Isotopic Indications of Late Pleistocene and Holocene Paleoenvironmental Changes at Boodie Cave Archaeological Site, Barrow Island, Western Australia
Abstract
1. Introduction
2. Materials and Methods
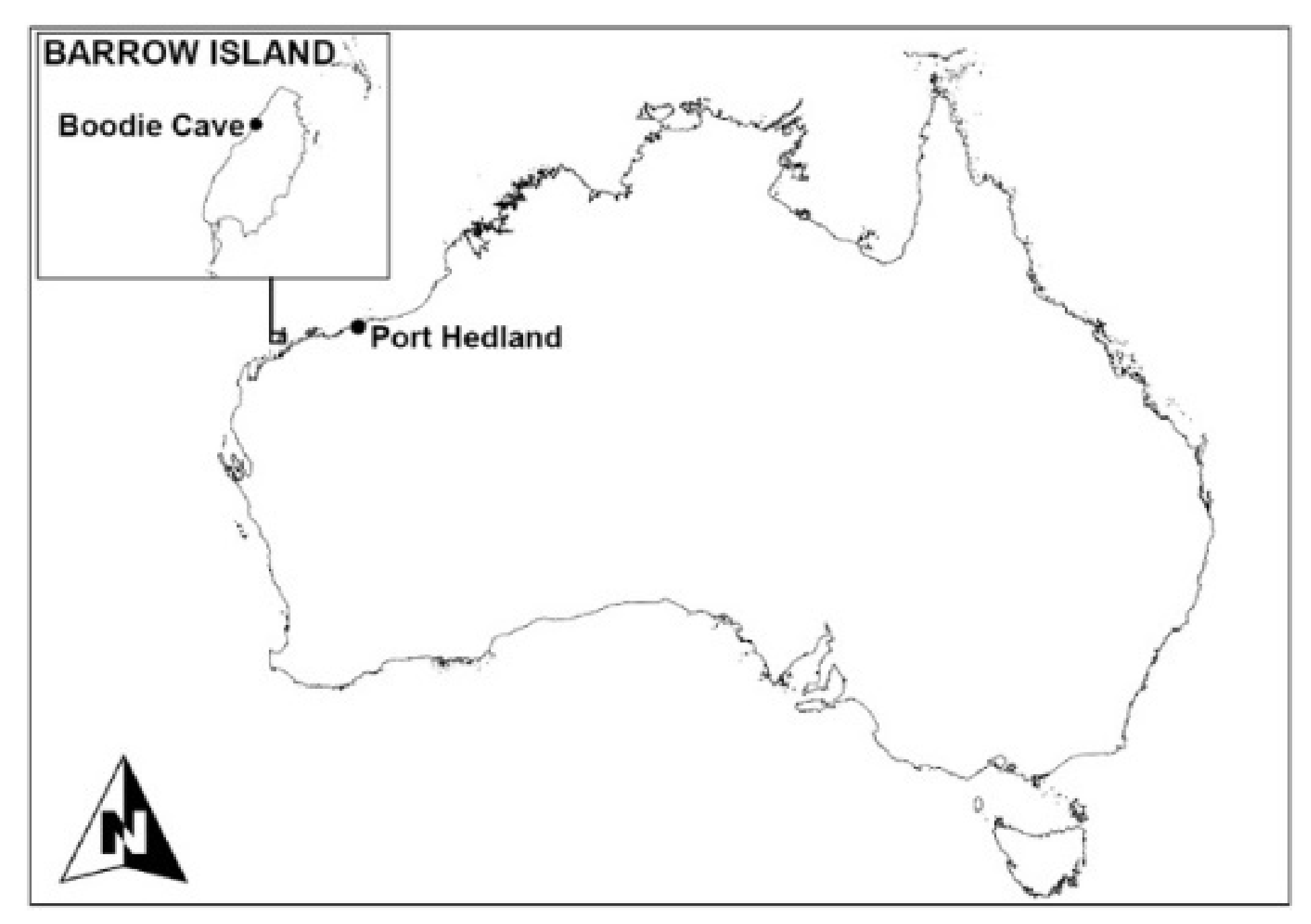
2.1. Excavation
2.2. Sampling
2.3. Preanatyical Processing and Pre-Treatments
2.4. Isotopic Analysis
2.5. Statistical Analysis
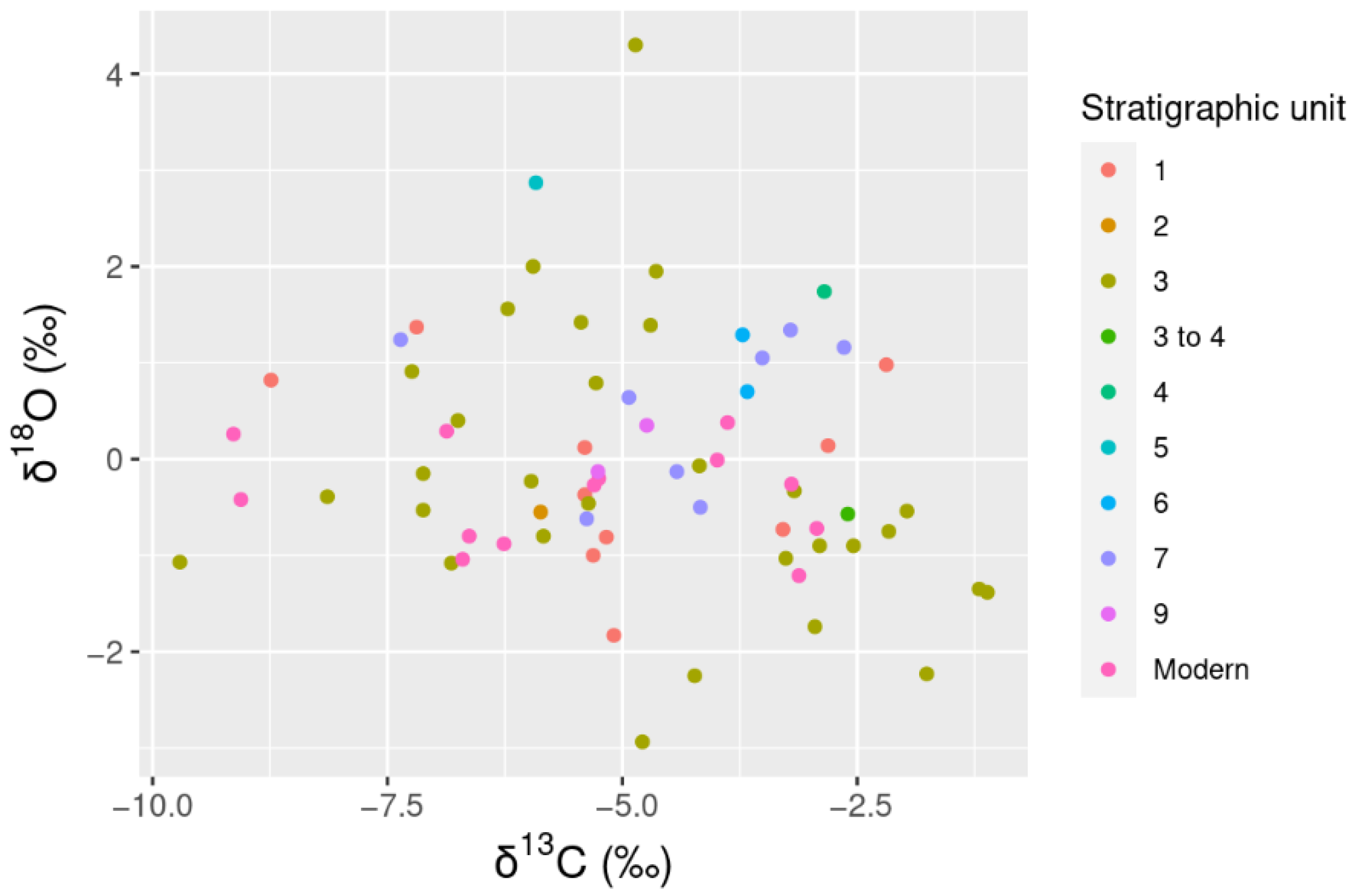
3. Results
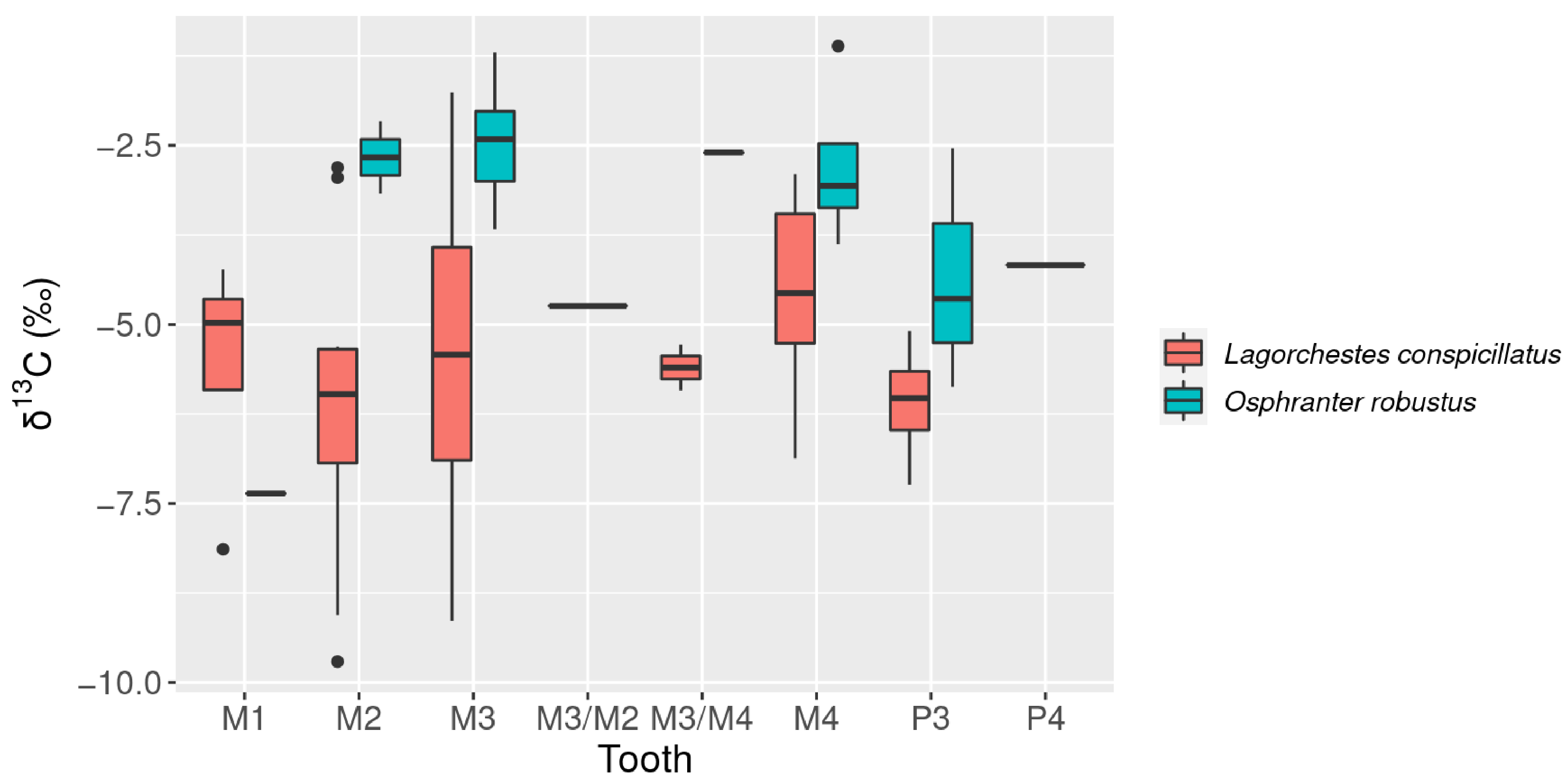
3.1. Carbon Isotopes
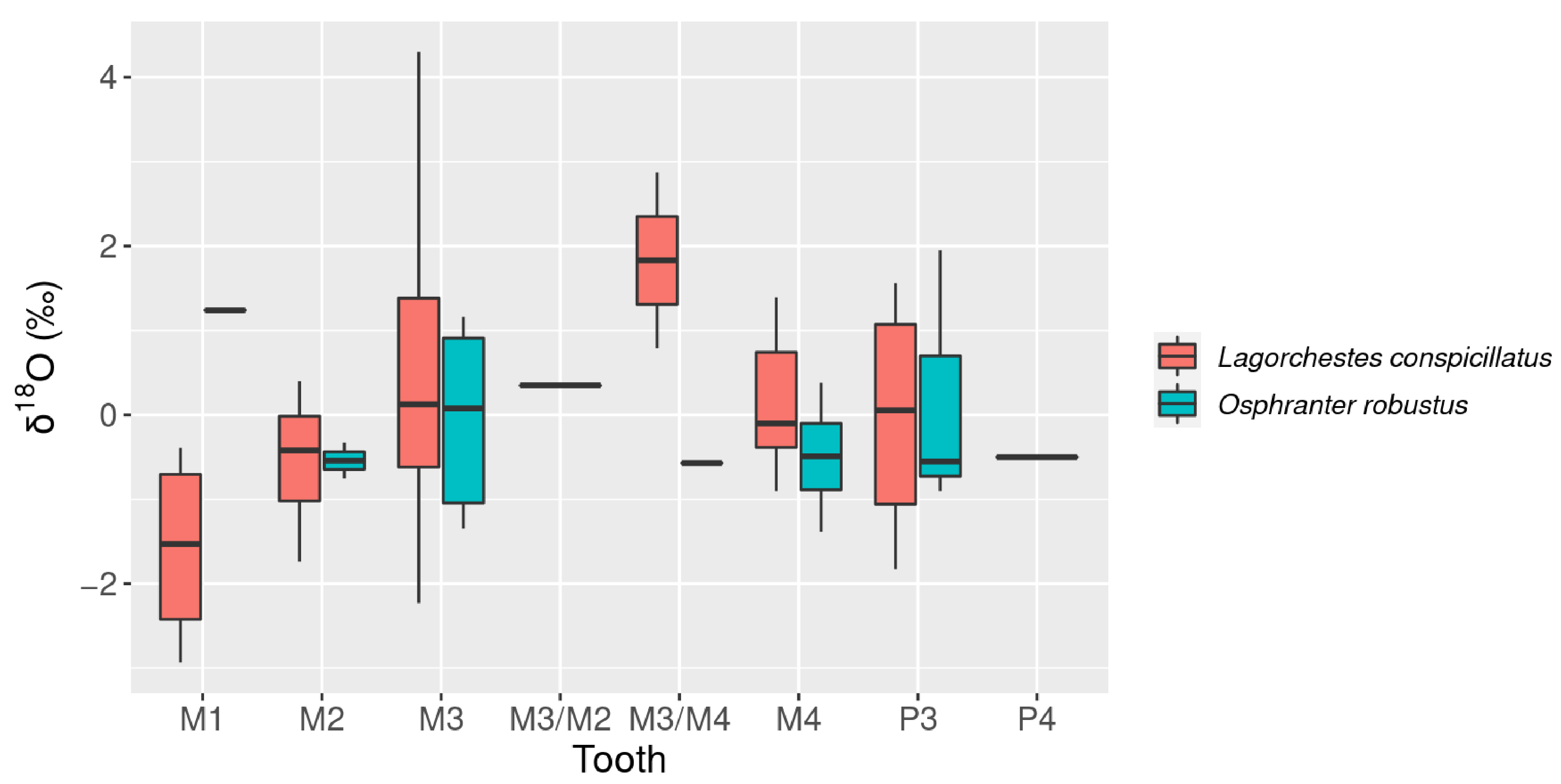
3.2. Oxygen Isotopes
4. Discussion
4.1. Feeding Ecology and Paleo-Vegetation
4.2. Paleohumidity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Denniston, R.F.; Asmerom, Y.; Lachniet, M.; Polyak, V.J.; Hope, P.; An, N.; Rodzinyak, K.; Humphreys, W.F. A Last Glacial Maximum through middle Holocene stalagmite record of coastal Western Australia climate. Quat. Sci. Rev. 2013, 77, 101–112. [Google Scholar] [CrossRef]
- Hattersley, P.W. The distribution of C3 and C4 grasses in Australia in relation to climate. Oecologia 1983, 57, 113–128. [Google Scholar] [CrossRef]
- Short, S.; Turner, B. Distribution and Abundance of Spectacled Hare-wallabies and Euros on Barrow Island, Western Australia. Wildl. Res. 1991, 18, 421–429. [Google Scholar] [CrossRef]
- Veth, P.; Kendrick, P.; Ward, I.; Manne, T.; Ulm, S.; Ditchfeild, K.; Dortch, J.; Hook, F.; Petchey, F.; Hogg, A.; et al. Early human occupation of a Maritime Desert, Barrow Island, North-West Australia. Quat. Sci. Rev. 2017, 168, 19–29. [Google Scholar] [CrossRef]
