Purification, Structural Characterization and Immunomodulatory Effects of Polysaccharides from Amomumvillosum Lour. on RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Results and Discussion
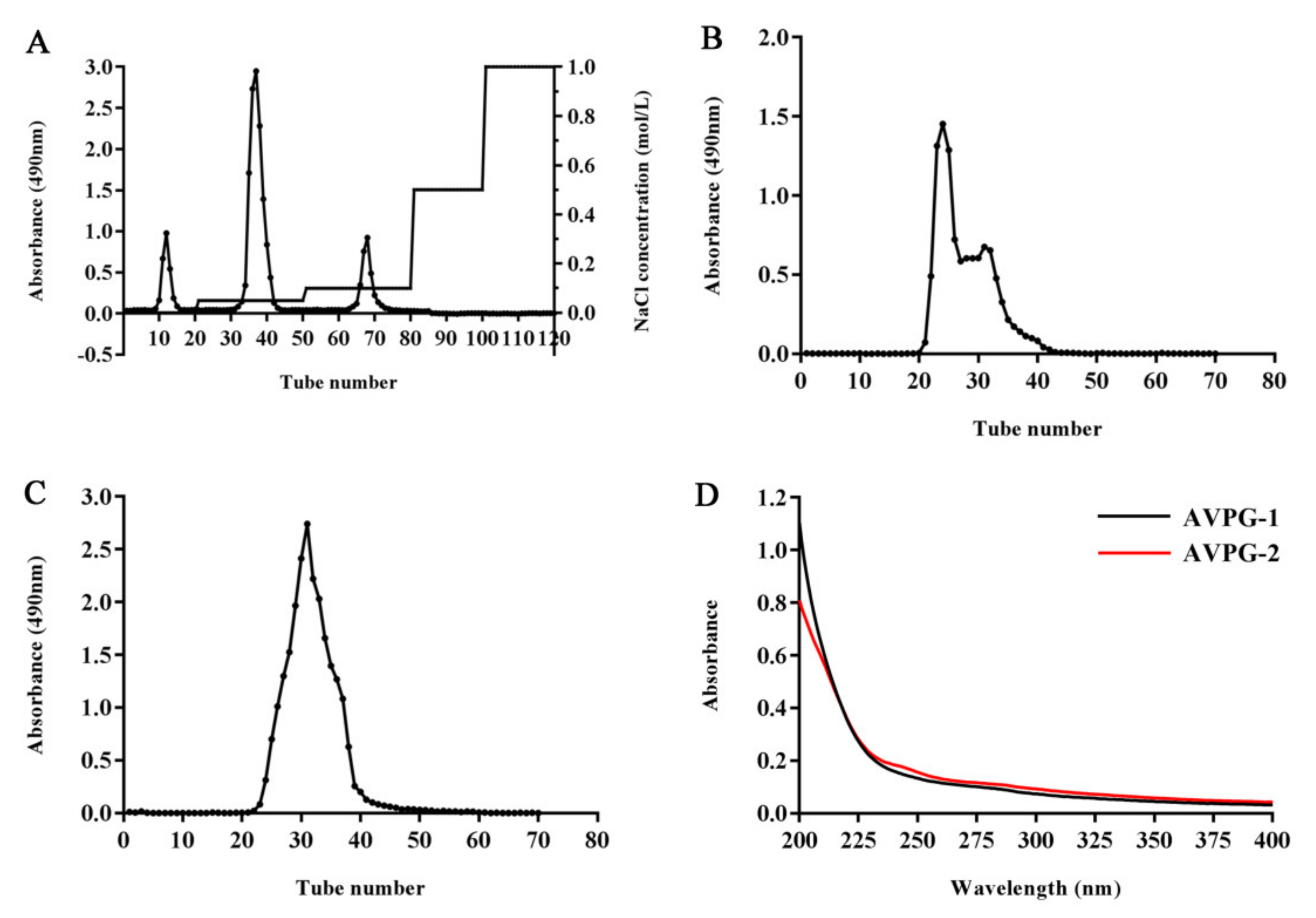
