Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice
Abstract
:1. Introduction
2. Results
2.1. Monitoring of the Release of Casomorphin from Examined Dressing
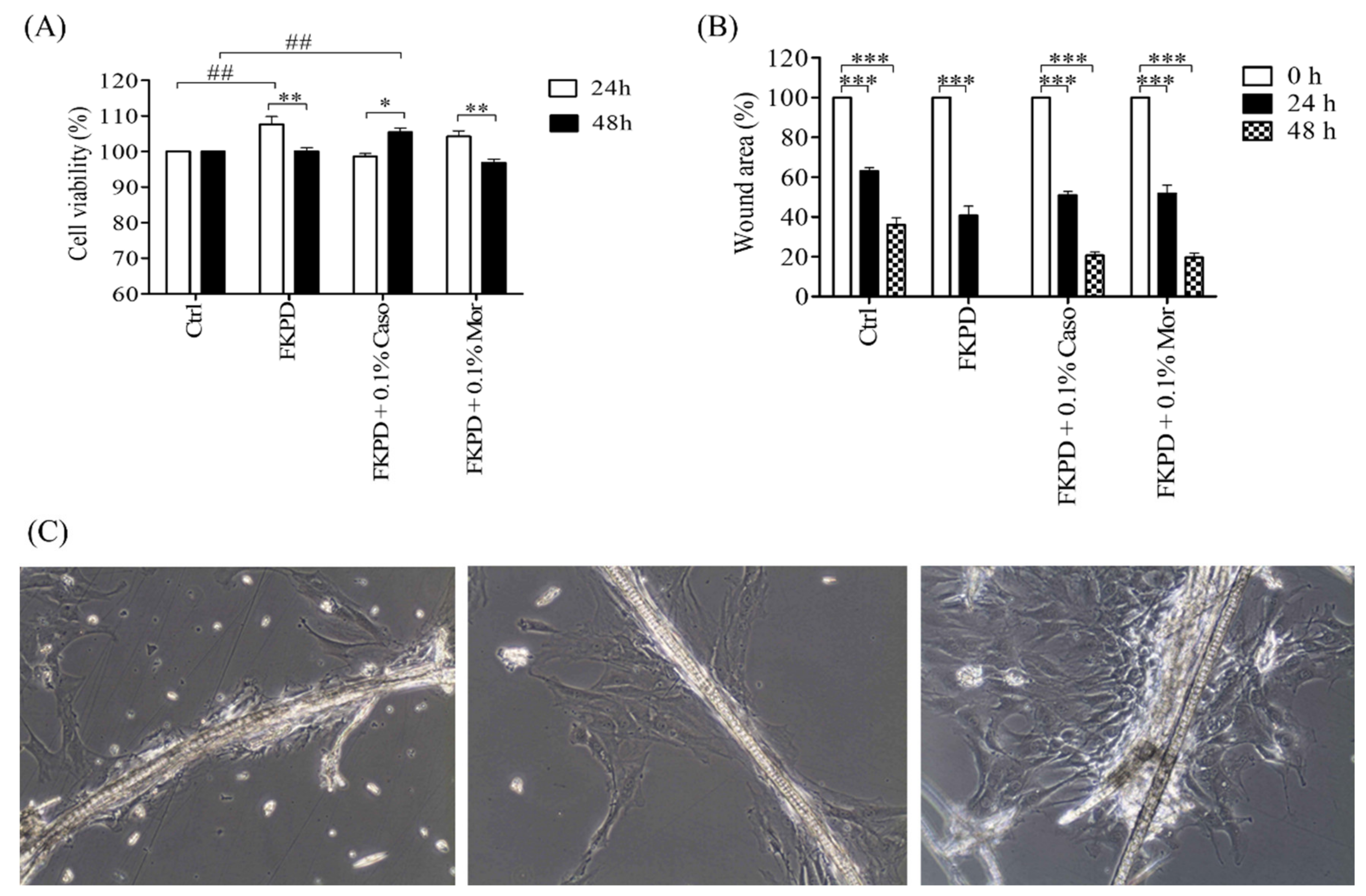
2.2. Effect of Experimental Dressing on Viability and Migration of Murine Fibroblasts
2.3. Diabetes Studies
2.4. Influence of Examined Dressing on Skin Wound Healing in Diabetic Mice
2.5. Changes in Cytokine Level during the Healing Course
2.6. Histopathological Studies
2.6.1. Oozing and Microhemorrhages
2.6.2. The Healing Course
2.6.3. Cell Infiltrate into the FKDP-0.1%Caso and the Control Wounds
2.6.4. Tissue Remodeling
2.7. Immunohistochemistry Staining
3. Discussion
4. Materials and Methods
4.1. Preparation of Experimental Wound Dressings
4.2. In Vitro Drug Release from the Wound Dressing
4.3. Electrophoretic Measurements
4.4. Cell Proliferation Assay
4.5. In Vitro Wound-Healing Assay
4.6. Animals
4.7. Iatrogenically Induced Diabetes
4.8. Surgical Procedure
4.9. Histopathological Analysis
4.10. Cytokine Analysis
4.11. Immunohistochemistry Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugam, V.K.; Couch, K.S.; McNish, S.; Amdur, R.L. Relationship between opioid treatment and rate of healing in chronic wounds. Wound Repair Regen. 2017, 25, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Lesniak, A.; Bochynska-Czyz, M.; Sacharczuk, M.; Benhye, S.; Misicka, A.; Bujalska-Zadrożny, M.; Lipkowski, A.W. Biphalin preferentially recruits peripheral opioid receptors to facilitate analgesia in a mouse model of cancer pain—A comparison with morphine. Eur. J. Pharm. Sci. 2016, 89, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.-D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-W.; Chen, Y.-K.; Tang, K.-C.; Yang, K.-C.; Cheng, N.-C.; Yu, J. Keratin scaffolds with human adipose stem cells: Physical and biological effects toward wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef] [PubMed]
- Bochynska-Czyz, M.; Redkiewicz, P.; Kozlowska, H.; Matalinska, J.; Konop, M.; Kosson, P. Can keratin scaffolds be used for creating three-dimensional cell cultures? Open Med. 2020, 15, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef]
- Rook, J.M.; Hasan, W.; McCarson, K.E. Morphine-induced early delays in wound closure: Involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochem. Pharmacol. 2009, 77, 1747–1755. [Google Scholar] [CrossRef] [Green Version]
- Flock, P. Pilot study to determine the effectiveness of diamorphine gel to control pressure ulcer pain. J. Pain Symptom Manag. 2003, 25, 547–554. [Google Scholar] [CrossRef]
- Long, T.D.; Cathers, T.A.; Twillman, R.; O’Donnell, T.; Garrigues, N.; Jones, T. Morphine-Infused Silver Sulfadiazine (MISS) cream for burn analgesia: A pilot study. J. Burn. Care Rehabil. 2001, 22, 118–123. [Google Scholar] [CrossRef]