- Ward, I.; Veth, P.; Prossor, L.; Denham, T.; Ditchfeild, K.; Manne, T.; Kendrick, P.; Byrne, C.; Hook, F.; Troitzsch, U. 50,000 years of archaeological site stratigraphy and micromorphology in Boodie Cave, Barrow Island, Western Australia. J. Archaeol. Sci. Rep. 2017, 15, 344–369. [Google Scholar] [CrossRef]
- Manne, T.; Veth, P.M. Late Pleistocene and early Holocene exploitation of estuarine communities in northwestern Australia. Quat. Int. 2015, 385, 112–123. [Google Scholar] [CrossRef]
- Balasse, M.; Ambrose, S. The seasonal mobility model for prehistoric herders in the south-western cape of south Africa assessed by isotopic analysis of sheep tooth enamel. J. Archaeol. Sci. 2002, 29, 917–932. [Google Scholar] [CrossRef]
- Balasse, M.; Smith, A.B.; Ambrose, S.H.; Leigh, S.R. Determining sheep birth seasonality by analysis of tooth enamel oxygen isotope ratios: The late Stone Age site of Kasteelberg (South Africa). J. Archaeol. Sci. 2003, 30, 205–215. [Google Scholar] [CrossRef]
- Cerling, T.E.; Harris, J.M. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 1999, 120, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Clementz, M.T. New Insights from Old Bones: Stable Isotope Analysis of Fossil Mammals. Am. Soc. Mammal. 2012, 93, 368–380. [Google Scholar] [CrossRef]
- Fraser, R.A.; Grun, R.; Privat, K.; Gagan, M.K. Stable-isotope microprofiling of wombat tooth enamel records seasonal changes in vegetation and environmental conditions in eastern Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 269, 66–77. [Google Scholar] [CrossRef]
- Pilaar Birch, S.E. Stable isotopes in zooarchaeology: An introduction. Archaeol. Anthropol. Sci. 2013, 5, 81–83. [Google Scholar] [CrossRef]
