Evaluation of Small Molecule Combinations against Respiratory Syncytial Virus In Vitro
Abstract
1. Introduction
2. Results
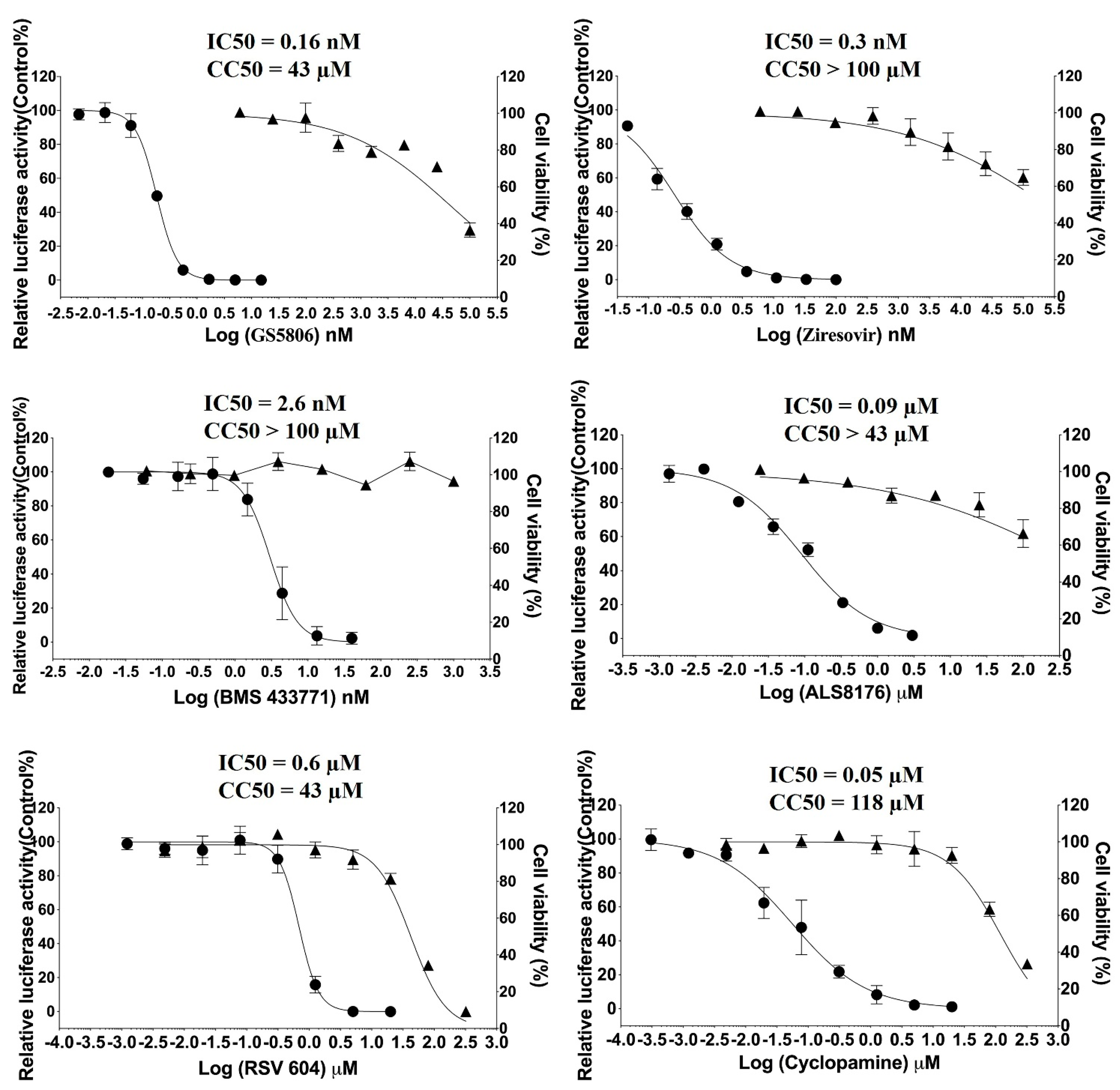
2.1. Antiviral Activity of Individual Compounds against RSV In Vitro
2.2. Antiviral Activity of GS5806 Combined with RdRp Inhibitors against RSV In Vitro
2.3. Antiviral Activity of Ziresovir Ccombined with RdRp Inhibitors against RSV In Vitro
2.4. Antiviral Activity of BMS433771 Combined with RdRp Inhibitors against RSV In Vitro
2.5. Antiviral Activity of Two RdRp Inhibitors in Combination against RSV In Vitro
2.6. Antiviral Activity of Two Fusion Inhibitors in Combination against RSV In Vitro
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell and Virus
4.3. Virus Iinhibition and Cytotoxicity Assays
4.4. Quantitative PCR
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Yassine, H.M.; Sohail, M.U.; Younes, N.; Nasrallah, G.K. Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms 2020, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Schildgen, O. The lack of protective immunity against RSV in the elderly. Epidemiol. Infect. 2009, 137, 1687–1690. [Google Scholar] [CrossRef]
- Rey-Jurado, E.; Kalergis, A.M. Immunological Features of Respiratory Syncytial Virus-Caused Pneumonia-Implications for Vaccine Design. Int. J. Mol. Sci. 2017, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of severe RSV disease among immunocompromised children and adults: A 10 year retrospective study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Heylen, E.; Neyts, J.; Jochmans, D. Drug candidates and model systems in respiratory syncytial virus antiviral drug discovery. Biochem. Pharmacol. 2017, 127, 1–12. [Google Scholar] [CrossRef]
- Zuccotti, G.; Fabiano, V. Indications to respiratory syncytial virus immunoprophylaxis in the 29-32 wGA group: Is there still room for debating? Ital. J. Pediatr. 2017, 43, 17. [Google Scholar] [CrossRef]
- Jorquera, P.A.; Tripp, R.A. Respiratory syncytial virus: Prospects for new and emerging therapeutics. Expert Rev. Respir. Med. 2017, 11, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Rameix-Welti, M.-A.; Le Goffic, R.; Hervé, P.-L.; Sourimant, J.; Rémot, A.; Riffault, S.; Yu, Q.; Galloux, M.; Gault, E.; Eléouët, J.-F. Visualizing the replication of respiratory syncytial virus in cells and in living mice. Nat. Commun. 2014, 5, 5104. [Google Scholar] [CrossRef]
