The Enantiomeric Discrimination of 5-Hexyl-2-methyl-3,4-dihydro-2H-pyrrole by Sulfobutyl ether-β-cyclodextrin: A Case Study
Abstract
1. Introduction
2. Results and Discussion
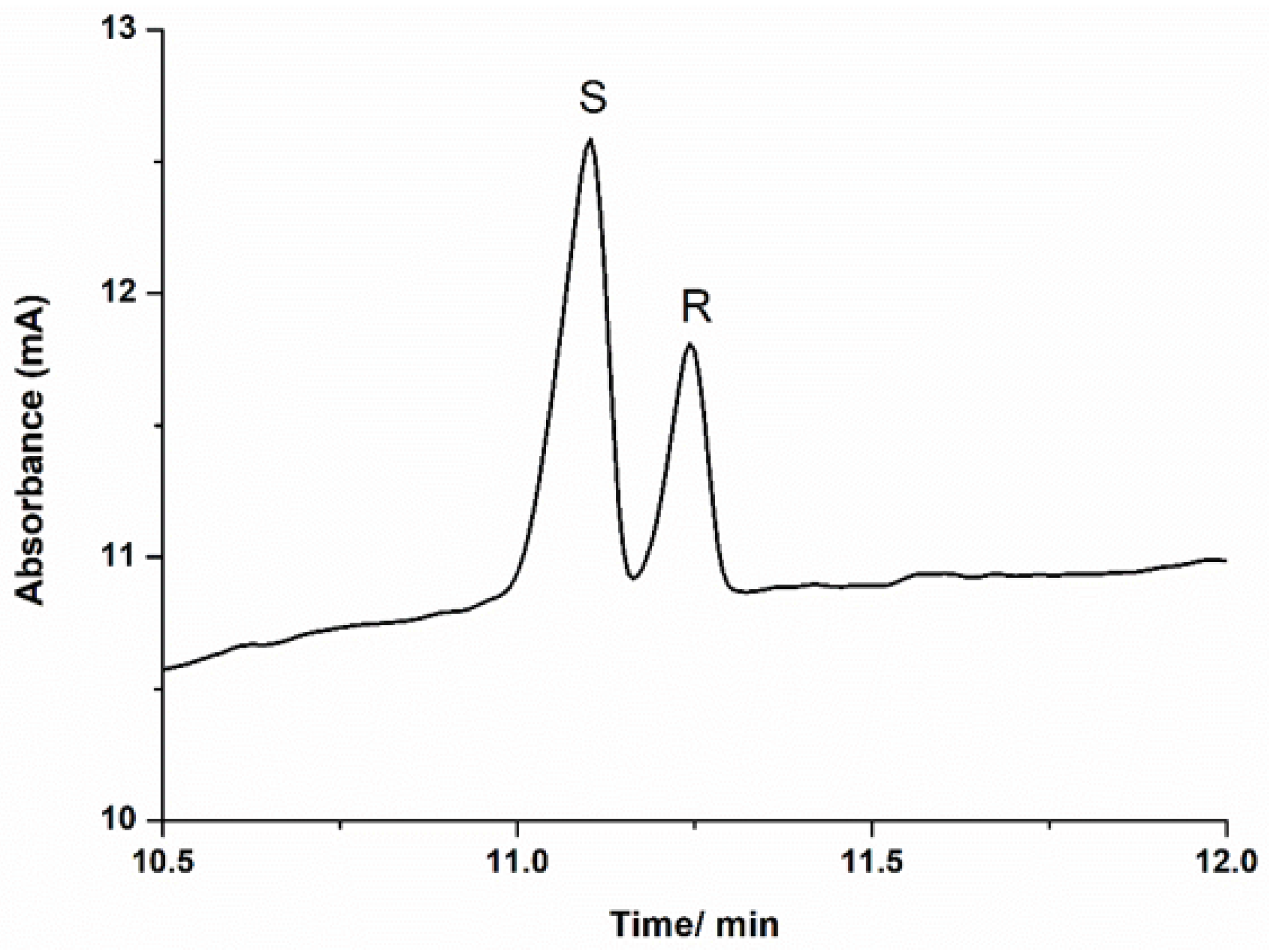
2.1. CE Analysis
2.2. Docking Simulations
3. Experimental Section
3.1. General Methods
3.2. Synthesis of (R)- and (S)-5-Hexyl-2-methyl-3,4-dihydro-2H-pyrrole 2
3.3. Docking Simulations
3.4. Electrophoresis Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daly, J.W.; Spande, T.F.; Garraffo, H.M. Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J. Nat. Prod. 2005, 68, 1556–1575. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cantrell, C.L.; Oi, D.; Grodowitz, M.J. Update on the defensive chemicals of the little black ant, Monomorium minimum (Hymenoptera: Formicidae). Toxicon 2016, 122, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.Z.; Galman, J.L.; Slabu, I.; France, S.P.; Marsaioli, A.J.; Turner, N.J. Synthesis of 2,5-Disubstituted Pyrrolidine Alkaloids via A One-Pot Cascade Using Transaminase and Reductive Aminase Biocatalysts. ChemCatChem 2018, 10, 4733–4738. [Google Scholar] [CrossRef]
- Juvancz, Z.; Kendrovics, R.B.; Iványi, R.; Szente, L. The role of cyclodextrins in chiral capillary electrophoresis. Electrophoresis 2008, 29, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.; Hou, T. End-point binding free energy calculation with MM/PBSA and MM/GBSA: Strategies and applications in drug design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, R.T. Structure-Based Drug Design: Docking and Scoring. Curr. Protein Pept. Sci. 2007, 8, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK, 2009; Volume 23. [Google Scholar]
- Ferreira De Freitas, R.; Schapira, M. A systematic analysis of atomic protein-ligand interactions in the PDB. MedChemComm 2017, 8, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.S.; Date, A.A.; Pissurlenkar, R.R.; Coutinho, E.C.; Nagarsenker, M.S. Sulfobutyl ether 7 β-cyclodextrin (SBE 7 β-CD) carbamazepine complex: Preparation, characterization, molecular modeling, and evaluation of in vivo anti-epileptic activity. AAPS PharmSciTech 2011, 12, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Rudolph, C.; Sadowski, J. Automatic generation of 3D-atomic coordinates for organic molecules. Tetrahedron Comput. Methodol. 