- Balasse, M. Reconstructing dietary and environmental history from enamel isotopic analysis: Time resolution of intra-tooth sequential sampling. Int. J. Osteoarchaeol. 2002, 12, 155–165. [Google Scholar] [CrossRef]
- Koch, P. Isotopic Studies of the Biology of Modern and Fossil Vertebrates. In Stable Isotopes in Ecology and Environmental Science; Michener, R., Lajtha, K., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 99–154. [Google Scholar]
- Wang, Y.; Cerling, T.E. A model of fossil tooth and bone diagenesis: Implications for paleodiet reconstruction from stable isotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 107, 281–289. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M.J.S.; Gagan, M.K. Sources of carbon isotope variation in kangaroo bone collagen and tooth enamel. Geochim. Cosmochim. Acta 2007, 71, 3847–3858. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M.J.S.; Gagan, M.K. The interactive effect of temperature and humidity on the oxygen isotope composition of kangaroos. Funct. Ecol. 2007, 21, 757–766. [Google Scholar] [CrossRef]
- Prideaux, G.J.; Long, J.A.; Ayliffe, L.K.; Hellstrom, J.C.; Pillans, B.; Boles, W.E.; Hutchinson, M.N.; Roberts, R.G.; Cupper, M.L.; Arnold, L.J.; et al. An arid-adapted middle Pleistocene vertebrate fauna from south-central Australia. Nature 2007, 445, 422–425. [Google Scholar] [CrossRef]
- Forbes, M.S.; Kohn, M.J.; Bestland, E.A.; Wells, R.T. Late Pleistocene environmental change interpreted from δ13C and δ18O of tooth enamel from the Black Creek Swamp Megafauna site, Kangaroo Island, South Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 319–327. [Google Scholar] [CrossRef]