- Pickles, R.J.; DeVincenzo, J.P. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J. Pathol. 2015, 235, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Noton, S.L.; Deflube, L.R.; Tremaglio, C.Z.; Fearns, R. The respiratory syncytial virus polymerase has multiple RNA synthesis activities at the promoter. PLoS Pathog. 2012, 8, e1002980. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, T.; He, H.; Qiu, H.; Wu, X.; Chen, J.; Wang, M.; Chen, C.; Huang, S. Respiratory syncytial virus prolifically infects N2a neuronal cells, leading to TLR4 and nucleolin protein modulations and RSV F protein co-localization with TLR4 and nucleolin. J. Biomed. Sci. 2018, 25, 13. [Google Scholar] [CrossRef]
- Sperandio, D.; Mackman, R. CHAPTER 2. Respiratory Syncytial Virus Fusion Inhibitors. In Successful Strategies for the Discovery of Antiviral Drugs; Royal Society of Chemistry: London, UK, 2013; pp. 29–62. [Google Scholar]
- Fearns, R.; Deval, J. New antiviral approaches for respiratory syncytial virus and other mononegaviruses: Inhibiting the RNA polymerase. Antivir. Res. 2016, 134, 63–76. [Google Scholar] [CrossRef]
- Fearns, R.; Plemper, R.K. Polymerases of paramyxoviruses and pneumoviruses. Virus Res. 2017, 234, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Pichon, M.; Valette, M.; Schuffenecker, I.; Billaud, G.; Lina, B. Analytical Performances of the Panther Fusion System for the Detection of Respiratory Viruses in the French National Reference Centre of Lyon, France. Microorganisms 2020, 8, 9. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, H.C.; Tripp, R.A. Emerging small and large molecule therapeutics for respiratory syncytial virus. Expert Opin. Investig. Drugs 2020, 29, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Bailly, B.; Richard, C.A.; Sharma, G.; Wang, L.; Johansen, L.; Cao, J.; Pendharkar, V.; Sharma, D.C.; Galloux, M.; Wang, Y.; et al. Targeting human respiratory syncytial virus transcription anti-termination factor M2-1 to inhibit in vivo viral replication. Sci. Rep. 2016, 6, 25806. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.; Scott, A.D.; Yuzhakov, O.; Zhou, Y.; Tiong-Yip, C.L.; Gao, N.; Thresher, J.; Yu, Q. Mechanism of Action for Respiratory Syncytial Virus Inhibitor RSV604. Antimicrob. Agents Chemother. 2015, 59, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Abbott, E.; Alber, D.G.; Baxter, R.C.; Bithell, S.K.; Henderson, E.A.; Carter, M.C.; Chambers, P.; Chubb, A.; Cockerill, G.S.; et al. RSV604, a Novel Inhibitor of Respiratory Syncytial Virus Replication. Antimicrob. Agents Chemother. 2007, 51, 3346–3353. [Google Scholar] [CrossRef]