2.1. Preparation and Physicochemical Features of Polysaccharides
2.2. Structural Characterization of Polysaccharides
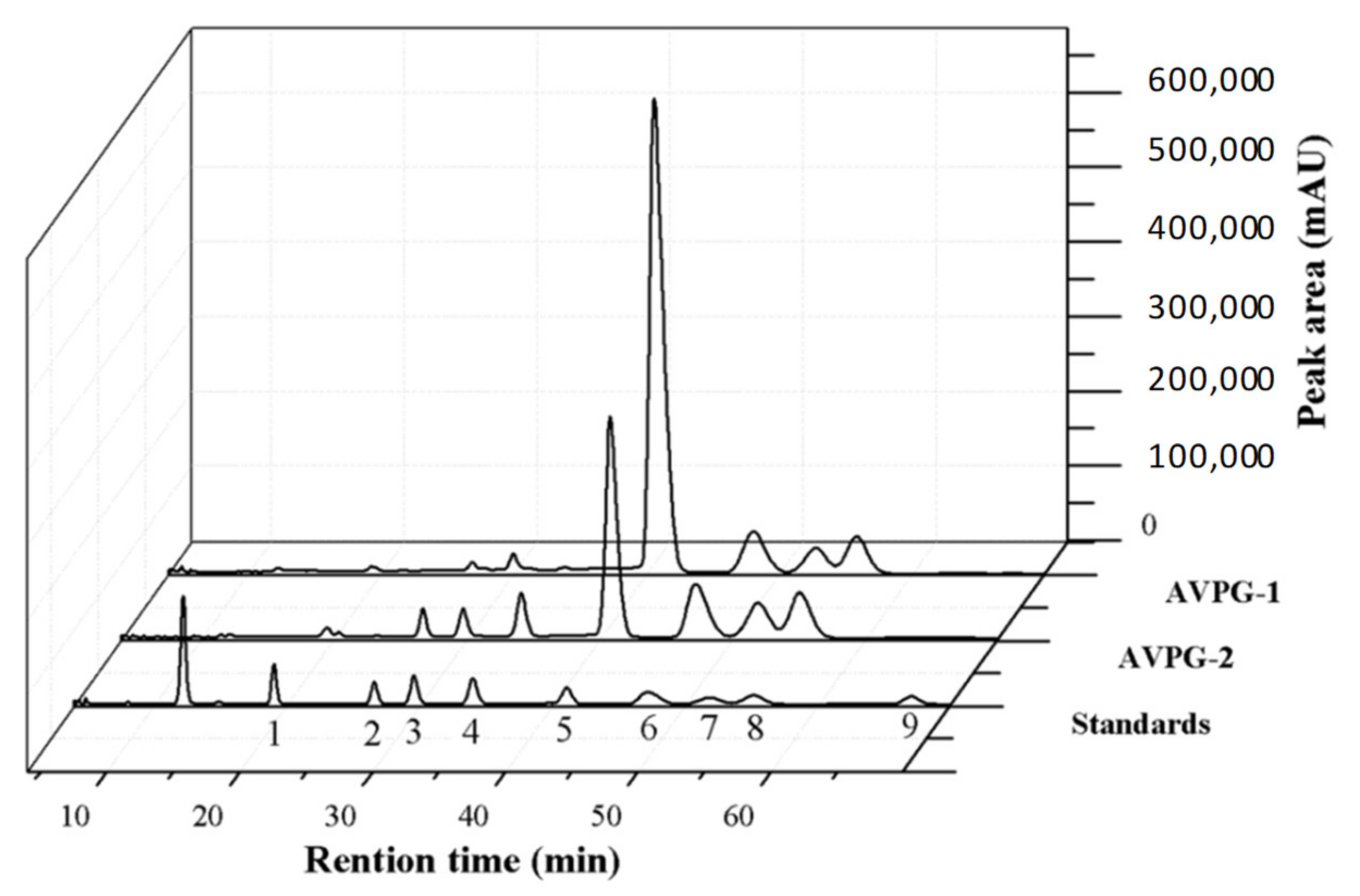
2.2.1. Monosaccharide Composition Identification
2.2.2. Determination of Homogeneity and Molecular Weight