- Brookman, T.H.; Ambrose, S.H. Seasonal variation in kangaroo tooth enamel oxygen and carbon isotopes in southern Australia. Quat. Res. 2012, 78, 256–265. [Google Scholar] [CrossRef]
- Montanari, S.; Louys, J.; Price, G. Pliocene paleoenvironments of Southeastern Queensland, Australia inferred from stable isotopes of marsupial tooth enamel. PLoS ONE 2013, 8, e66221. [Google Scholar] [CrossRef]
- Garvie-Lok, S.J.; Varney, T.L.; Katzenberg, M.A. Preparation of bone carbonate for stable isotope analysis: The effects of treatment time and acid concentration. J. Archaeol. Sci. 2004, 31, 763–776. [Google Scholar] [CrossRef]
- Koch, P.L.; Tuross, N.; Fogel, M.L. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J. Archaeol. Sci. 1997, 24, 417–429. [Google Scholar] [CrossRef]
- Skippington, J.; Veth, P.; Manne, T.; Slack, M. Preanalytical processing of archaeological mammal enamel apatite carbonates for stable isotope investigations: A comparative analysis of the effect of acid treatment on samples from Northwest Australia. International. Int. J. Osteoarchaeol. 2019, 29, 760–771. [Google Scholar] [CrossRef]
- Paul, D.; Skrzypek, G. Assessment of carbonate-phosphoric acid analytical technique performed using GasBench II in continuous flow isotope ratio mass spectrometry. Int. J. Mass Spectrom. 2007, 262, 180–186. [Google Scholar] [CrossRef]
- Skrzypek, G. Normalization procedures and reference material selection in stable HCNOS isotope analyses—An overview. Anal. Bioanal. Chem. 2013, 405, 2815–2823. [Google Scholar] [CrossRef]
