Ralph, M.R. Regulation of circadian photic responses by nitric oxide. J. Biol. Rhythm. 1997, 12, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Baidanoff, F.M.; Plano, S.A.; Doctorovich, F.; Suárez, S.A.; Golombek, D.A.; Chiesa, J.J. N-nitrosomelatonin enhances photic synchronization of mammalian circadian rhythms. J. Neurochem. 2014, 129, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.R.; Fernández, I.C.; Ordenes, P.P.; Treuer, A.V.; Eller, G.; Boric, M.P. Differential role of S-nitrosylation and the NO–cGMP–PKG pathway in cardiac contractility. Nitric Oxide 2008, 18, 157–167. [Google Scholar] [CrossRef]
- Watanabe, A.; Ono, M.; Shibata, S.; Watanabe, S. Effect of a nitric oxide synthase inhibitor, N-nitro-l-arginine methylester, on light-induced phase delay of circadian rhythm of wheel-running activity in golden hamsters. Neurosci. Lett. 1995, 192, 25–28. [Google Scholar] [CrossRef]
- Plano, S.A.; Golombek, D.A.; Chiesa, J.J. Circadian entrainment to light-dark cycles involves extracellular nitric oxide communication within the suprachiasmatic nuclei. Eur. J. Neurosci. 2010, 31, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, J.J.; Baidanoff, F.M.; Golombek, D.A. Don’t just say no: Differential pathways and pharmacological responses to diverse nitric oxide donors. Biochem. Pharmacol. 2018, 156, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; Marletta, M.A. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 2012, 16, 498–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ruiz, A.; Araújo, I.M.; Izquierdo-Álvarez, A.; Hernansanz-Agustín, P.; Lamas, S.; Serrador, J.M. Specificity in S-Nitrosylation: A Short-Range Mechanism for NO Signaling? Antioxid. Redox Signal. 2013, 19, 1220–1235. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.N. Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Turell, L.; Zeida, A.; Trujillo, M. Mechanisms and consequences of protein cysteine oxidation: The role of the initial short-lived intermediates. Essays Biochem. 2020, 64, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Arnelle, D.R.; Stamler, J.S. NO+, NO, and NO− Donation by S-Nitrosothiols: Implications for Regulation of Physiological Functions by S-Nitrosylation and Acceleration of Disulfide Formation. Arch. Biochem. Biophys. 1995, 318, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Meissner, G. Physiology of Nitric Oxide in Skeletal Muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Cammack, R.; Shergill, J.K.; Inalsingh, V.A.; Hughes, M.N. Applications of electron paramagnetic resonance spectroscopy to study interactions of iron proteins in cells with nitric oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 2393–2402. [Google Scholar] [CrossRef]
- Parissis, J.; Bistola, V.; Ikonomidis, I.; Triposkiadis, F. Nitroxyl donors for acute heart failure: Promising newcomers. Eur. J. Heart Fail. 2017, 19, 1333–1334. [Google Scholar] [CrossRef] [Green Version]
- Fukuto, J.M. A recent history of nitroxyl chemistry, pharmacology and therapeutic potential. Br. J. Pharmacol. 2019, 176, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Paolocci, N.; Saavedra, W.F.; Miranda, K.M.; Martignani, C.; Isoda, T.; Hare, J.M.; Espey, M.G.; Fukuto, J.M.; Feelisch, M.; Wink, D.A.; et al. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 10463–10468. [Google Scholar] [CrossRef] [Green Version]
- Choe, C.-U.; Lewerenz, J.; Gerloff, C.; Magnus, T.; Donzelli, S. Nitroxyl in the Central Nervous System. Antioxid. Redox Signal. 2011, 14, 1699–1711. [Google Scholar] [CrossRef]
- Dutton, A.S.; Fukuto, J.M.; Houk, K.N. Mechanisms of HNO and NO Production from Angeli’s Salt: Density Functional and CBS-QB3 Theory Predictions. J. Am. Chem. Soc. 2004, 126, 3795–3800. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S.; Ducrocq, C.; Hu, S.; Tapsoba, I. Angeli’s Salt (Na2N2O3) is a Precursor of HNO and NO: A Voltammetric Study of the Reactive Intermediates Released by Angeli’s Salt Decomposition. ChemMedChem 2007, 2, 898–903. [Google Scholar] [CrossRef]