- DeVincenzo, J.P.; McClure, M.W.; Symons, J.A.; Fathi, H.; Westland, C.; Chanda, S.; Lambkin-Williams, R.; Smith, P.; Zhang, Q.; Beigelman, L.; et al. Activity of Oral ALS-008176 in a Respiratory Syncytial Virus Challenge Study. N. Engl. J. Med. 2015, 373, 2048–2058. [Google Scholar] [CrossRef]
- Mackman, R.L.; Sangi, M.; Sperandio, D.; Parrish, J.P.; Eisenberg, E.; Perron, M.; Hui, H.; Zhang, L.; Siegel, D.; Yang, H.; et al. Discovery of an Oral Respiratory Syncytial Virus (RSV) Fusion Inhibitor (GS-5806) and Clinical Proof of Concept in a Human RSV Challenge Study. J. Med. Chem. 2015, 58, 1630–1643. [Google Scholar] [CrossRef]
- Perron, M.; Stray, K.; Kinkade, A.; Theodore, D.; Lee, G.; Eisenberg, E.; Sangi, M.; Gilbert, B.E.; Jordan, R.; Piedra, P.A.; et al. GS-5806 Inhibits a Broad Range of Respiratory Syncytial Virus Clinical Isolates by Blocking the Virus-Cell Fusion Process. Antimicrob. Agents Chemother. 2015, 60, 1264–1273. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, L.; Wang, L.; Liang, C.; Wang, B.; Liu, Y.; Feng, S.; Zhang, B.; Zhou, M.; Yu, X.; et al. Discovery of Ziresovir as a Potent, Selective, and Orally Bioavailable Respiratory Syncytial Virus Fusion Protein Inhibitor. J. Med. Chem. 2019, 62, 6003–6014. [Google Scholar] [CrossRef]
- Cianci, C.; Meanwell, N.; Krystal, M. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. J. Antimicrob. Chemother. 2005, 55, 289–292. [Google Scholar] [CrossRef]
- Cianci, C.; Yu, K.-L.; Combrink, K.; Sin, N.; Pearce, B.; Wang, A.; Civiello, R.; Voss, S.; Luo, G.; Kadow, K.; et al. Orally Active Fusion Inhibitor of Respiratory Syncytial Virus. Antimicrob. Agents Chemother. 2004, 48, 413–422. [Google Scholar] [CrossRef]
- Cox, R.; Plemper, R.K. Structure-guided design of small-molecule therapeutics against RSV disease. Expert Opin. Drug Discov. 2016, 11, 543–556. [Google Scholar] [CrossRef]
- Caidi, H.; Harcourt, J.L.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Combination therapy using monoclonal antibodies against respiratory syncytial virus (RSV) G glycoprotein protects from RSV disease in BALB/c mice. PLoS ONE 2012, 7, e51485. [Google Scholar] [CrossRef]
- Rehman, S.; Ashfaq, U.A.; Ijaz, B.; Riazuddin, S. Anti-hepatitis C virus activity and synergistic effect of Nymphaea alba extracts and bioactive constituents in liver infected cells. Microb. Pathog. 2018, 121, 198–209. [Google Scholar] [CrossRef]
- O’Brien, M.S.; Markovich, K.C.; Selleseth, D.; DeVita, A.V.; Sethna, P.; Gentry, B.G. In vitro evaluation of current and novel antivirals in combination against human cytomegalovirus. Antivir. Res. 2018, 158, 255–263. [Google Scholar] [CrossRef]