- Fitzsimmons, K.E.; Cohen, T.J.; Hesse, P.P.; Jansen, J.; Nanson, G.C.; May, J.; Barrows, T.T.; Haberlah, D.; Hilgers, A.; Kelly, T.; et al. Late Quaternary palaeoenvironmental change in the Australian drylands. Quat. Sci. Rev. 2013, 74, 78–96. [Google Scholar] [CrossRef]
- Friedli, H.; Loetscher, H.; Oeschger, H.; Siegenthaler, U.; Stauffer, B. Ice core record of the 13C/12C ratio of atmospheric CO2 in the past two centuries. Nature 1986, 324, 237–238. [Google Scholar] [CrossRef]
- Leuenberger, M.; Siegenthaler, U.; Langway, C. Carbon isotope compositionof atmospheric CO2 during the last ice age from an Antarctic ice core. Nature 1992, 357, 488–490. [Google Scholar] [CrossRef]
- O’Leary, M. Carbon isotopes in photosynthesis. Bioscience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Johnson, B.J.; Miller, G.H.; Fogel, M.L.; Beaumont, P.B. The determination of late Quaternary paleoenvironments at Equus Cave, South Africa, using stable isotopes and amino acid racemization in ostrich eggshell. Palaeogeog. Palaeoclim. Palaeoecol. 1997, 136, 121–137. [Google Scholar] [CrossRef]
- Ealey, E.H.M. Ecology of the Euro, Macropus robustus (Gould) in north-western Australia: Age and growth. CSIRO Wildl. Res. 1967, 12, 67–80. [Google Scholar] [CrossRef]
- Hume, I. Digestive Physiology and Nutrition of Marsupial; Cambridge University Press: London, UK, 1982. [Google Scholar]
- Jackson, S. Australian Mammals: Biology and Captive Management; CSIRO Publishing: Collingwood, Australia, 2003. [Google Scholar]
- Jackson, S.; Groves, C. Taxonomy of Australian Mammals; CSIRO Publishing: Clayton, Australia, 2015. [Google Scholar]
