Oxidation of Ethanol in Cu-Faujasites Studied by IR Spectroscopy
Abstract
1. Introduction
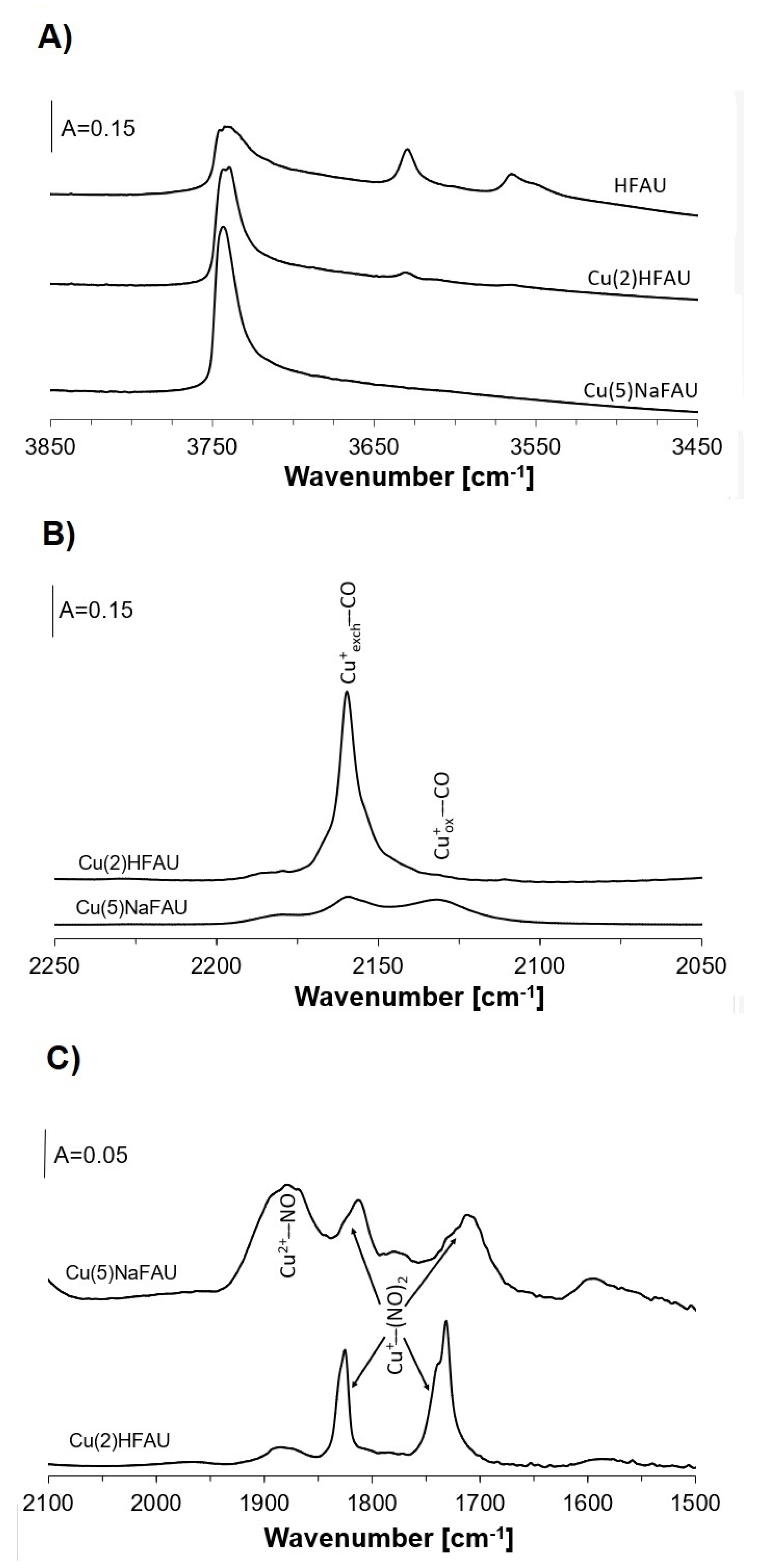
2. Results and Discussion
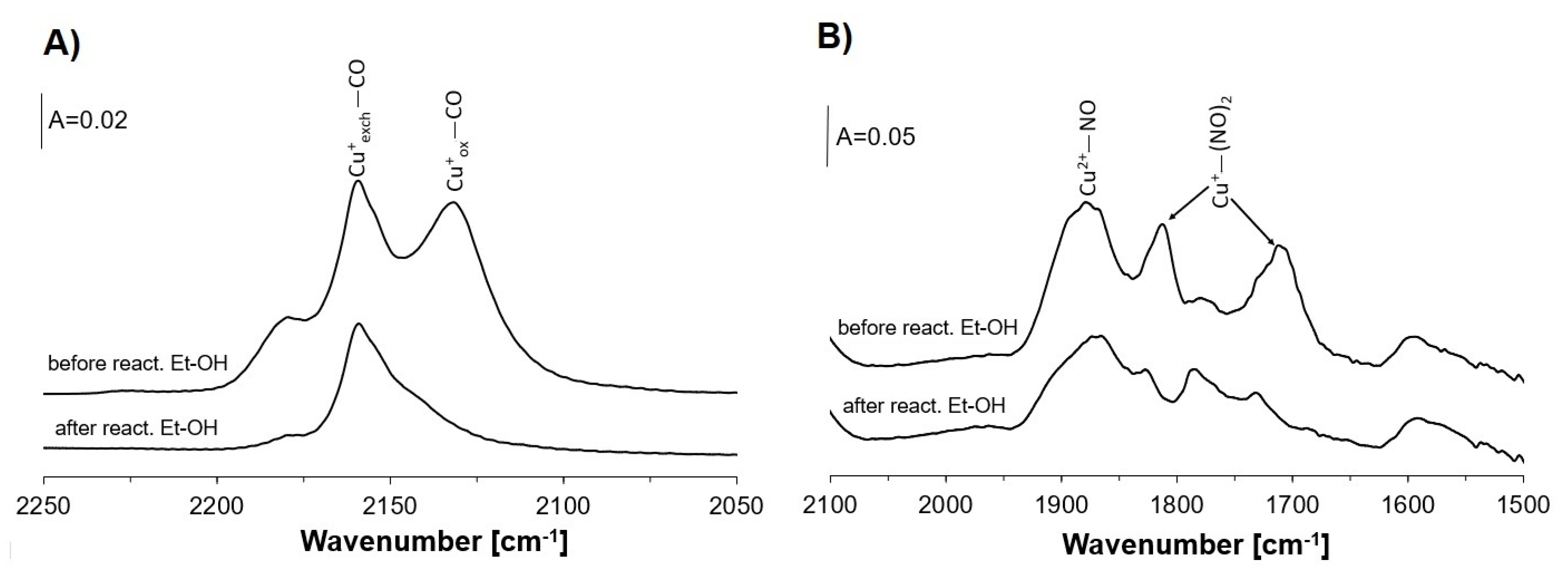
2.1. Coadsorption of Ethanol and CO
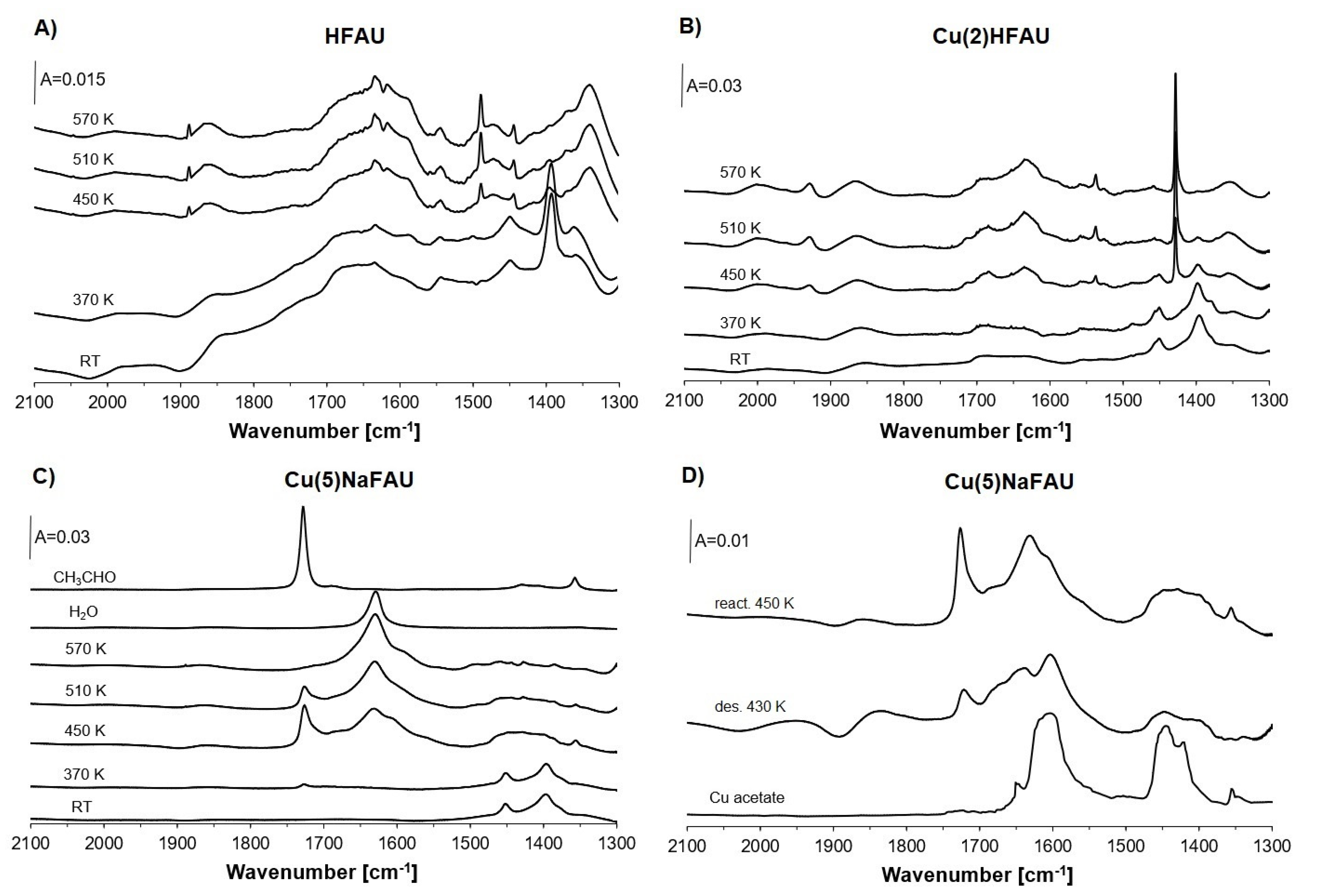
2.2. Reaction of Ethanol in HFAU
2.3. Reaction of Ethanol in Cu(2)HFAU
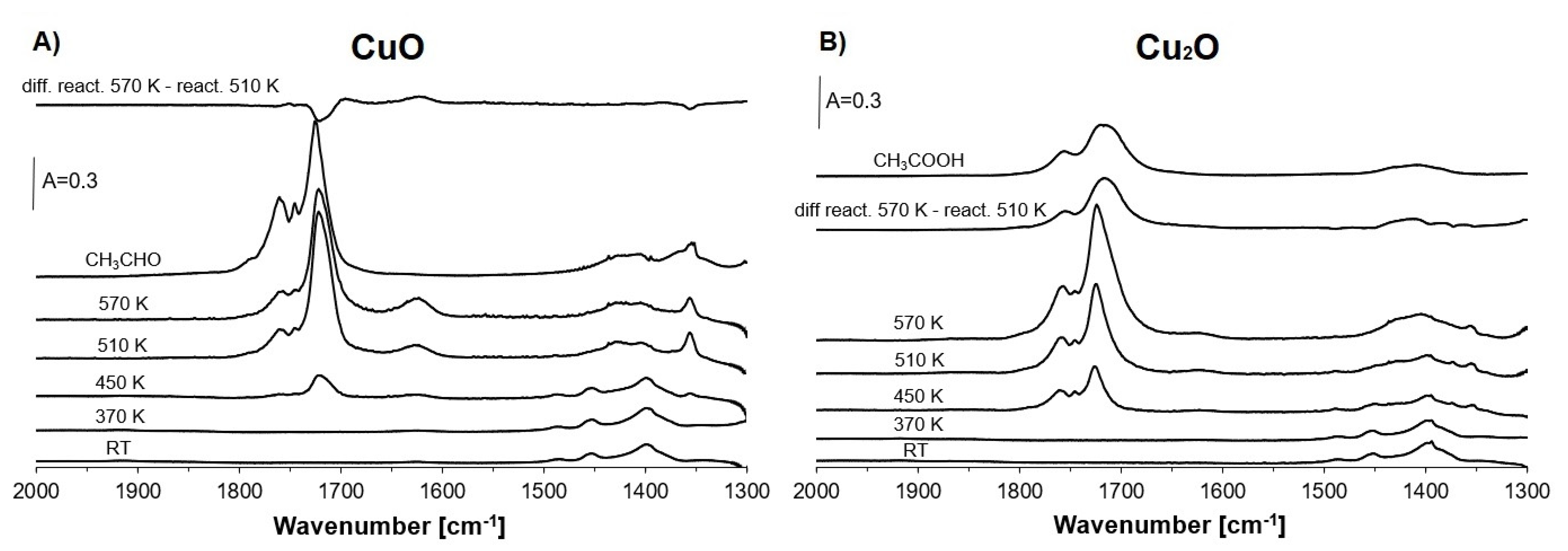
2.4. Reaction of Ethanol in Cu(5)NaFAU
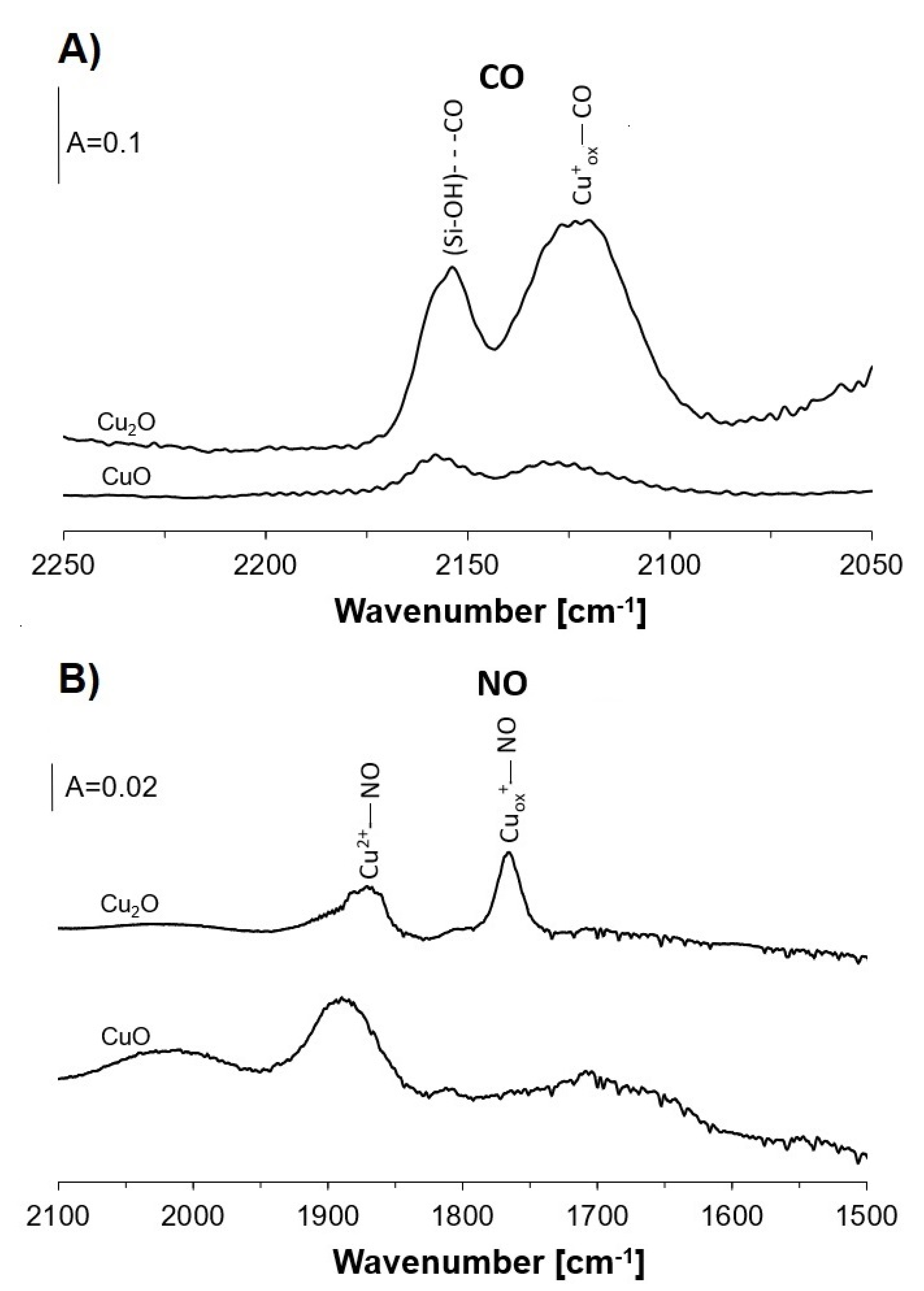
2.5. Oxygen Donors in Ethanol Oxidation
3. Materials and Methods
3.1. Materials
3.2. IR Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Iwamoto, M.; Furukawa, H.; Mine, Y.; Uemura, F.; Mikuriya, S.; Kagawa, S. Copper(II) ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide. J. Chem. Soc. Chem. Commun. 1986, 16, 1272–1273. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yokoo, S.; Sakai, K.; Kagawa, S. Catalytic decomposition of nitric oxide over copper(II)-exchanged, Y-type zeolites. J. Chem. Soc. Faraday Trans. 1981, 77, 1629–1638. [Google Scholar] [CrossRef]
- Spoto, G.; Zecchina, A.; Bordiga, S.; Ricchiardi, G.; Martra, G.; Leofanti, G.; Petrini, G. Cu(I)-ZSM-5 zeolites prepared by reaction of H-ZSM-5 with gaseous CuCl: Spectroscopic characterization and reactivity towards carbon monoxide and nitric oxide. Appl. Catal. B 1994, 3, 151–172. [Google Scholar] [CrossRef]
- Mizumoto, M.; Yamazoe, N.; Seiyama, T. Efects of coexisting gases on the catalytic reduction of NO with NH3 over Cu(II) NaY. J. Catal. 1979, 59, 319–324. [Google Scholar] [CrossRef]
