Low Pre-Transplant Caveolin-1 Serum Concentrations Are Associated with Acute Cellular Tubulointerstitial Rejection in Kidney Transplantation
Abstract
:1. Introduction
2. Results
2.1. Patients
2.2. Cav-1 Serum Concentration in Patients Prior to Kidney Transplantation and Its Correlation with Patients’ Medication
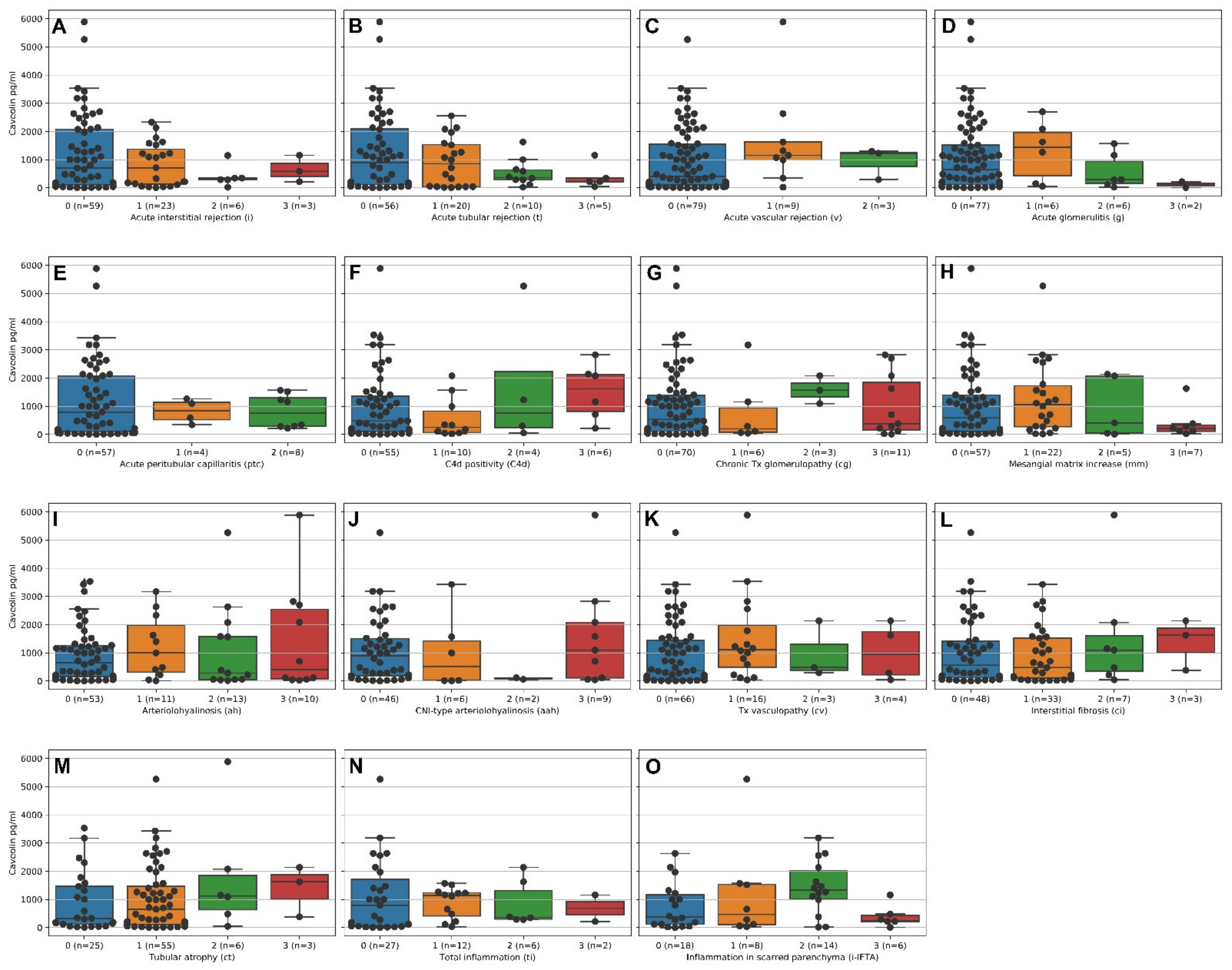
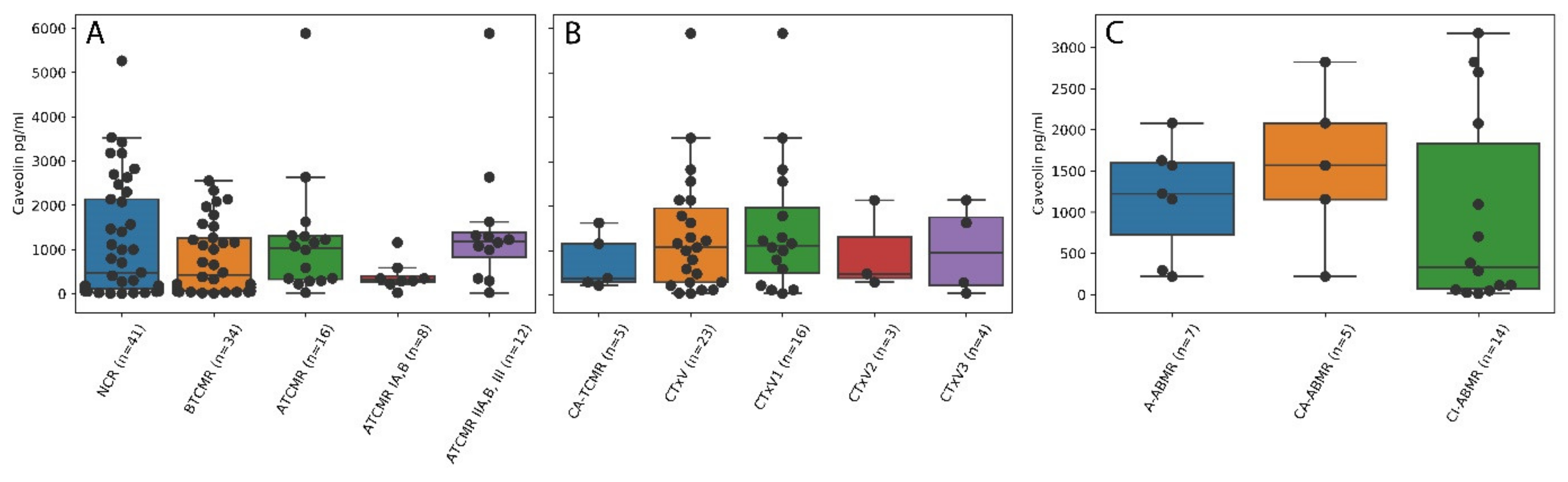
2.3. Higher Caveolin Serum Levels Are Associated with Lower Intensities of Acute Cellular Tubulointerstitial Rejections (ATCMR IA,B) but Not Acute Vascular Rejection, Acute Humoral Rejection and Chronic Damage
2.4. Pre-Transplant Cav-1 Serum Levels Are Superior Indicators to Pre-Transplant HLA Mismatches for the Prediction of Acute Cellular Tubulointerstitial Rejections in the Posttransplant Setting
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics
4.2. Caveolin Serum Analyses
4.3. Histological Analyses
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Valenzuela, N.M.; McNamara, J.T.; Reed, E.F. Antibody-mediated graft injury: Complement-dependent and complement-independent mechanisms. Curr. Opin. Organ Transplant. 2014, 19, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.M.; Gill, R.G. Direct and indirect allograft recognition: Pathways dictating graft rejection mechanisms. Curr. Opin. Organ Transplant. 2016, 21, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Mikhalski, D.; Wissing, K.M.; Ghisdal, L.; Broeders, N.; Touly, M.; Hoang, A.D.; Loi, P.; Mboti, F.; Donckier, V.; Vereerstraeten, P.; et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation 2008, 85, S3–S9. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion-from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuquay, R.; Renner, B.; Kulik, L.; McCullough, J.W.; Amura, C.; Strassheim, D.; Pelanda, R.; Torres, R.; Thurman, J.M. Renal ischemia-reperfusion injury amplifies the humoral immune response. J. Am. Soc. Nephroli. 2013, 24, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, J.; Lu, L.; Zhai, Y. T cells in organ ischemia reperfusion injury. Curr. Opin. Organ Transplant. 2014, 19, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Postalcioglu, M.; Kaze, A.D.; Byun, B.C.; Siedlecki, A.; Tullius, S.G.; Milford, E.L.; Paik, J.M.; Abdi, R. Association of cold ischemia time with acute renal transplant rejection. Transplantation 2018, 102, 1188–1194. [Google Scholar] [CrossRef]