- Cerchietti, L.C.A.; Navigante, A.H.; Bonomi, M.R.; Zaderajko, M.A.; Menéndez, P.R.; Pogany, C.E.; Roth, B.M.C. Effect of topical morphine for mucositis-associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer 2002, 95, 2230–2236. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Thirupathi, P.; Chung, I.-M.; Subramanian, U. β-Casomorphin: A complete health perspective. Food Chem. 2021, 337, 127765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, S.; Liu, F.; Liu, Y.; Zhang, Y. Beta-casomorphin-7 prevents epithelial-mesenchymal transdifferentiation of NRK-52E cells at high glucose level: Involvement of AngII-TGF-β1 pathway. Peptides 2015, 70, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Zheng, A.J.; Chen, Z.M.; Zhang, S.; Cai, H.Y.; Liu, G.H. β-Casomorphin increases fat deposition in broiler chickens by modulating expression of lipid metabolism genes. Animal 2019, 13, 777–783. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, J.; Wang, S.; Zhang, Y. The protective effects of beta-casomorphin-7 against glucose-induced renal oxidative stress in vivo and vitro. PLoS ONE 2013, 8, e63472. [Google Scholar] [CrossRef]
- Yin, H.; Miao, J.; Zhang, Y. Protective effect of β-casomorphin-7 on type 1 diabetes rats induced with streptozotocin. Peptides 2010, 31, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.M.; McCarson, K.E. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem. Pharmacol. 2007, 74, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Poonawala, T.; Levay-Young, B.K.; Hebbel, R.P.; Gupta, K. Opioids heal ischemic wounds in the rat. Wound Repair Regen. 2005, 13, 165–174. [Google Scholar] [CrossRef]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Zielenkiewicz, U.; Brzozowska, I.; Nabavi, S.M.; Rudnicka, L. Development of a novel keratin dressing which accelerates full-thickness skin wound healing in diabetic mice: In vitro and in vivo studies. J. Biomater. Appl. 2018, 33, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Küchler, S. Targeting inflammation and wound healing by opioids. Trends Pharmacol. Sci. 2013, 34, 303–312. [Google Scholar] [CrossRef]
- Bechert, K.; Abraham, S.E. Pain management and wound care. J. Am. Coll. Certif. Wound Spec. 2009, 1, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Stein, C. Non-analgesic effects of opioids: Peripheral opioid effects on inflammation and wound healing. Curr. Pharm. Des. 2012, 18, 6053–6069. [Google Scholar] [CrossRef]
- Konop, M.; Damps, T.; Misicka, A.; Rudnicka, L. Certain aspects of silver and silver nanoparticles in wound care: A minireview. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Muchowska, A.; Redkiewicz, P.; Różycki, K.; Matalińska, J.; Lipiński, P.F.; Czuwara, J.; Kosson, P. The analgesic hybrid of dermorphin/substance P and analog of enkephalin improve wound healing in streptozotocin-induced diabetic rats. Wound Repair Regen. 2019, 28, 177–184. [Google Scholar] [CrossRef]
- Taylor, R.; Low, A.; Reid, R. Determination of opiates in urine by capillary electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1996, 675, 213–223. [Google Scholar] [CrossRef]
- Ding, Y.; Garcia, C.D. Determination of nonsteroidal anti-inflammatory drugs in serum by microchip capillary electrophoresis with electrochemical detection. Electroanalysis 2006, 18, 2202–2209. [Google Scholar] [CrossRef]