1990, 3, 537–547. [Google Scholar] [CrossRef]
- Sadowski, J.; Gasteiger, J.; Klebe, G. Comparison of automatic three-dimensional model builders using 639 X-ray structures. J. Chem. Inf. Comput. Sci. 1994, 34, 1000–1008. [Google Scholar] [CrossRef]
- Discovery Studio Modeling Environment; Release 4.0; Accelrys Software Inc.: San Diego, CA, USA, 2006.
- Wavefunction. Spartan, Version 14; Wavefunction Inc.: Irvine, CA, USA, 2014. [Google Scholar]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, D.F.; Costa, B.Z.; Romero-Orejón, K.L.; Loureiro, H.C.; de Jesus, D.P.; Marsaioli, A.J. The Enantiomeric Discrimination of 5-Hexyl-2-methyl-3,4-dihydro-2H-pyrrole by Sulfobutyl ether-β-cyclodextrin: A Case Study. Molecules 2021, 26, 2611. https://doi.org/10.3390/molecules26092611
Kawano DF, Costa BZ, Romero-Orejón KL, Loureiro HC, de Jesus DP, Marsaioli AJ. The Enantiomeric Discrimination of 5-Hexyl-2-methyl-3,4-dihydro-2H-pyrrole by Sulfobutyl ether-β-cyclodextrin: A Case Study. Molecules. 2021; 26(9):2611. https://doi.org/10.3390/molecules26092611
Chicago/Turabian StyleKawano, Daniel F., Bruna Z. Costa, Katherine L. Romero-Orejón, Hugo C. Loureiro, Dosil P. de Jesus, and Anita J. Marsaioli. 2021. "The Enantiomeric Discrimination of 5-Hexyl-2-methyl-3,4-dihydro-2H-pyrrole by Sulfobutyl ether-β-cyclodextrin: A Case Study" Molecules 26, no. 9: 2611. https://doi.org/10.3390/molecules26092611
APA StyleKawano, D. F., Costa, B. Z., Romero-Orejón, K. L., Loureiro, H. C., de Jesus, D. P., & Marsaioli, A. J. (2021). The Enantiomeric Discrimination of 5-Hexyl-2-methyl-3,4-dihydro-2H-pyrrole by Sulfobutyl ether-β-cyclodextrin: A Case Study. Molecules, 26(9), 2611. https://doi.org/10.3390/molecules26092611