- Chou, S.; Ercolani, R.J.; Derakhchan, K. Antiviral activity of maribavir in combination with other drugs active against human cytomegalovirus. Antivir. Res. 2018, 157, 128–133. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Yuan, S.; Gao, Q.; Lan, K.; Altmeyer, R.; Zou, G. In VitroAssessment of Combinations of Enterovirus Inhibitors against Enterovirus 71. Antimicrob. Agents Chemother. 2016, 60, 5357–5367. [Google Scholar] [CrossRef]
- Checkmahomed, L.; Padey, B.; Pizzorno, A.; Terrier, O.; Rosa-Calatrava, M.; Abed, Y.; Baz, M.; Boivin, G. In Vitro Combinations of Baloxavir Acid and Other Inhibitors against Seasonal Influenza A Viruses. Viruses 2020, 12, 10. [Google Scholar] [CrossRef]
- Schloer, S.; Goretzko, J.; Pleschka, S.; Ludwig, S.; Rescher, U. Combinatory Treatment with Oseltamivir and Itraconazole Targeting Both Virus and Host Factors in Influenza A Virus Infection. Viruses 2020, 12, 7. [Google Scholar] [CrossRef]
- Snyder, B.; Goebel, S.; Koide, F.; Ptak, R.; Kalkeri, R. Synergistic antiviral activity of Sofosbuvir and type-I interferons (alpha and beta) against Zika virus. J. Med. Virol. 2018, 90, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Shipman, C. A 3-dimensional model to analyze drug-drug interactions. Antivir. Res. 1990, 14, 181–206. [Google Scholar] [CrossRef]
- Mirabelli, C.; Jaspers, M.; Boon, M.; Jorissen, M.; Koukni, M.; Bardiot, D.; Chaltin, P.; Marchand, A.; Neyts, J.; Jochmans, D. Differential antiviral activities of respiratory syncytial virus (RSV) inhibitors in human airway epithelium. J. Antimicrob. Chemother. 2018, 73, 1823–1829. [Google Scholar] [CrossRef]
- Huntjens, D.R.H.; Ouwerkerk-Mahadevan, S.; Brochot, A.; Rusch, S.; Stevens, M.; Verloes, R. Population Pharmacokinetic Modeling of JNJ-53718678, a Novel Fusion Inhibitor for the Treatment of Respiratory Syncytial Virus: Results from a Phase I, Double-Blind, Randomized, Placebo-Controlled First-in-Human Study in Healthy Adult Subjects. Clin. Pharm. 2017, 56, 1331–1342. [Google Scholar] [CrossRef]
- DeVincenzo, J.P.; Whitley, R.J.; Mackman, R.L.; Scaglioni-Weinlich, C.; Harrison, L.; Farrell, E.; McBride, S.; Lambkin-Williams, R.; Jordan, R.; Xin, Y.; et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N. Engl. J. Med. 2014, 371, 711–722. [Google Scholar] [CrossRef]