- Cui, X.; Ni, C.; Liang, C.; Gong, F.; Wang, R.; Chen, G.; Zhang, Y. Screening and quantitation of forty-six drugs of abuse and toxic compounds in human whole blood by capillary electrophoresis: Application to forensic cases. Microchem. J. 2019, 144, 403–410. [Google Scholar] [CrossRef]
- Nishiwada, T.; Kawaraguchi, Y.; Uemura, K.; Kawaguchi, M. Morphine inhibits cell viability and growth via suppression of vascular endothelial growth factor in human oral cancer HSC-3 cells. J. Anesthesia 2019, 33, 408–415. [Google Scholar] [CrossRef]
- Kampa, M.; Bakogeorgou, E.; Hatzoglou, A.; Damianaki, A.; Martin, P.-M.; Castanas, E. Opioid alkaloids and casomorphin peptides decrease the proliferation of prostatic cancer cell lines (LNCaP, PC3 and DU145) through a partial interaction with opioid receptors. Eur. J. Pharmacol. 1997, 335, 255–265. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yussof, S.J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Trengove, N.J.; Bielefeldt-Ohmann, H.; Stacey, M.C. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2001, 8, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Monin, L.; Gaffen, S.L. Interleukin 17 family cytokines: Signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb. Perspect. Biol. 2018, 10, a028522. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Luo, G.; He, W. Functions of Vγ4 T cells and dendritic epidermal t cells on skin wound healing. Front. Immunol. 2018, 9, 1099. [Google Scholar] [CrossRef] [Green Version]
- Akitsu, A.; Iwakura, Y. Interleukin-17-producing γδ T (γδ17) cells in inflammatory diseases. Immunology 2018, 155, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLeod, A.S.; Hemmers, S.; Garijo, O.; Chabod, M.; Mowen, K.; Witherden, D.A.; Havran, W.L. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J. Clin. Investig. 2013, 123, 4364–4374. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C.; Nauseef, W.M. IFN-γ targets macrophage-mediated immune responses toward Staphylococcus aureus. J. Leukoc. Biol. 2016, 101, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.-C.; Li, C.-Y.; Kuo, C.-Y.; Kuo, Y.-Z.; Fang, W.-Y.; Huang, Y.-H.; Hsieh, T.-C.; Kao, H.-Y.; Kuo, Y.; Kang, Y.-R.; et al. The p53-S100A2 positive feedback loop negatively regulates epithelialization in cutaneous wound healing. Sci. Rep. 2018, 8, 5458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausmann, R.; Nerlich, A.; Betz, P. The time-related expression of p53 protein in human skin wounds—A quantitative immunohistochemical analysis. Int. J. Leg. Med. 1998, 111, 169–172. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Park, Y.R.; Sultan, T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nat. Cell Biol. 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Chen, Y.; Yang, Z.; You, B.; Ruan, Y.C.; Peng, Y. Epidermal CFTR suppresses MAPK/NF-κB to promote cutaneous wound healing. Cell. Physiol. Biochem. 2016, 39, 2262–2274. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Shi, T.; Lu, L. Epidermal Growth Factor (EGF)-induced corneal epithelial wound healing through nuclear factor κB subtype-regulated CCCTC binding factor (CTCF) activation. J. Biol. Chem. 2013, 288, 24363–24371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, S.C.; Jeon, E.S.; Lee, I.H.; Kim, H.S.; Kim, M.B.; Kim, J.H. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J. Investig. Dermatol. 2011, 131, 1559–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stęplewski, W.; Wawro, D.; Ratajska, M.; Wrześniewska-Tosik, K. Novel biocomposites with feather keratin. Fibres Text. East. Eur. 2007, 15, 64–65. [Google Scholar]
- Katoh, K.; Shibayama, M.; Tanabe, T.; Yamauchi, K. Preparation and properties of keratin-poly(vinyl alcohol) blend fiber. J. Appl. Polym. Sci. 2003, 91, 756–762. [Google Scholar] [CrossRef]
- Konop, M.; Sulejczak, D.; Czuwara, J.; Kosson, P.; Misicka, A.; Lipkowski, A.W.; Rudnicka, L. The role of allogenic keratin-derived dressing in wound healing in a mouse model. Wound Repair Regen. 2017, 25, 62–74. [Google Scholar] [CrossRef]
- Yuan, J.; Geng, J.; Xing, Z.; Shim, K.-J.; Han, I.; Kim, J.-C.; Kang, I.-K.; Shen, J. Novel wound dressing based on nanofibrous PHBV-keratin mats. J. Tissue Eng. Regen. Med. 2015, 9, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, O.; Ahmed, K.S.Z.; Sujatha, K.; Ponnmurugan, P.; Srivastava, A.; Ramesh, R.; Sukumar, R.; Elanithi, K. Fabrication and characterization of chicken feather keratin/polysaccharides blended polymer coated nonwoven dressing materials for wound healing applications. Mater. Sci. Eng. C 2018, 92, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Veerasubramanian, P.K.; Thangavel, P.; Kannan, R.; Chakraborty, S.; Ramachandran, B.; Suguna, V.L. Muthuvijayan, Corrigendum to “An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing”. Colloids Surf. B Biointerfaces 2018, 165, 92–102. [Google Scholar] [CrossRef]
- Martin, J.L.; Charboneau, R.; Barke, R.A.; Roy, S. Chronic morphine treatment inhibits LPS-induced angiogenesis: Implications in wound healing. Cell. Immunol. 2010, 265, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.L.; Koodie, L.; Krishnan, A.G.; Charboneau, R.; Barke, R.A.; Roy, S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am. J. Pathol. 2010, 176, 786–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habiboallah, G.; Mahdi, Z.; Majid, Z.; Nasroallah, S.; Taghavi, A.M.; Forouzanfar, A.; Arjmand, N. Enhancement of gingival wound healing by local application of silver nanoparticles periodontal dressing following surgery: A histological assessment in animal model. Mod. Res. Inflamm. 2014, 3, 128–138. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.; Ge, D.; Wang, S.; Qi, B. β-casomorphin-7 ameliorates sepsis-induced acute kidney injury by targeting NF-κB pathway. Med Sci. Monit. 2019, 25, 121–127. [Google Scholar] [CrossRef]