- Main, A.R.; Yadav, M. Conservation of macropods in reserves in Western Australia. Biol. Conserv. 1971, 3, 123–133. [Google Scholar] [CrossRef]
- Burbidge, A.A.; Johnson, K.A. Spectacled Hare-wallaby, Lagorchestes conspicillatus. In Complete Book of Australian Mammals; Strahan, R., Ed.; Angus and Robertson: Sydney, Australia, 1983; pp. 197–198. [Google Scholar]
- Lngleby, S.; Westoby, M. Habitat Requirements of the Spectacled Hare-wallaby (Lagorchestes conspicillatus) in the Northern Territory and Western Australia. Wildl. Res. 1992, 19, 721–741. [Google Scholar] [CrossRef]
- Strahan, R. (Ed.) The Mammals of Australia; Reed Books: Chatswood, Australia, 1995. [Google Scholar]
- Morton, S.R.; Smith, D.M.S.; Dickman, C.R.; Dunkerley, D.L.; Friedel, M.H.; McAllister, R.R.J.; Reid, J.R.W.; Roshier, D.A.; Smith, M.A.; Walsh, F.J.; et al. A fresh framework for the ecology of arid Australia. J. Arid Environ. 2011, 75, 313–329. [Google Scholar] [CrossRef]
- Williams, A.N.; Veth, P.; Steffan, W.; Ulm, S.; Turney, C.S.; Reeves, J.M.; Phipps, S.J.; Smith, M. A continental narrative: Human settlement patterns and Australian climate change over the last 35,000 years. Quat. Sci. Rev. 2015, 28, 91–112. [Google Scholar] [CrossRef]
- van Etten, E.J.B. Inter-annual rainfall variability of arid Australia: Greater than elsewhere? Aust. Geogr. 2009, 40, 109–120. [Google Scholar] [CrossRef]
- King, J.M.; Bradshaw, S.D. Stress in an island kangaroo? The Barrow Island euro, Macropus robustus isabellinus. Gen. Comp. Endocrinol. 2010, 167, 60–67. [Google Scholar] [CrossRef]
- Ayliffe, L.K.; Chivas, A.R. Oxygen isotope composition of the bone phosphate of Australian kangaroos: Potential as a palaeoenvironmental recorder. Geochim. Cosmochim. 1990, 54, 2603–2609. [Google Scholar] [CrossRef]
