Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry Analysis of Exhaled Breath Compounds after Whole Grain Diets
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
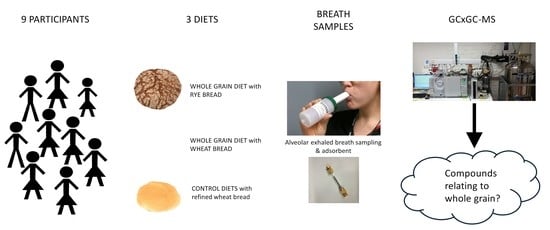
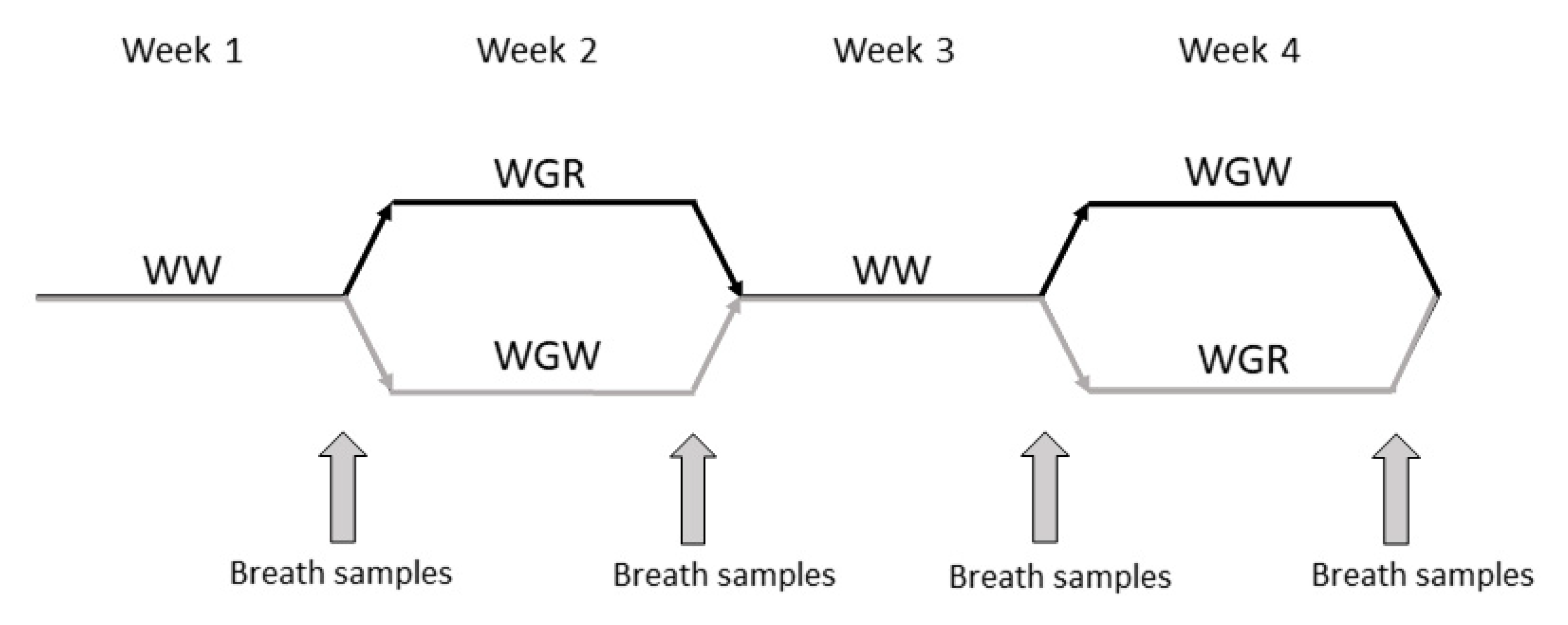
4.1. Protocol
4.2. Study Participants
4.3. Diets
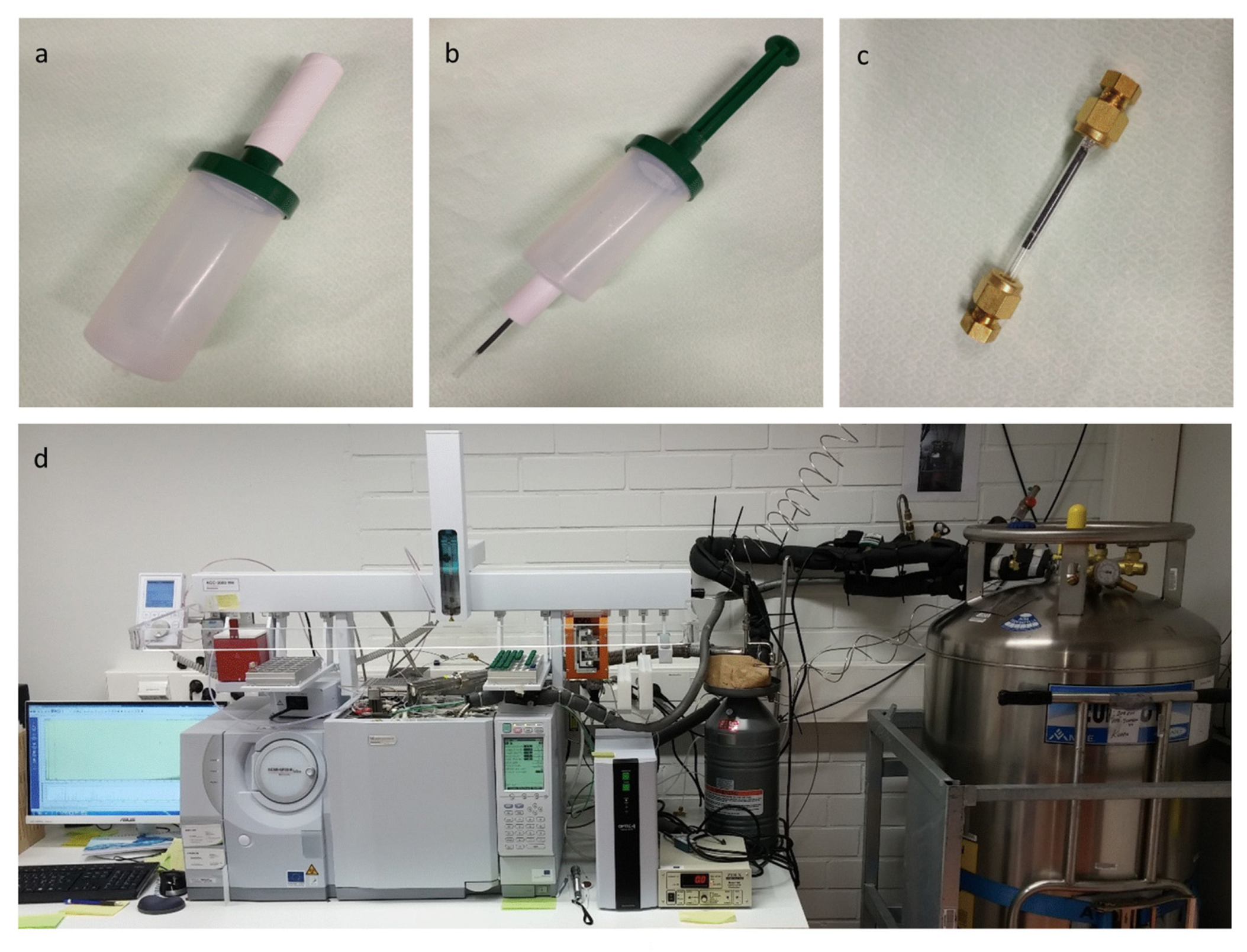
4.4. Exhaled Breath Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Mykkänen, H.; Kolehmainen, M.; Poutanen, K. Wholegrain foods and health. In Fibre-Rich and Wholegrain Foods; Delcour, J.A., Poutanen, K., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 76–95. [Google Scholar]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Q.; Guo, W.; Bao, W.; Wang, X. Association of whole grain intake with all-cause, cardiovascular, and cancer mortality: A systematic review and dose-response meta-analysis from prospective cohort studies. Eur. J. Clin. Nutr. 2018, 72, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Maki, K.C.; Palacios, O.M.; Koecher, K.; Sawicki, C.M.; Livingston, K.A.; Bell, M.; Nelson Cortes, H.; McKeown, N.M. The relationship between whole grain intake and body weight: Results of meta-analyses of observational studies and randomized controlled trials. Nutrients 2019, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food groups and risk of overweight, obesity, and weight gain: A systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Chanson-Rolle, A.; Meynier, A.; Aubin, F.; Lappi, J.; Poutanen, K.; Vinoy, S.; Braesco, V. Systematic review and meta-analysis of human studies to support a quantitative recommendation for whole grain intake in relation to type 2 diabetes. PLoS ONE 2015, 10, e0131377. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Hartley, L.; Loveman, E.; Colquitt, J.L.; Jones, H.M.; Al-Khudairy, L.; Clar, C.; Germanò, R.; Lunn, H.R.; Frost, G.; et al. Whole grain cereals for the primary or secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017, 8, CD005051. [Google Scholar] [CrossRef]
- Benisi-Kohansal, S.; Saneei, P.; Salehi-Marzijarani, M.; Larijani, B.; Esmaillzadeh, A. Whole-grain intake and mortality from all causes, cardiovascular disease, and cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Adv. Nutr. 2016, 7, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Gallaher, D.D. Whole grain intake and cardiovascular disease: A review. Curr. Atheroscler. Rep. 2004, 6, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, J.; Tsao, R.; Wang, Z.; Sun, B.; Wang, J. Whole grain consumption for the prevention and treatment of breast cancer. Nutrients 2019, 11, 1769. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Why whole grains are protective: Biological mechanisms. Proc. Nutr. Soc. 2003, 62, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Kamal-Eldin, A.; Aman, P. Dietary alkylresorcinols: Absorption, bioactivities, and possible use as biomarkers of whole-grain wheat- and rye-rich foods. Nutr. Rev. 2004, 62, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of personalized nutrition in chronic-degenerative diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef]