- Wichterlová, B.; Sobalík, Z.; Skokánek, M. Effect of water vapour and ammonia on the solid-solid interaction of Cu oxide with Y-type zeolite: Preparation of catalyst for reduction of nitric oxide with ammonia at low temperature. Appl. Catal. A 1993, 103, 269–280. [Google Scholar] [CrossRef]
- Petunchi, O.; Sill, G.; Hall, W.K. Studies of the selective reduction of nitric oxide by hydrocarbons. Appl. Catal. B 1993, 2, 303–321. [Google Scholar] [CrossRef]
- Burch, R.; Scire, S. Selective catalytic reduction of nitric oxide with ethane and methane on some metal exchanged ZSM-5 zeolites. Appl. Catal. B 1994, 3, 295–318. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Gerlock, J.L.; Jen, H.W.; Shelef, M. In Situ ESR Monitoring of CuH-ZSM-5 Up to 500°C in Flowing Dry Mixtures of No(NO2), C3H6(C2H5OH), and Excess O. J. Catal. 1995, 152, 63–69. [Google Scholar] [CrossRef]
- Choi, E.Y.; Nam, I.S.; Kim, Y.G. TPD Study of Mordenite-Type Zeolites for Selective Catalytic Reduction of NO by NH. J. Catal. 1996, 161, 597–604. [Google Scholar] [CrossRef]
- Broclawik, E.; Datka, J.; Gil, B.; Kozyra, P. Why Cu+ in ZSM-5 framework is active in DeNOx reaction-Quantum chemical calculations and IR studies. Catal. Today 2002, 75, 353–357. [Google Scholar] [CrossRef]
- Rejmak, P.; Broclawik, E.; Góra-Marek, K.; Radoń, M.; Datka, J. Nitrogen Monoxide Interaction with Cu(I) Sites in Zeolites X and Y: Quantum Chemical Calculations and IR Studies. J. Phys. Chem. C 2008, 112, 17998–18010. [Google Scholar] [CrossRef]
- Hessou, E.P.; Kanhounnon, W.G.; Rocca, D.; Monnier, H.; Vallières, C.; Lebègue, S.; Badawi, M. Adsorption of NO, NO2, CO, H2O and CO2 over isolated monovalent cations in faujasite zeolite: A periodic DFT investigation. Theor. Chem. Acc. 2018, 137, 161. [Google Scholar] [CrossRef]