- Wu, H.; Chadban, S.J. Roles of Toll-like receptors in transplantation. Curr. Opin. Organ Transplant. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sowa, G. Caveolae, caveolins, cavins, and endothelial cell function: New insights. Front. Physiol. 2012, 2, 120. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.W.; Lee, S.M. Impaired expression of caveolin-1 contributes to hepatic ischemia and reperfusion injury. Biochem. Biophys. Res. Commun. 2014, 450, 1351–1357. [Google Scholar] [CrossRef]
- Liu, M.; Wu, Y.; Liu, Y.; Chen, Z.; He, S.; Zhang, H.; Wu, L.; Tu, F.; Zhao, Y.; Liu, C.; et al. Basic fibroblast growth factor protects astrocytes against ischemia/reperfusion injury by upregulating the caveolin-1/VEGF signaling pathway. J. Mol. Neurosci. 2018, 64, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jia, L.; Zhou, H.; Wang, X.; Zhang, J. Caveolin-1 promotes the transformation and anti-apoptotic ability of mouse hepatoma cells. IUBMB Life 2008, 60, 693–699. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, W.W.; Liu, C.S.; Hsu, L.S.; Lin, Y.M.; Su, S.L. Caveolin-1 expression ameliorates nephrotic damage in a rabbit model of cholesterol-induced hypercholesterolemia. PLoS ONE 2016, 11, e0154210. [Google Scholar] [CrossRef] [PubMed]
- Percy, C.J.; Pat, B.K.; Healy, H.; Johnson, D.W.; Gobe, G.C. Phosphorylation of caveolin-1 is anti-apoptotic and promotes cell attachment during oxidative stress of kidney cells. Pathology 2008, 40, 694–701. [Google Scholar] [CrossRef]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 2018, 175, 695–708.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 kidney meeting report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Tahir, S.A.; Ren, C.; Timme, T.L.; Gdor, Y.; Hoogeveen, R.; Morrisett, J.D.; Frolov, A.; Ayala, G.; Wheeler, T.M.; Thompson, T. Development of an immunoassay for serum caveolin-1: A novel biomarker for prostate cancer. Clin. Cancer Res. 2003, 3653–3659. [Google Scholar]
- Goes, N.; Hobart, M.; Ramassar, V.; Urmson, J.; Halloran, P.F. Many forms of renal injury induce a stereotyped response with increased expression of MHC, IFN-gamma, and adhesion molecules. Transplant. Proc. 1997, 29, 1085. [Google Scholar] [CrossRef]
- Daemen, M.A.R.C.; Veer, C.V.; Wolfs, T.G.A.M.; Buurman, W.A. Ischemia/reperfusion-induced IFN-g up-regulation: Involvement of IL-12 and IL-18. J. Immunol. 1999, 162, 5506–5510. [Google Scholar]
- Zhang, X.; Beduhn, M.; Zheng, X.; Lian, D.; Chen, D.; Li, R.; Siu, L.K.S.; Marleau, A.; French, P.W.; Ichim, T.E.; et al. Induction of alloimmune tolerance in heart transplantation through gene silencing of TLR adaptors. Am. J. Transplant. 2012, 12, 2675–2688. [Google Scholar] [CrossRef]