- Amann, A.; Costello Bde, L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Van Malderen, K.; De Winter, B.Y.; De Man, J.G.; De Schepper, H.U.; Lamote, K. Volatomics in inflammatory bowel disease and irritable bowel syndrome. EBioMed. 2020, 54, 102725. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Lockman, K.A.; Homer, N.Z.M.; Bower, E.; Brinkman, P.; Knobel, H.H.; Fallowfield, J.A.; Jaap, A.J.; Hayes, P.C.; Plevris, J.N. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100137. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perdoni, F.; Infantino, V.; Faliva, M.A.; Peroni, G.; Iannello, G.; Nichetti, M.; Alalwan, T.A.; Perna, S.; Cocuzza, C. Volatile organic compounds as biomarkers of gastrointestinal diseases and nutritional status. J. Anal. Methods Chem. 2019, 2019, 7247802. [Google Scholar] [CrossRef]
- Ajibola, O.A.; Smith, D.; Spaněl, P.; Ferns, G.A. Effects of dietary nutrients on volatile breath metabolites. J. Nutr. Sci. 2013, 2, e34. [Google Scholar] [CrossRef]
- Rattray, N.J.W.; Hamrang, Z.; Trivedi, D.K.; Goodacre, R.; Fowler, S.J. Taking your breath away: Metabolomics breathes life in to personalized medicine. Trends Biotechnol. 2014, 32, 538–548. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Pleil, J.D.; Stiegel, M.A.; Risby, T.H. Clinical breath analysis: Discriminating between human endogenous compounds and exogenous (environmental) chemical confounders. J. Breath Res. 2013, 7, 017107. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Španěl, P.; Davies, S. Trace gases in breath of healthy volunteers when fasting and after a protein-calorie meal: A preliminary study. J. Appl. Physiol. 1999, 87, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Parekh, B.; Walton, C.; Spaněl, P.; Smith, D.; Evans, M. An exploratory comparative study of volatile compounds in exhaled breath and emitted by skin using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Spaněl, P.; Dryahina, K.; Rejskova, A.; Chippendale, T.W.; Smith, D. Breath Acetone Concentration; Biological Variability and the Influence of Diet. Physiol. Meas. 2011, 32, N23–N31. [Google Scholar] [CrossRef] [PubMed]
- Dowlaty, N.; Yoon, A.; Galassetti, P. Monitoring states of altered carbohydrate metabolism via breath analysis: Are times ripe for transition from potential to reality? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef]