- Broclawik, E.; Datka, J.; Gil, B.; Kozyra, P. Molecular modelling of copper sites in ZSM-5: DFT and IR studies on the properties of Cu2+ and Cu+ Centres and their interaction with NO. Metal-Ligand Interact. 2003, 116, 371–384. [Google Scholar] [CrossRef]
- Datka, J.; Kukulska-Zając, E. IR Studies of the Activation of CC Bond in Alkenes by Cu+ Ions in Zeolites. J. Phys. Chem. B 2004, 108, 17760–17766. [Google Scholar] [CrossRef]
- Datka, J.; Kukulska-Zając, E.; Kobyzewa, W. The activation of acetylene by Cu+ ions in zeolites studied by IR spectroscopy. Catal. Today 2005, 101, 123–129. [Google Scholar] [CrossRef]
- Datka, J.; Kozyra, P.; Kukulska-Zając, E.; Kobyzewa, W. The activation of CO bond in acetone by Cu+ cations in zeolites: IR studies and quantum chemical DFT calculations. Catal. Today 2005, 101, 117–122. [Google Scholar] [CrossRef]
- Kukulska-Zając, E.; Datka, J. Transformations of Formaldehyde Molecules in Cu−ZSM-5 Zeolites. J. Phys. Chem. 2007, 111, 3471. [Google Scholar] [CrossRef]
- Kukulska-Zając, E.; Kozyra, P.; Datka, J. The interaction of benzene with Cu+ sites in zeolites: IR studies and DFT quantum chemical calculations. Appl. Catal. A 2006, 307, 46–50. [Google Scholar] [CrossRef]
- Hübner, G.; Rauhut, G.; Stoll, H. FTIR measurements and quantum chemical calculations of ethylene adsorbed on CuNaY. Phys. Chem. Chem. Phys. 2002, 4, 3112–3121. [Google Scholar] [CrossRef]