- Veeren, B.; Bringart, M.; Turpin, C.; Rondeau, P.; Planesse, C.; Ait-Arsa, I.; Gimié, F.; Marodon, C.; Meilhac, O.; Gonthier, M.-P.; et al. Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis. Biomedicines 2021, 9, 358. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Wu, D.; Li, B.; Dayang, W.; Bing, L. The protective effect of beta-casomorphin-7 via promoting Foxo1 activity and nuclear translocation in human lens epithelial cells. Cutan. Ocul. Toxicol. 2018, 37, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Fujimori, T.; Satoh, T.; Matsumura, E. Effects of β-casomorphins on neuronal survival in culture of embryonic chick dorsal root ganglion neurons. Jpn. J. Pharmacol. 2001, 86, 363–365. [Google Scholar] [CrossRef] [PubMed]
 -epidermis;
-epidermis;  -hair follicle;
-hair follicle;  -empty zones;
-empty zones;  -blood extravasation. (B) The effect of fur keratin-derived powder (FKDP) containing 0.1% casomorphin on skin wound healing. Because of the decreasing number of surviving mice, the data were only tested by the t-test for dependent samples (for each post-wounding day separately), with no prior two-way analysis of variance. Mice numbers at Day 5, N = 20; Day 8, N = 12; Day 15, N = 6. (C) Changes in cytokine level during wound healing in diabetic mice (p-value < 0.05 was considered significant, were * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001). (D) Changes in blood glucose level (BGL) during diabetes induction. (The data were statistically significant if p < 0.05 two-way analysis of variance, followed by Bonferroni post hoc tests, mean ± standard error of the mean) (color online, black and white in print).
-blood extravasation. (B) The effect of fur keratin-derived powder (FKDP) containing 0.1% casomorphin on skin wound healing. Because of the decreasing number of surviving mice, the data were only tested by the t-test for dependent samples (for each post-wounding day separately), with no prior two-way analysis of variance. Mice numbers at Day 5, N = 20; Day 8, N = 12; Day 15, N = 6. (C) Changes in cytokine level during wound healing in diabetic mice (p-value < 0.05 was considered significant, were * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001). (D) Changes in blood glucose level (BGL) during diabetes induction. (The data were statistically significant if p < 0.05 two-way analysis of variance, followed by Bonferroni post hoc tests, mean ± standard error of the mean) (color online, black and white in print).
 -epidermis;
-epidermis;  -hair follicle;
-hair follicle;  -empty zones;
-empty zones;  -blood extravasation. (B) The effect of fur keratin-derived powder (FKDP) containing 0.1% casomorphin on skin wound healing. Because of the decreasing number of surviving mice, the data were only tested by the t-test for dependent samples (for each post-wounding day separately), with no prior two-way analysis of variance. Mice numbers at Day 5, N = 20; Day 8, N = 12; Day 15, N = 6. (C) Changes in cytokine level during wound healing in diabetic mice (p-value < 0.05 was considered significant, were * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001). (D) Changes in blood glucose level (BGL) during diabetes induction. (The data were statistically significant if p < 0.05 two-way analysis of variance, followed by Bonferroni post hoc tests, mean ± standard error of the mean) (color online, black and white in print).