- Novak, B.J.; Blake, D.R.; Meinardi, S.; Rowland, F.S.; Pontello, A.; Cooper, D.M.; Galassetti, P.R. Exhaled Methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15613–15618. [Google Scholar] [CrossRef]
- Minh, T.D.; Oliver, S.R.; Ngo, J.; Flores, R.; Midyett, J.; Meinardi, S.; Carlson, M.K.; Rowland, F.S.; Blake, D.R.; Galassetti, P.R. Noninvasive measurement of plasma glucose from exhaled breath in healthy and type 1 diabetic subjects. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1166–E1175. [Google Scholar] [CrossRef]
- Lee, J.; Ngo, J.; Blake, D.; Meinardi, S.; Pontello, A.M.; Newcomb, R.; Galassetti, P.R. Improved predictive models for plasma glucose estimation from multi-linear regression analysis of exhaled volatile organic compounds. J. Appl. Physiol. 2009, 107, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath ethanol and acetone as indicators of serum glucose levels: An initial report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.; Oliver, S.R.; Flores, R.L.; Ngo, J.; Meinardi, S.; Carlson, M.K.; Midyett, J.; Rowland, F.S.; Blake, D.R.; Galassetti, P.R. Noninvasive measurement of plasma triglycerides and free fatty acids from exhaled breath. J. Diabetes Sci. Technol. 2012, 6, 86–101. [Google Scholar] [CrossRef]
- Baranska, A.; Mujagic, Z.; Smolinska, A.; Dallinga, J.W.; Jonkers, D.M.; Tigchelaar, E.F.; Dekens, J.; Zhernakova, A.; Ludwig, T.; Masclee, A.A.; et al. Volatile organic compounds in breath as markers for irritable bowel syndrome: A metabolomic approach. Aliment. Pharmacol. Ther. 2016, 44, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bodelier, A.G.; Smolinska, A.; Baranska, A.; Dallinga, J.W.; Mujagic, Z.; Vanhees, K.; van den Heuvel, T.; Masclee, A.A.; Jonkers, D.; Pierik, M.J.; et al. Volatile organic compounds in exhaled air as novel marker for disease activity in crohn’s disease: A metabolomic approach. Inflamm. Bowel Dis. 2015, 21, 1776–1785. [Google Scholar] [CrossRef]
- Van Berkel, J.J.; Dallinga, J.W.; Moller, G.M.; Godschalk, R.W.; Moonen, E.J.; Wouters, E.F.; Van Schooten, F.J. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir. Med. 2010, 104, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Baranska, A.; Tigchelaar, E.; Smolinska, A.; Dallinga, J.W.; Moonen, E.J.; Dekens, J.A.; Wijmenga, C.; Zhernakova, A.; van Schooten, F.J. Profile of volatile organic compounds in exhaled breath changes as a result of gluten-free diet. J. Breath Res. 2013, 7, 037104. [Google Scholar] [CrossRef]