- Hübner, G.; Rauhut, G.; Stoll, H.; Roduner, E. Ethyne Adsorbed on CuNaY Zeolite: FTIR Spectra and Quantum Chemical Calculations. J. Phys Chem. B 2003, 107, 8568–8573. [Google Scholar] [CrossRef]
- Tsuruya, S.; Okamoto, Y.; Kuwada, T. Benzyl alcohol oxidation over Y-type zeolite ion-exchanged with copper(II) ion. J. Catal. 1979, 56, 52–64. [Google Scholar] [CrossRef]
- Tsuruya, S.; Tsukamoto, M.; Watanabe, M.; Masai, M. Ethanol oxidation over Y-type zeolite ion-exchanged with copper(II) and cobalt(II) ions. J. Catal. 1985, 3, 303–311. [Google Scholar] [CrossRef]
- Bun, S.; Nishiyama, S.; Tsuruya, S.; Masai, M. Ethanol conversion over ion-exchanged ZSM-5 zeolites. Appl. Catal. 1990, 59, 13–29. [Google Scholar] [CrossRef]
- Nakao, M.; Nishiyama, S.; Tsuruya, S.; Masai, M. Catalysis of copper(II)-NaZSM-5 zeolites during benzyl alcohol oxidation. Inorg. Chem. 1992, 31, 4662–4668. [Google Scholar] [CrossRef]
- Sueto, S.; Nishiyama, S.; Tsuruya, S.; Masai, M. Catalytic activity of NaZSM-5 supported Cu catalysts with or without added alkali metal in benzyl alcohol oxidation. J. Chem. Soc. Faraday Trans. 1997, 93, 659–664. [Google Scholar] [CrossRef]
- Azizi, S.N.; Tilami, S.E. Cu-modified analcime as a catalyst for oxidation of benzyl alcohol: Experimental and theoretical. Microporous Mesoporous Mater. 2013, 167, 89–93. [Google Scholar] [CrossRef]
- Xu, J.; Ekblad, M.; Nishiyama, S.; Tsuruya, S.; Masai, M. Effect of alkali metals added to Cu ion-exchanged Y-type zeolite catalysts in the gas-phase catalytic oxidation of benzyl alcohol. J. Chem. Soc. Faraday Trans. 1998, 94, 473–479. [Google Scholar] [CrossRef]