-blood extravasation. (B) The effect of fur keratin-derived powder (FKDP) containing 0.1% casomorphin on skin wound healing. Because of the decreasing number of surviving mice, the data were only tested by the t-test for dependent samples (for each post-wounding day separately), with no prior two-way analysis of variance. Mice numbers at Day 5, N = 20; Day 8, N = 12; Day 15, N = 6. (C) Changes in cytokine level during wound healing in diabetic mice (p-value < 0.05 was considered significant, were * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001). (D) Changes in blood glucose level (BGL) during diabetes induction. (The data were statistically significant if p < 0.05 two-way analysis of variance, followed by Bonferroni post hoc tests, mean ± standard error of the mean) (color online, black and white in print).




| The Concentration of Released Casomorphin FKDP + 0.1%Caso Dressing into PBS [mmol/mL] (C ± SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 2 h | 3 h | 4 h | 24 h | 48 h | 72 h | 96 h | 120 h |
| 0.628 | 0.523 | 0.508 | 0.520 | 0.526 | 0.599 | 0.591 | 0.567 | 0.569 | 0.599 |
| ± 0.103 | ± 0.023 | ± 0.033 | ± 0.024 | ± 0.043 | ± 0.053 | ± 0.058 | ± 0.058 | ± 0.081 | ± 0.122 |
| Experiment Day (Number of Mice) | Wound Status | Epidermal Status | Number of Micro-BloodExtravasations (mean ± SD) | ANOVA Results |
|---|---|---|---|---|
| Day 5 (N = 3) | Undressed | − | 16.56 ± 3.87 | Experimental day effect: F2.6 = 139.10 p = 0.001 Dressing effect: F1.6 = 0.65 p = 0.452 Interaction effect (Dressing × Experiment Day) F2.6 = 1.81 p = 0.242 |
| Dressed | − | 19.00 ± 2.19 | ||
| Day 8 (N = 3) | Undressed | + | 14.39 ± 2.10 | |
| Dressed | + | 11.17 ± 1.86 | ||
| Day 15 (N = 3) | Undressed | + | 6.39 ± 0.84 | |
| Dressed | ++ | 4.00 ± 0.33 **,# |
| Cells Tyee | |||||
|---|---|---|---|---|---|
| Neutrophils | Macrophages | Lymphocytes | |||
| Histiocytes | Foreign-Body Giant Cell (FBGC) | ||||
| 5D (N = 3) | Control wound | +++ | + | − | + |
| Dressed wound | ++ | ++ | + | − | |
| 8D (N = 3) | Control wound | +++ | + | − | + |
| Dressed wound | + | ++ | + | + | |
| 15D (N = 3) | Control wound | ++ | ++ | + | ++ |
| Dressed wound | + | ++ | +++ | ++ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konop, M.; Laskowska, A.K.; Rybka, M.; Kłodzińska, E.; Sulejczak, D.; Schwartz, R.A.; Czuwara, J. Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice. Molecules 2021, 26, 2554. https://doi.org/10.3390/molecules26092554
Konop M, Laskowska AK, Rybka M, Kłodzińska E, Sulejczak D, Schwartz RA, Czuwara J. Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice. Molecules. 2021; 26(9):2554. https://doi.org/10.3390/molecules26092554
Chicago/Turabian StyleKonop, Marek, Anna K. Laskowska, Mateusz Rybka, Ewa Kłodzińska, Dorota Sulejczak, Robert A. Schwartz, and Joanna Czuwara. 2021. "Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice" Molecules 26, no. 9: 2554. https://doi.org/10.3390/molecules26092554
APA StyleKonop, M., Laskowska, A. K., Rybka, M., Kłodzińska, E., Sulejczak, D., Schwartz, R. A., & Czuwara, J. (2021). Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice. Molecules, 26(9), 2554. https://doi.org/10.3390/molecules26092554