- Cardinal, H.; Dieude, M.; Hebert, M.J. The emerging importance of non-HLA autoantibodies in kidney transplant complications. J. Am. Soc. Nephrol. 2017, 28, 400–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Reed, E.F. The importance of non-HLA antibodies in transplantation. Nat. Rev. Nephrol. 2016, 12, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.T.; Tsapepas, D.S.; Martin, S.T.; Hardy, M.A.; Ratner, L.E. Managing renal transplant ischemia reperfusion injury: Novel therapies in the pipeline. Clin. Transplant. 2013, 27, 484–491. [Google Scholar] [CrossRef]
- Razani, B.; Zhang, X.L.; Bitzer, M.; von Gersdorff, G.; Bottinger, E.P.; Lisanti, M.P. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J. Biol. Chem. 2001, 276, 6727–6738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Yang, J.; Liu, Y. Transforming growth factor-beta1 potentiates renal tubular epithelial cell death by a mechanism independent of smad signaling. J. Biol. Chem. 2003, 278, 12537–12545. [Google Scholar] [CrossRef] [Green Version]
- Granata, S.; Benedetti, C.; Gambaro, G.; Zaza, G. Kidney allograft fibrosis: What we learned from latest translational research studies. J. Nephrol. 2020, 33, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Young, L.H.; Ikeda, Y.; Lefer, A.M. Caveolin-1 peptide exerts cardioprotective effects in myocardial ischemia-reperfusion via nitric oxide mechanism. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2489–H2495. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Guo, L.; Song, Z.; Gao, H.; Wang, D.; Fu, W.; Han, J.; Li, Z.; Huang, B.; Li, X.A. Caveolin-1 protects against sepsis by modulating inflammatory response, alleviating bacterial burden, and suppressing thymocyte apoptosis. J. Biol. Chem. 2010, 285, 25154–25160. [Google Scholar] [CrossRef] [Green Version]
- Sellers, S.L.; Trane, A.E.; Bernatchez, P.N. Caveolin as a potential drug target for cardiovascular protection. Front. Physiol. 2012, 3, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ma, Z.; Hu, W.; Wang, D.; Jiang, S.; Fan, C.; Di, S.; Liu, D.; Sun, Y.; Yi, W. Caveolin-1/-3: Therapeutic targets for myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2016, 111, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Head, B.P. Caveolin-1 in stroke neuropathology and neuroprotection: A novel molecular therapeutic target for ischemic-related injury. Curr. Vasc. Pharmacol. 2019, 17, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chinnakkannu, P.; Reese, C.; Gaspar, J.A.; Panneerselvam, S.; Pleasant-Jenkins, D.; Mukherjee, R.; Baicu, C.; Tourkina, E.; Hoffman, S.; Kuppuswamy, D. Suppression of angiotensin II-induced pathological changes in heart and kidney by the caveolin-1 scaffolding domain peptide. PLoS ONE 2018, 13, e0207844. [Google Scholar] [CrossRef]