- Konda, T.; Nishiyama, S.; Tsuruya, S. Infuence and role of added alkali metals on the gas-phase oxidation of benzyl alcohol catalyzed by Cu ion-exchanged NaX zeolites. Phys. Chem. Chem. Phys. 1999, 1, 5393–5399. [Google Scholar] [CrossRef]
- Bhagya, K.N.; Gayathri, V. Metal complexes of 2-methylimidazole encapsulated in zeolite-Y as efficient and reusable catalysts for oxidation of phenol and benzyl alcohol. J. Porous Mater. 2013, 20, 257–266. [Google Scholar] [CrossRef]
- Itho, Y.; Nishiyama, S.; Tsuruya, S.; Masai, M. Redox Behavior and Mobility of Copper Ions in NaZSM-5 Zeolite during Oxidation. Phys. Chem. 1994, 98, 960–967. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Zendehdel, M.; Safari, P. Synthesis and characterization of some novel transition metal Schiff base complexes encapsulated in zeolite Y: Effective catalysts for the selective oxidation of benzyl alcohol. React. Kinet. Mech. Cat. 2013, 110, 497–514. [Google Scholar] [CrossRef]
- Hayashibara, H.; Nishiyama, S.; Tsuruya, S.; Masai, M. The Effect of Alkali Promoters on Cu-Na-ZSM-5 Catalysts in the Oxidation of Benzyl Alcohol. J. Catal. 1995, 153, 254–264. [Google Scholar] [CrossRef]
- Li, F.; Hu, D.; Yuan, Y.; Luo, B.; Song, Y.; Xiao, S.; Chen, G.; Fang, Y.; Lu, F. Zeolite Y encapsulated Cu (II) and Zn (II)-imidazole-salen catalysts for benzyl alcohol oxidation. Mol. Catal. 2018, 452, 75–82. [Google Scholar] [CrossRef]
- Xavier, K.O.; Chacko, J.; Yusuff, K.K.M. Zeolite-encapsulated Co(II), Ni(II) and Cu(II) complexes as catalysts for partial oxidation of benzyl alcohol and ethylbenzene. Appl. Catal. A Gen. 2004, 258, 251–259. [Google Scholar] [CrossRef]