- Raninen, K.; Lappi, J.; Kolehmainen, M.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K.; Raatikainen, O. Diet-derived changes by sourdough-fermented rye bread in exhaled breath aspiration ion mobility spectrometry profiles in individuals with mild gastrointestinal symptoms. Int. J. Food Sci. Nutr. 2017, 68, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Raninen, K.J.; Kolehmainen, M.; Tuomainen, T.; Mykkänen, H.; Poutanen, K.; Raatikainen, O. Exhaled breath aspiration ion mobility spectrometry profiles reflect metabolic changes induced by diet. J. Physiobiochem. Metab. 2015, 3. [Google Scholar] [CrossRef]
- Mathew, T.L.; Pownraj, P.; Abdulla, S.; Pullithadathil, B. Technologies for Clinical Diagnosis using Expired Human Breath Analysis. Diagnostics 2015, 5, 27–60. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Kaplan, P.D.; Libardoni, M.; Mundada, M.; Patel, U.; Zhang, X. Detection of an extended human volatome with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. PLoS ONE 2013, 8, e75274. [Google Scholar] [CrossRef]
- Raninen, K.J.; Lappi, J.E.; Mukkala, M.L.; Tuomainen, T.; Mykkänen, H.M.; Poutanen, K.S.; Raatikainen, O.J. Fiber content of diet affects exhaled breath volatiles in fasting and postprandial state in a pilot crossover study. Nutr. Res. 2016, 36, 612–619. [Google Scholar] [CrossRef]
- Fischer, S.; Bergmann, A.; Steffens, M.; Trefz, P.; Ziller, M.; Miekisch, W.; Schubert, J.S.; Kohler, H.; Reinhold, P. Impact of food intake on in vivo voc concentrations in exhaled breath assessed in a caprine animal model. J. Breath Res. 2015, 9, 047113. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; King, J.; Haas, M.; Unterkofler, K.; Amann, A.; Mayer, G. Blood and breath profiles of volatile organic compounds in patients with end-stage renal disease. BMC Nephrol. 2014, 15, 43. [Google Scholar] [CrossRef]
- Marco, E.; Grimalt, J.O. A rapid method for the chromatographic analysis of volatile organic compounds in exhaled breath of tobacco cigarette and electronic cigarette smokers. J. Chromatogr. A 2015, 1410, 51–59. [Google Scholar] [CrossRef]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef]
- Salerno-Kennedy, R.; Cashman, K.D. Potential applications of breath isoprene as a biomarker in modern medicine: A concise overview. Wien. Klin. Wochenschr. 2005, 117, 180–186. [Google Scholar] [CrossRef]
- King, J.; Mochalski, P.; Unterkofler, K.; Teschl, G.; Klieber, M.; Stein, M.; Amann, A.; Baumann, M. Breath isoprene: Muscle dystrophy patients support the concept of a pool of isoprene in the periphery of the human body. Biochem. Biophys. Res. Commun. 2012, 423, 526–530. [Google Scholar] [CrossRef]
- Hibbard, T.; Killard, A.J. Breath ammonia levels in a normal human population study as determined by photoacoustic laser spectroscopy. J. Breath Res. 2011, 5, 037101. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.; Low, K.; Khoshini, R.; Melmed, G.; Sahakian, A.; Makhani, M.; Pokkunuri, V.; Pimentel, M. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig. Dis. Sci. 2010, 55, 398–403. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Hanhineva, K. Microbial and endogenous metabolic conversions of rye phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600627. [Google Scholar] [CrossRef]