- Wang, G.; Deval, J.; Hong, J.; Dyatkina, N.; Prhavc, M.; Taylor, J.; Fung, A.; Jin, Z.; Stevens, S.K.; Serebryany, V.; et al. Discovery of 4′-chloromethyl-2′-deoxy-3′,5′-di-O-isobutyryl-2′-fluorocytidine (ALS-8176), a first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J. Med. Chem. 2015, 58, 1862–1878. [Google Scholar] [CrossRef] [PubMed]
- Deval, J.; Fung, A.; Stevens, S.K.; Jordan, P.C.; Gromova, T.; Taylor, J.S.; Hong, J.; Meng, J.; Wang, G.; Dyatkina, N.; et al. Biochemical Effect of Resistance Mutations against Synergistic Inhibitors of RSV RNA Polymerase. PLoS ONE 2016, 11, e0154097. [Google Scholar] [CrossRef] [PubMed]
- Wildum, S.; Zimmermann, H.; Lischka, P. In vitro drug combination studies of letermovir (AIC246, MK-8228) with approved anti-human cytomegalovirus (HCMV) and anti-HIV compounds in inhibition of HCMV and HIV replication. Antimicrob. Agents Chemother. 2015, 59, 3140–3148. [Google Scholar] [CrossRef]






| Compound A | Compound B | Synergy/Antagonism (μM2%) a by MacSynergy II Analysis | Synergy/Antagonism a |
|---|---|---|---|
| GS5806 c | ALS8176 d | 31.53/0 | Additivity |
| ALS8176 b,d | 58/0 | Slight synergy | |
| RSV604 d | 31/−5 | Additivity | |
| Cyclopamine d | 0/−19 | Additivity | |
| Ziresovir c | 0/−50 | Additivity | |
| BMS433771 c | 0/−55 | Slight antagonism | |
| Ziresovir c | ALS8176 d | 50/0 | Additivity |
| RSV604 d | 0/−5 | Additivity | |
| Cyclopamine d | 0/−25 | Additivity | |
| BMS433771 c | 0/−176 | Strong antagonism | |
| BMS433771 c | ALS8176 d | 16/0 | Additivity |
| RSV604 d | 64/0 | Slight synergy | |
| Cyclopamine d | 0/−18 | Additivity | |
| ALS8176 d | RSV604 d | 20/−5 | Additivity |
| Cyclopamine d | 0/−20 | Additivity | |
| RSV604 d | Cyclopamine d | 0/−50 | Additivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Cao, J.; Xing, P.; Altmeyer, R.; Zhang, Y. Evaluation of Small Molecule Combinations against Respiratory Syncytial Virus In Vitro. Molecules 2021, 26, 2607. https://doi.org/10.3390/molecules26092607
Gao Y, Cao J, Xing P, Altmeyer R, Zhang Y. Evaluation of Small Molecule Combinations against Respiratory Syncytial Virus In Vitro. Molecules. 2021; 26(9):2607. https://doi.org/10.3390/molecules26092607
Chicago/Turabian StyleGao, Yuzhen, Jingjing Cao, Pan Xing, Ralf Altmeyer, and Youming Zhang. 2021. "Evaluation of Small Molecule Combinations against Respiratory Syncytial Virus In Vitro" Molecules 26, no. 9: 2607. https://doi.org/10.3390/molecules26092607
APA StyleGao, Y., Cao, J., Xing, P., Altmeyer, R., & Zhang, Y. (2021). Evaluation of Small Molecule Combinations against Respiratory Syncytial Virus In Vitro. Molecules, 26(9), 2607. https://doi.org/10.3390/molecules26092607