- Hu, W.; Li, D.; Yang, Y.; Li, T.; Chen, H.; Liu, P. Copper ferrite supported gold nanoparticles as efficient and recyclable catalyst for liquid-phase ethanol oxidation. J. Catal. 2018, 357, 108–117. [Google Scholar] [CrossRef]
- Mirzai, J.I.; Nadirov, P.A.; Velieva, A.D.; Muradkhanli, V.G. Oxidation of Ethanol on NaX Zeolite Modified with Transition Metals. Russ. J. Phys. Chem. A 2017, 91, 1005–1009. [Google Scholar] [CrossRef]
- Ahmad, J.U. Copper Catalysts for Alcohol Oxidation. Ph.D. Thesis, the Faculty of Science of the University of Helsinki, Yliopistonkatu, Helsinki, 2012. [Google Scholar]
- Janas, J.; Gurgul, J.; Socha, R.P.; Dzwigaj, S. Effect of Cu content on the catalytic activity of CuSiBEA zeolite in the SCR of NO by ethanol: Nature of the copper species. Appl. Catal. B Environ. 2009, 91, 217–224. [Google Scholar] [CrossRef]
- Kuterasiński, Ł.; Podobiński, J.; Rutkowska-Zbik, D.; Datka, J. IR Studies of the Cu ions in Cu-Faujasites. Molecules 2019, 24, 4250. [Google Scholar] [CrossRef] [PubMed]
- Kuterasiński, Ł.; Podobiński, J.; Madej, E.; Smoliło-Utrata, M.; Rutkowska-Zbik, D.; Datka, J. Reduction and Oxidation of Cu Species in Cu-faujasites Studied by IR Spectroscopy. Molecules 2020, 25, 4765. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Yahiro, H.; Tanada, K.; Mizumo, N.; Mine, Y.; Kagawa, S. Removal of nitrogen monoxide through a novel catalytic process. Decomposition on excessively copper-ion-exchanged ZSM-5 zeolites. J. Phys. Chem. 1991, 95, 3727. [Google Scholar] [CrossRef]