- Nordlund, E.; Aura, A.M.; Mattila, I.; Kosso, T.; Rouau, X.; Poutanen, K. Formation of phenolic microbial metabolites and short-chain fatty acids from rye, wheat, and oat bran and their fractions in the metabolical in vitro colon model. J. Agric. Food Chem. 2012, 60, 8134–8145. [Google Scholar] [CrossRef] [PubMed]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath analysis: Comparison among methodological approaches for breath sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef] [PubMed]
- Braden, B. Methods and functions: Breath tests. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 1. C1 through C29 organic compounds from meat charbroiling. Environ. Sci. Technol. 1999, 33, 1566–1577. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 4. C1-C27 organic compounds from cooking with seed oils. Environ. Sci. Technol. 2002, 36, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wahala, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Hallmans, G.; Zhang, J.X.; Lundin, E.; Stattin, P.; Johansson, A.; Johansson, I.; Hulten, K.; Winkvist, A.; Aman, P.; Lenner, P.; et al. Rye, lignans and human health. Proc. Nutr. Soc. 2003, 62, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hålldin, E.; Eriksen, A.K.; Brunius, C.; da Silva, A.B.; Bronze, M.; Hanhineva, K.; Aura, A.M.; Landberg, R. Factors explaining interpersonal variation in plasma enterolactone concentrations in humans. Mol. Nutr. Food Res. 2019, 63, e1801159. [Google Scholar] [CrossRef] [PubMed]
| Detected in % of Samples in | ||||
|---|---|---|---|---|
| Compounds | WGR | WGW | WW | BG |
| Carbon dioxide | 100 | 100 | 100 | 100 |
| Ethanol | 100 | 100 | 100 | 100 |
| Hexanoic acid | 100 | 100 | 100 | 100 |
| Acetophenone | 100 | 100 | 100 | 100 |
| 1-Butanol | 100 | 100 | 100 | 97 |
| Benzene | 100 | 100 | 100 | 97 |
| Benzaldehyde | 100 | 100 | 100 | 97 |
| Methyl vinyl ketone | 100 | 100 | 100 | 97 |
| 2-Butanone | 100 | 100 | 100 | 97 |
| Acetone | 100 | 100 | 100 | 94 |
| Phenol | 100 | 100 | 100 | 94 |
| 2-Propanol | 100 | 100 | 100 | 90 |
| Acetonitrile | 100 | 100 | 100 | 87 |
| Isoprene | 100 | 100 | 100 | 42 |
| 2,3-Butanedione | 100 | 89 | 100 | 68 |
| Toluene | 100 | 33 | 81 | 74 |
| 3-Pentanol/2-Propanol, 2-methyl | 86 | 100 | 100 | 97 |
| Butanal | 86 | 100 | 100 | 87 |
| Hexanal | 86 | 100 | 88 | 87 |
| Heptanal | 86 | 100 | 81 | 74 |
| Octanal | 86 | 89 | 94 | 23 |
| Benzaldehyde, 2/4-methyl | 86 | 89 | 75 | 90 |
| Pentanal | 86 | 89 | 69 | 68 |