- Veth, P.; Smith, M.; Bowler, J.M.; Fitzsimmons, K.E.; Williams, A.; Hiscock, P. Excavations at Parnkupirti, Lake Gregory, Great Sandy Desert: OSL ages for occupation before the Last Glacial Maximum. Aust. Archaeol. 2009, 69, 1–10. [Google Scholar] [CrossRef]
- De Deckker, P.; Moros, M.; Perner, K.; Blanz, T.; Wacker, L.; Schneider, R.; Barrows, T.T.; O’Loingsigh, T.; Jansen, E. Climatic evolution in the Australian region over the last 94 ka—Spanning human occupancy—And unveiling the Last Glacial Maximum. Quat. Sci. Rev. 2020, 249, 106593. [Google Scholar] [CrossRef]




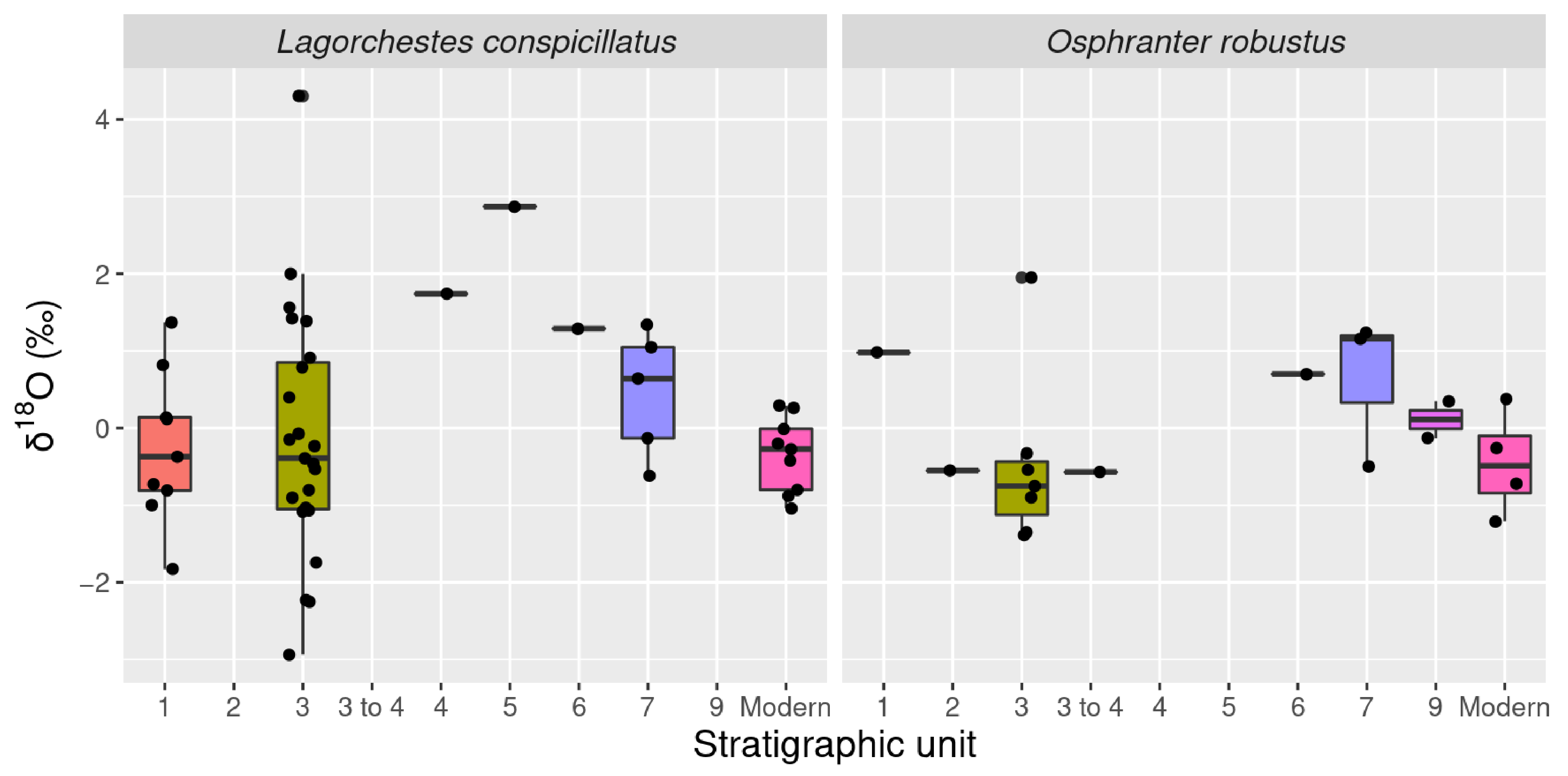
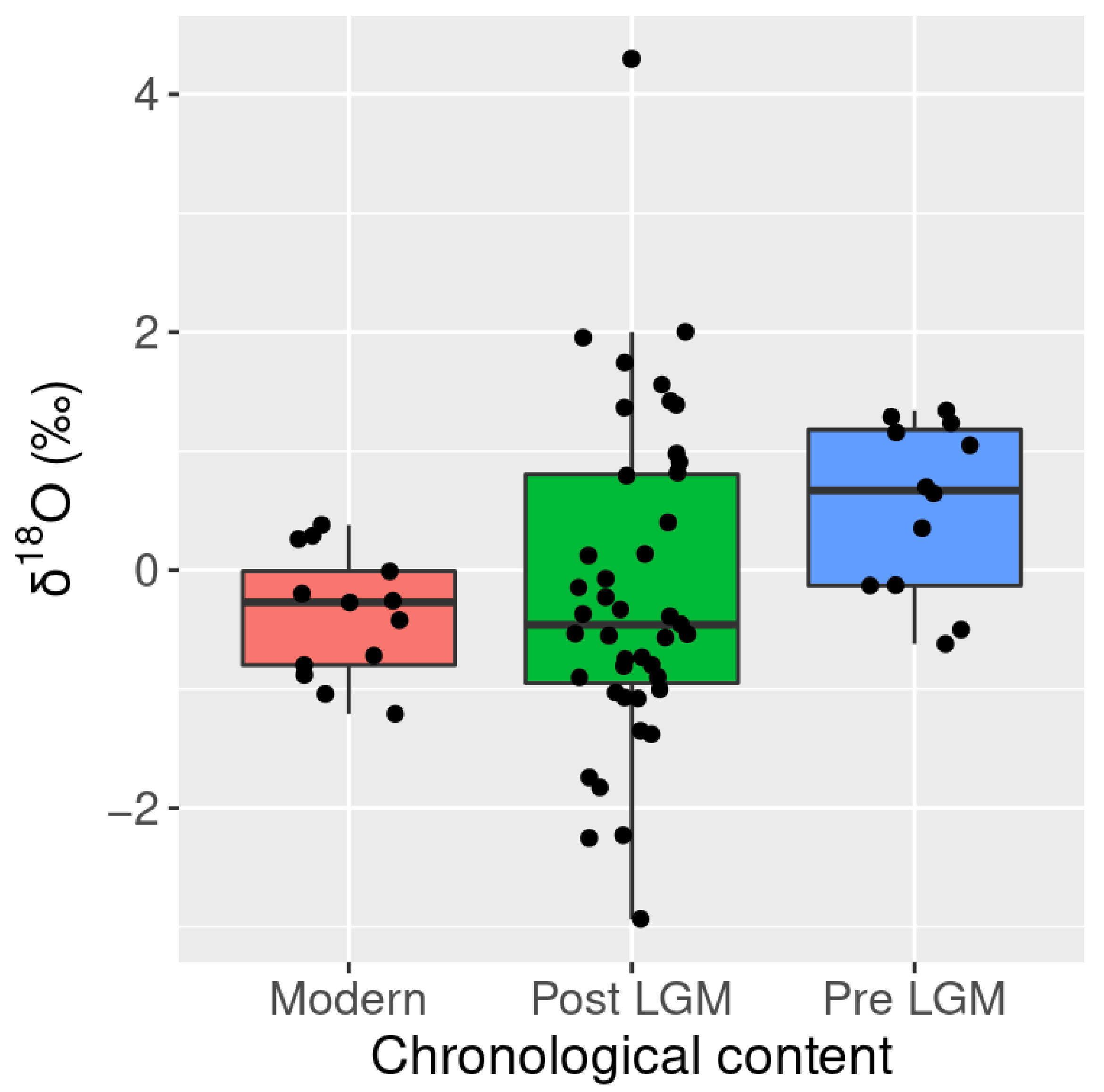
| Species | Type | n | δ13C 1 | sd | δ18O 1 | sd |
|---|---|---|---|---|---|---|
| L. conspicillatus | Archaeological | 40 | −5.22 | 1.74 | −0.07 | 1.45 |
| Modern | 9 | −6.58 | 1.70 | −0.34 | 0.49 | |
| O. robustus | Archaeological | 16 | −3.46 | 1.76 | −0.04 | 0.99 |
| Modern | 4 | −3.28 | 0.41 | −0.45 | 0.68 |
| Stratigraphic Unit | Chronology (ka BP) 1 | Local Paleoenvironmental Context 1 | Regional Climate Context 2 |
|---|---|---|---|
| 1 | 1.7–2.5 | Fully marine (island) | MIS1, Holocene variability |
| 2 | 2.5−6.8 | Fully marine (island) | |
| 3 | 6.8–7.2 | Islandization | |
| 4 | 7.2–7.4 | Islandization | |
| 5 | 7.4–22.4 | Transgressing shoreline | Start of MIS1 at 14 ka, deglacial variability |
| Discontinuity | MIS2, LGM aridity and complex glacial response | ||
| 6 | 36.6–42.6 | Regressing Shoreline | Late MIS3, humid phase, and initiation of cooler conditions |