- Tranquada, J.M.; Heald, S.M.; Moodenbaugh, A.R. X-ray-absorption near-edge-structure study of La2−x(Ba,Sr)xCuO4−y superconductors. Phys. Rev. B 1987, 36, 5263. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, C.; Bordiga, S.; Salvalaggio, M.; Sopto, G.; Zecchina, A. XAFS, IR, and UV-Vis Study of the CuI Environment in CuI-ZSM-5. J. Phys. Chem. B 1997, 101, 344–360. [Google Scholar] [CrossRef]
- Datka, J.; Kukulska-Zając, E.; Kobyzewa, W. IR Studies of Co-adsorption of Organic Molecules and CO on Cu+ Cations in Zeolites. Catal. Today 2006, 114, 169–173. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heisse, H.M.; Akcelrud, L. Polyethylene Characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Kozyra, P.; Góra-Marek, K.; Datka, J. Extinction coefficients of C=C and C≡C bands in Ethene and Ethyne Molecules Interacting with Cu+ and Ag+ in zeolites–IR studies and quantumchemical calculations. Spectrochim. Acta A 2014, 136, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, F.; Zhan, E.; Van Veen, A.C.; Mirodatos, C.; Shen, W. Hydrogen production from ethanol over Ir/CeO2 catalysts: A comparative study of steam reforming, partial oxidation and oxidative steam reforming. J. Catal. 2008, 257, 96–107. [Google Scholar] [CrossRef]
- Sheng, P.Y.; Yee, A.; Bowmaker, G.A.; Idriss, H. H2 Production from Ethanol over Rh–Pt/CeO2 Catalysts: The Role of Rh for the Efficient Dissociation of the Carbon–Carbon Bond. J. Catal. 2002, 208, 393–403. [Google Scholar] [CrossRef]
- Yee, A.; Morrison, S.J.; Idriss, H. A Study of Ethanol Reactions over Pt/CeO2 by Temperature-Programmed Desorption and in Situ FT-IR Spectroscopy: Evidence of Benzene Formation. J. Catal. 2000, 191, 30–45. [Google Scholar] [CrossRef]