- Dragun, D.; Müller, D.M.; Bräsen, J.H.; Fritsche, L.; Nieminen-Kelhä, M.; Dechend, R.; Kintscher, U.; Rudolph, B.; Hoebeke, J.; Eckert, E.; et al. Angiotensin II Type 1–Receptor Activating Antibodies in Renal-Allograft Rejection. N. Engl. J. Med. 2005, 352, 558–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Clinical Data | n | Mean | std | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
| Age at Serum Draw | 91 | 47.90 | 14.39 | 7 | 39 | 50 | 59.5 | 72 |
| Age at Biopsy 1 | 91 | 50.10 | 14.34 | 7 | 43 | 51 | 61 | 72 |
| Age at Biopsy 2 | 18 | 46.06 | 16.52 | 12 | 37.25 | 49 | 55 | 75 |
| Age at Biopsy 3 | 2 | 40.00 | 35.36 | 15 | 27.50 | 40 | 52.5 | 65 |
| Age at Biopsy 4 | 1 | 69.00 | 69 | 69 | 69 | 69 | 69 | |
| Sum HLA-Mismatch | 86 | 2.71 | 1.63 | 0 | 2 | 3 | 4 | 6 |
| HLA-Mismatch A | 86 | 0.79 | 0.74 | 0 | 0 | 1 | 1 | 2 |
| HLA-Mismatch B | 86 | 0.95 | 0.65 | 0 | 1 | 1 | 1 | 2 |
| HLA-Mismatch DR | 86 | 0.97 | 0.69 | 0 | 0.25 | 1 | 1 | 2 |
| Caveolin Classifier (CC) | 91 | 0.35 | 0.67 | 0 | 0 | 0 | 1 | 3 |
| Cav-1(pg/mL) | 91 | 1029.23 | 1180.46 | 3.57 | 117.39 | 586.65 | 1543.82 | 5882.83 |
| Antibodies developed (1 = yes, 0 = no) | 86 | 0.17 | 0.38 | 0 | 0 | 0 | 0 | 1 |
| Sex (m = 1, f = 2) | 91 | 1.34 | 0.48 | 1 | 1 | 1 | 2 | 2 |
| Patient Aggregation | n | Mean | std | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
| One biopsy per patient | 91 | 1.09 | 0.38 | 1 | 1 | 1 | 1 | 4 |
| i | 91 | 0.48 | 0.77 | 0 | 0 | 0 | 1 | 3 |
| t | 91 | 0.60 | 0.89 | 0 | 0 | 0 | 1 | 3 |
| v | 91 | 0.16 | 0.45 | 0 | 0 | 0 | 0 | 2 |
| g | 91 | 0.26 | 0.68 | 0 | 0 | 0 | 0 | 3 |
| ptc | 69 | 0.29 | 0.67 | 0 | 0 | 0 | 0 | 2 |
| ti | 47 | 0.64 | 0.87 | 0 | 0 | 0 | 1 | 3 |
| i-IFTA | 46 | 1.17 | 1.10 | 0 | 0 | 1 | 2 | 3 |
| C4d | 75 | 0.48 | 0.92 | 0 | 0 | 0 | 1 | 3 |
| cg | 90 | 0.50 | 1.03 | 0 | 0 | 0 | 0 | 3 |
| mm | 91 | 0.58 | 0.91 | 0 | 0 | 0 | 1 | 3 |
| ah | 87 | 0.77 | 1.09 | 0 | 0 | 0 | 2 | 3 |
| aah | 63 | 0.59 | 1.09 | 0 | 0 | 0 | 1 | 3 |
| cv | 89 | 0.38 | 0.76 | 0 | 0 | 0 | 1 | 3 |
| ci | 91 | 0.62 | 0.77 | 0 | 0 | 0 | 1 | 3 |
| ct | 89 | 0.85 | 0.68 | 0 | 0 | 1 | 1 | 3 |
| Age at Serum Draw | 91 | 47.90 | 14.39 | 7 | 39 | 50 | 59.50 | 72 |