| 7 | 42.6–46.2 | Regressing Shoreline | |
| Discontinuity | MIS3 | ||
| 8 | 46.2–51.1 | Transgressing Shoreline; cave opens | MIS3 |
| 9 | ~77 | Continental–regressing shoreline; cave closed | MIS4 |
| Species | Type | δ13Cenamel | δ13Cdiet 1 | %C3 Diet 2 |
|---|---|---|---|---|
| L. conspicillatus | Archaeological | −5.22 | −17.22 | 23.00% |
| Modern | −6.58 | −18.58 | 32.71% | |
| O. robustus | Archaeological | −3.46 | −15.46 | 10.43% |
| Modern | −3.28 | −15.28 | 9.14% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skippington, J.; Manne, T.; Veth, P. Isotopic Indications of Late Pleistocene and Holocene Paleoenvironmental Changes at Boodie Cave Archaeological Site, Barrow Island, Western Australia. Molecules 2021, 26, 2582. https://doi.org/10.3390/molecules26092582
Skippington J, Manne T, Veth P. Isotopic Indications of Late Pleistocene and Holocene Paleoenvironmental Changes at Boodie Cave Archaeological Site, Barrow Island, Western Australia. Molecules. 2021; 26(9):2582. https://doi.org/10.3390/molecules26092582
Chicago/Turabian StyleSkippington, Jane, Tiina Manne, and Peter Veth. 2021. "Isotopic Indications of Late Pleistocene and Holocene Paleoenvironmental Changes at Boodie Cave Archaeological Site, Barrow Island, Western Australia" Molecules 26, no. 9: 2582. https://doi.org/10.3390/molecules26092582
APA StyleSkippington, J., Manne, T., & Veth, P. (2021). Isotopic Indications of Late Pleistocene and Holocene Paleoenvironmental Changes at Boodie Cave Archaeological Site, Barrow Island, Western Australia. Molecules, 26(9), 2582. https://doi.org/10.3390/molecules26092582