- Yee, A.; Morrison, S.J.; Idriss, H. The reactions of ethanol over M/CeO2 catalysts: Evidence of carbon–carbon bond dissociation at low temperatures over Rh/CeO2. Catal. Today 2000, 63, 327–335. [Google Scholar] [CrossRef]
- Idriss, H. Ethanol Reactions over the Surfaces of Noble Metal/Cerium Oxide Catalysts. Platin. Met. Rev. 2004, 48, 105–115. [Google Scholar] [CrossRef]
- Silchenkova, O.N.; Matyshak, V.A.; Bychkov, V.Y.; Korchak, V.N. Mechanism of Ethanol Conversion on a 5% CuO/ZrO2 Catalyst According to In Situ IR-Spectroscopic Data. Kinet. Catal. 2020, 61, 460–465. [Google Scholar] [CrossRef]
- Kukulska-Zając, E.; Góra-Marek, K.; Datka, J. IR and TPD studies of the reaction of acetic acid In zeolite NaHY. Microporous Mesoporous Mater. 2006, 96, 216–221. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuterasiński, Ł.; Podobiński, J.; Datka, J. Oxidation of Ethanol in Cu-Faujasites Studied by IR Spectroscopy. Molecules 2021, 26, 2669. https://doi.org/10.3390/molecules26092669
Kuterasiński Ł, Podobiński J, Datka J. Oxidation of Ethanol in Cu-Faujasites Studied by IR Spectroscopy. Molecules. 2021; 26(9):2669. https://doi.org/10.3390/molecules26092669
Chicago/Turabian StyleKuterasiński, Łukasz, Jerzy Podobiński, and Jerzy Datka. 2021. "Oxidation of Ethanol in Cu-Faujasites Studied by IR Spectroscopy" Molecules 26, no. 9: 2669. https://doi.org/10.3390/molecules26092669
APA StyleKuterasiński, Ł., Podobiński, J., & Datka, J. (2021). Oxidation of Ethanol in Cu-Faujasites Studied by IR Spectroscopy. Molecules, 26(9), 2669. https://doi.org/10.3390/molecules26092669