- Suarez, S.A.; Vargas, P.; Doctorovich, F.A. Updating NO•/HNO interconversion under physiological conditions: A biological implication overview. J. Inorg. Biochem. 2021, 216, 111333. [Google Scholar] [CrossRef]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free. Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, G.A.; Golombek, D.A. Rhythmicity of the cGMP-related signal transduction pathway in the mammalian circadian system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1348–R1355. [Google Scholar] [CrossRef]
- Tischkau, S.A.; Mitchell, J.W.; Pace, L.A.; Barnes, J.W.; Barnes, J.A.; Gillette, M.U. Protein Kinase G Type II Is Required for Night-to-Day Progression of the Mammalian Circadian Clock. Neuron 2004, 43, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Tischkau, S.A.; Weber, E.T.; Abbott, S.M.; Mitchell, J.W.; Gillette, M.U. Circadian Clock-Controlled Regulation of cGMP-Protein Kinase G in the Nocturnal Domain. J. Neurosci. 2003, 23, 7543–7550. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free. Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Miranda, K.M.; Paolocci, N.; Katori, T.; Thomas, D.D.; Ford, E.; Bartberger, M.D.; Espey, M.G.; Kass, D.A.; Feelisch, M.; Fukuto, J.M.; et al. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2003, 100, 9196–9201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smulik, R.; Dębski, D.; Zielonka, J.; Michałowski, B.; Adamus, J.; Marcinek, A.; Kalyanaraman, B.; Sikora, A. Nitroxyl (HNO) Reacts with Molecular Oxygen and Forms Peroxynitrite at Physiological pH: Biological implications. J. Biol. Chem. 2014, 289, 35570–35581. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.H.H.W.; Hofmann, H.; Schindler, U.; Shutenko, Z.S.; Cunningham, D.D.; Feelisch, M. No ·NO from NO synthase. Proc. Natl. Acad. Sci. USA 1996, 93, 14492–14497. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Zweier, J.L. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1997, 94, 12705–12710. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.W.; Cherney, M.M.; Lee, A.J.; Francoleon, N.E.; Farmer, P.J.; King, S.B.; Hobbs, A.J.; Miranda, K.M.; Burstyn, J.N.; Fukuto, J.M. The Effects of Nitroxyl (HNO) on Soluble Guanylate Cyclase Activity: INTERACTIONS AT FERROUS HEME AND CYSTEINE THIOLS. J. Biol. Chem. 2009, 284, 21788–21796. [Google Scholar] [CrossRef] [Green Version]
- Cheong, E.; Tumbev, V.; Abramson, J.; Salama, G.; Stoyanovsky, D.A. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium 2005, 37, 87–96. [Google Scholar] [CrossRef]
- Sáenz, D.A.; Bari, S.E.; Salido, E.; Chianelli, M.; Rosenstein, R.E. Effect of nitroxyl on the hamster retinal nitridergic pathway. Neurochem. Int. 2007, 51, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, A.J.; Moed, M.; Tuominen, R.K.; Helkamaa, T.H.; Wiksten, M.; Liesi, P.; Chiueh, C.C.; Rauhala, P. Angeli’s Salt Induces Neurotoxicity in Dopaminergic Neurons In Vivo and In Vitro. Free. Radic. Res. 2003, 37, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F. What is the Mechanism of Nitric Oxide Conversion into Nitrosonium Ions Ensuring S-Nitrosating Processes in Living Organisms. Cell Biochem. Biophys. 2019, 77, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Zhitkovich, A. N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocernich, C.B.; Cardin, A.L.; Racine, C.L.; Lauderback, C.M.; Butterfield, D.A. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: Relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem. Int. 2001, 39, 141–149. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Butterfield, D.A. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Wrotek, S.; Jędrzejewski, T.; Piotrowski, J.; Kozak, W. N -Acetyl- l -cysteine exacerbates generation of IL-10 in cells stimulated with endotoxin in vitro and produces antipyresis via IL-10 dependent pathway in vivo. Immunol. Lett. 2016, 177, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pilz, L.K.; Trojan, Y.; Quiles, C.L.; Benvenutti, R.; Melo, G.; Levandovski, R.; Hidalgo, M.P.L.; Elisabetsky, E. Effects of N-acetylcysteine and imipramine in a model of acute rhythm disruption in BALB/c mice. Chronobiol. Int. 2015, 32, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ledezma, C.; Coria-Lucero, C.; Delsouc, M.B.; Casais, M.; Della Vedova, C.; Ramirez, D.; Devia, C.M.; Delgado, S.M.; Navigatore-Fonzo, L.; Anzulovich, A.C. Effect of an Intracerebroventricular Injection of Aggregated Beta-amyloid (1–42) on Daily Rhythms of Oxidative Stress Parameters in the Prefrontal Cortex. Neuroscience 2021, 458, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Bhatnagar, A. Protein S-glutathiolation: Redox-sensitive regulation of protein function. J. Mol. Cell. Cardiol. 2012, 52, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Naseri Kouzehgarani, G.; Bothwell, M.Y.; Gillette, M.U. Circadian rhythm of redox state regulates membrane excitability in hippocampal CA1 neurons. Eur. J. Neurosci. 2020, 51, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Schmalen, I.; Reischl, S.; Wallach, T.; Klemz, R.; Grudziecki, A.; Prabu, J.R.; Benda, C.; Kramer, A.; Wolf, E. Interaction of Circadian Clock Proteins CRY1 and PER2 Is Modulated by Zinc Binding and Disulfide Bond Formation. Cell 2014, 157, 1203–1215. [Google Scholar] [CrossRef] [Green Version]
- Putker, M.; Crosby, P.; Feeney, K.A.; Hoyle, N.P.; Costa, A.S.; Gaude, E.; Frezza, C.; O’Neill, J.S. Mammalian Circadian Period, But Not Phase and Amplitude, Is Robust Against Redox and Metabolic Perturbations. Antioxid. Redox Signal. 2018, 28, 507–520. [Google Scholar] [CrossRef]
- Pei, J.-F.; Li, X.-K.; Li, W.-Q.; Gao, Q.; Zhang, Y.; Wang, X.-M.; Fu, J.-Q.; Cui, S.-S.; Qu, J.-H.; Zhao, X.; et al. Diurnal oscillations of endogenous H2O2 sustained by p66Shc regulate circadian clocks. Nat. Cell Biol. 2019, 21, 1553–1564. [Google Scholar] [CrossRef]
- Miranda, K.M.; Dutton, A.S.; Ridnour, L.A.; Foreman, C.A.; Ford, E.; Paolocci, N.; Katori, T.; Tocchetti, C.G.; Mancardi, D.; Thomas, D.D.; et al. Mechanism of Aerobic Decomposition of Angeli’s Salt (Sodium Trioxodinitrate) at Physiological pH. J. Am. Chem. Soc. 2005, 127, 722–731. [Google Scholar] [CrossRef]
- Suarez, S.A.; Bikiel, D.E.; Wetzler, D.E.; Marti, M.A.; Doctorovich, F. Time-Resolved Electrochemical Quantification of Azanone (HNO) at Low Nanomolar Level. Anal. Chem. 2013, 85, 10262–10269. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plano, S.A.; Baidanoff, F.M.; Trebucq, L.L.; Suarez, S.Á.; Doctorovich, F.; Golombek, D.A.; Chiesa, J.J. Redox and Antioxidant Modulation of Circadian Rhythms: Effects of Nitroxyl, N-Acetylcysteine and Glutathione. Molecules 2021, 26, 2514. https://doi.org/10.3390/molecules26092514
Plano SA, Baidanoff FM, Trebucq LL, Suarez SÁ, Doctorovich F, Golombek DA, Chiesa JJ. Redox and Antioxidant Modulation of Circadian Rhythms: Effects of Nitroxyl, N-Acetylcysteine and Glutathione. Molecules. 2021; 26(9):2514. https://doi.org/10.3390/molecules26092514
Chicago/Turabian StylePlano, Santiago Andrés, Fernando Martín Baidanoff, Laura Lucía Trebucq, Sebastián Ángel Suarez, Fabio Doctorovich, Diego Andrés Golombek, and Juan José Chiesa. 2021. "Redox and Antioxidant Modulation of Circadian Rhythms: Effects of Nitroxyl, N-Acetylcysteine and Glutathione" Molecules 26, no. 9: 2514. https://doi.org/10.3390/molecules26092514