| Age at Biopsy | 91 | 50.31 | 14.33 | 7 | 43 | 51 | 61 | 72 |
| Biopsy Aggregation | n | Mean | std | Min | 25% | 50% | 75% | Max |
| All Biopsies | 112 | 1.00 | 0.00 | 1 | 1 | 1 | 1 | 1 |
| CC | 112 | 0.39 | 0.69 | 0 | 0 | 0 | 1 | 3 |
| Cav-1 (pg/mL) | 112 | 1099.64 | 1215.39 | 3.57 | 120.17 | 713.38 | 1625.97 | 5882.83 |
| i | 112 | 0.44 | 0.76 | 0 | 0 | 0 | 1 | 3 |
| t | 112 | 0.52 | 0.84 | 0 | 0 | 0 | 1 | 3 |
| v | 111 | 0.15 | 0.43 | 0 | 0 | 0 | 0 | 2 |
| g | 112 | 0.26 | 0.67 | 0 | 0 | 0 | 0 | 3 |
| ptc | 87 | 0.25 | 0.63 | 0 | 0 | 0 | 0 | 2 |
| ti | 51 | 0.63 | 0.85 | 0 | 0 | 0 | 1 | 3 |
| i-IFTA | 48 | 1.17 | 1.10 | 0 | 0 | 1 | 2 | 3 |
| C4d | 88 | 0.45 | 0.91 | 0 | 0 | 0 | 0.25 | 3 |
| cg | 111 | 0.55 | 1.04 | 0 | 0 | 0 | 0.5 | 3 |
| mm | 111 | 0.64 | 0.95 | 0 | 0 | 0 | 1 | 3 |
| ah | 108 | 0.83 | 1.11 | 0 | 0 | 0 | 2 | 3 |
| aah | 74 | 0.69 | 1.17 | 0 | 0 | 0 | 1 | 3 |
| cv | 110 | 0.40 | 0.78 | 0 | 0 | 0 | 1 | 3 |
| ci | 112 | 0.71 | 0.80 | 0 | 0 | 1 | 1 | 3 |
| ct | 110 | 0.91 | 0.71 | 0 | 0 | 1 | 1 | 3 |
| Age at Serum Draw | 112 | 46.54 | 15.39 | 7 | 37.75 | 48 | 59 | 72 |
| Age at Biopsy | 112 | 49.44 | 15.07 | 7 | 40 | 51 | 61 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmerich, F.; Zschiedrich, S.; Reichenbach-Braun, C.; Süsal, C.; Minguet, S.; Pauly, M.-C.; Seidl, M. Low Pre-Transplant Caveolin-1 Serum Concentrations Are Associated with Acute Cellular Tubulointerstitial Rejection in Kidney Transplantation. Molecules 2021, 26, 2648. https://doi.org/10.3390/molecules26092648
Emmerich F, Zschiedrich S, Reichenbach-Braun C, Süsal C, Minguet S, Pauly M-C, Seidl M. Low Pre-Transplant Caveolin-1 Serum Concentrations Are Associated with Acute Cellular Tubulointerstitial Rejection in Kidney Transplantation. Molecules. 2021; 26(9):2648. https://doi.org/10.3390/molecules26092648
Chicago/Turabian StyleEmmerich, Florian, Stefan Zschiedrich, Christine Reichenbach-Braun, Caner Süsal, Susana Minguet, Marie-Christin Pauly, and Maximilian Seidl. 2021. "Low Pre-Transplant Caveolin-1 Serum Concentrations Are Associated with Acute Cellular Tubulointerstitial Rejection in Kidney Transplantation" Molecules 26, no. 9: 2648. https://doi.org/10.3390/molecules26092648
APA StyleEmmerich, F., Zschiedrich, S., Reichenbach-Braun, C., Süsal, C., Minguet, S., Pauly, M. -C., & Seidl, M. (2021). Low Pre-Transplant Caveolin-1 Serum Concentrations Are Associated with Acute Cellular Tubulointerstitial Rejection in Kidney Transplantation. Molecules, 26(9), 2648. https://doi.org/10.3390/molecules26092648