| n-Hexane | 86 | 67 | 88 | 58 |
| Nonanal | 71 | 78 | 100 | 94 |
| Acetaldehyde | 71 | 56 | 81 | 77 |
| 3,4-Dimethyl heptane | 71 | 33 | 75 | 35 |
| D-Limonene | 71 | 33 | 69 | 10 |
| 1,3-Pentadiene | 71 | 33 | 69 | 0 |
| Benzene, 1,4-dimethyl- | 71 | 22 | 56 | 26 |
| Dimethyl sulfide | 57 | 78 | 81 | 0 |
| Decanal | 57 | 67 | 56 | 84 |
| Methyl cyclopentane | 57 | 56 | 63 | 42 |
| 6-Methyl-5-hepten-2-one | 57 | 56 | 88 | 68 |
| 1-Propanol | 57 | 44 | 81 | 39 |
| Octane | 57 | 44 | 56 | 17 |
| Ethyl acetate | 57 | 33 | 75 | 71 |
| Styrene | 57 | 33 | 69 | 29 |
| p-Cymene | 57 | 33 | 69 | 3 |
| Heptane | 43 | 56 | 75 | 71 |
| Detected in % of Samples in | ||||||
|---|---|---|---|---|---|---|
| Compounds | RT | SI | WGR | WGW | WW | BG |
| Phthalic acid/Phthalic anhydride | 40–69 | 93 | 57 | 11 | 0 | 6 |
| Benzoic acid | 30–69 | 94 | 29 | 11 | 0 | 6 |
| Diphenyl ethanedione | 61.7 | 92 | 29 | 0 | 0 | 0 |
| 5-Dodecyldihydro-(3H)-furanone | 63.7 | 92 | 29 | 0 | 0 | 0 |
| Benzamide | 65.5 | 94 | 14 | 11 | 0 | 0 |
| Dihydro-4-hydroxy-2(3H)-furanone | 57.5 | 85 | 14 | 11 | 0 | 0 |
| Dihydro-5-tetradecyl-2(3H)-furanone | 67.7 | 89 | 14 | 11 | 0 | 0 |
| WW1 | WW2 | WGR | WGW | p-Value 2 | |
|---|---|---|---|---|---|
| Energy, MJ | 9.0 ± 1.7 | 9.3 ± 1.6 | 9.0 ± 1.8 | 9.0 ± 1.5 | 0.865 |
| Carbohydrates, E% | 42 ± 2 | 41 ± 4 | 42 ± 3 | 41 ± 4 | 0.706 |
| Protein, E% | 20 ± 3 | 20 ± 3 | 19 ± 2 | 20 ± 3 | 0.254 |
| Fat, E% | 35 ± 4 | 36 ± 5 | 35 ± 4 | 35 ± 7 | 0.954 |
| Dietary fiber, g | 24 ± 8 | 25 ± 8 | 36 ± 6 * | 34 ± 1 * | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raninen, K.; Nenonen, R.; Järvelä-Reijonen, E.; Poutanen, K.; Mykkänen, H.; Raatikainen, O. Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry Analysis of Exhaled Breath Compounds after Whole Grain Diets. Molecules 2021, 26, 2667. https://doi.org/10.3390/molecules26092667
Raninen K, Nenonen R, Järvelä-Reijonen E, Poutanen K, Mykkänen H, Raatikainen O. Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry Analysis of Exhaled Breath Compounds after Whole Grain Diets. Molecules. 2021; 26(9):2667. https://doi.org/10.3390/molecules26092667
Chicago/Turabian StyleRaninen, Kaisa, Ringa Nenonen, Elina Järvelä-Reijonen, Kaisa Poutanen, Hannu Mykkänen, and Olavi Raatikainen. 2021. "Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry Analysis of Exhaled Breath Compounds after Whole Grain Diets" Molecules 26, no. 9: 2667. https://doi.org/10.3390/molecules26092667
APA StyleRaninen, K., Nenonen, R., Järvelä-Reijonen, E., Poutanen, K., Mykkänen, H., & Raatikainen, O. (2021). Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry Analysis of Exhaled Breath Compounds after Whole Grain Diets. Molecules, 26(9), 2667. https://doi.org/10.3390/molecules26092667