2.2.3. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
2.2.4. Determination of Triple Helical Structure
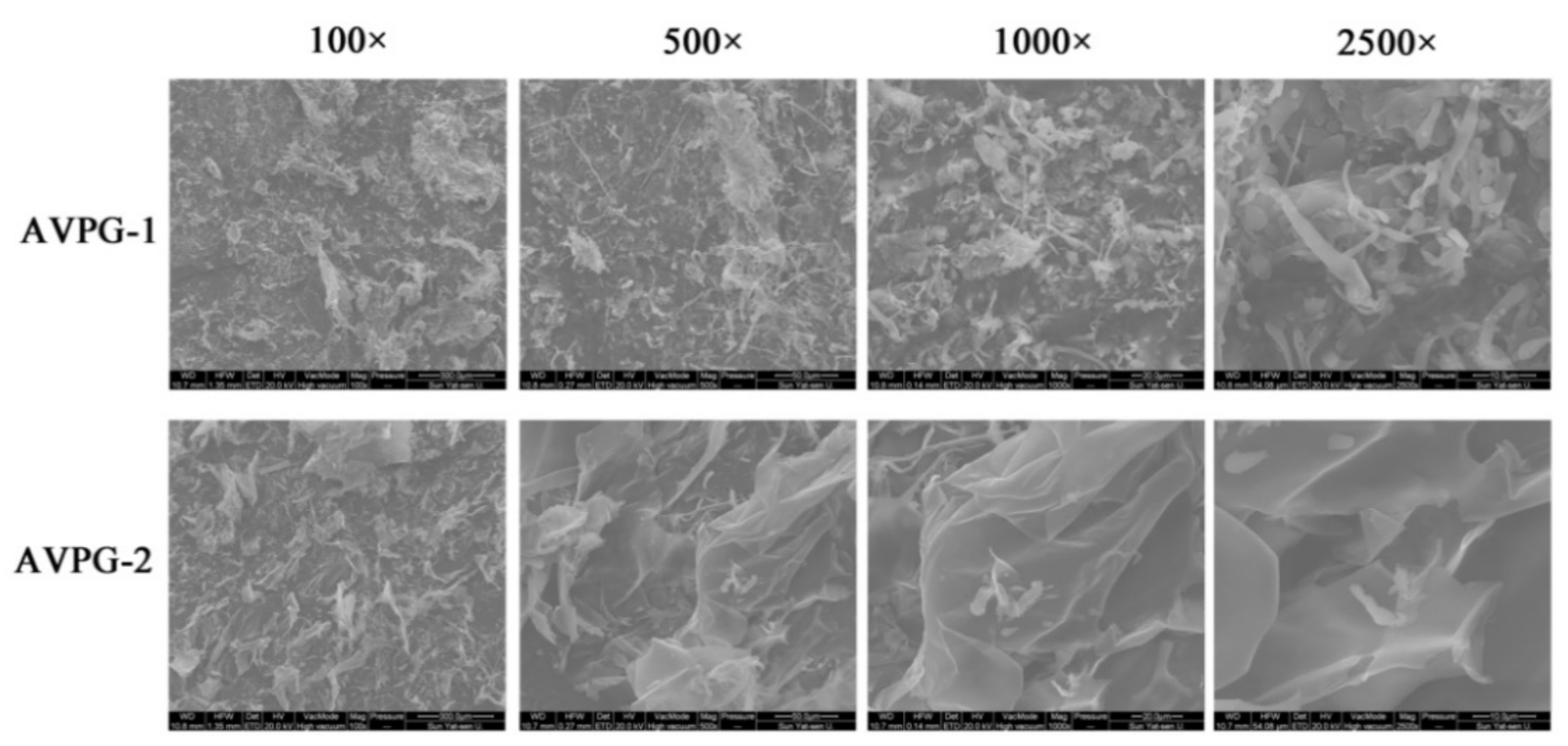
2.2.5. Surface Microscopic Analysis
2.2.6. Methylation Analysis
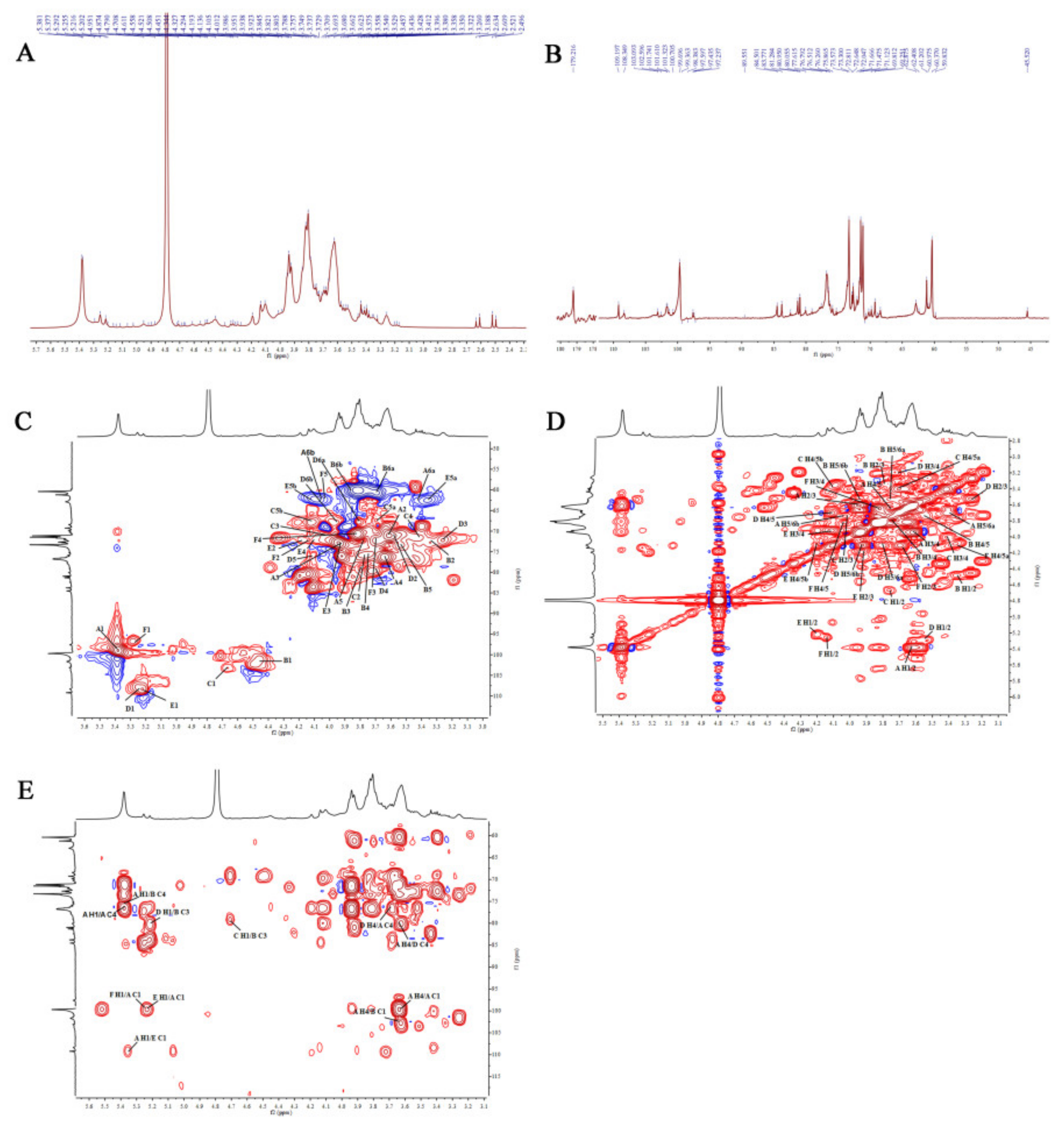
2.2.7. Nuclear Magnetic Resonance (NMR) Analysis
2.3. Immunomodulatory Activities
2.3.1. Effects of Polysaccharides on Cell Viability and NO Production of Macrophages
2.3.2. Effect of AVPG-2 on Cytokines Secretion of Macrophages
2.3.3. Effects of AVPG-2 on Polarization of Macrophages
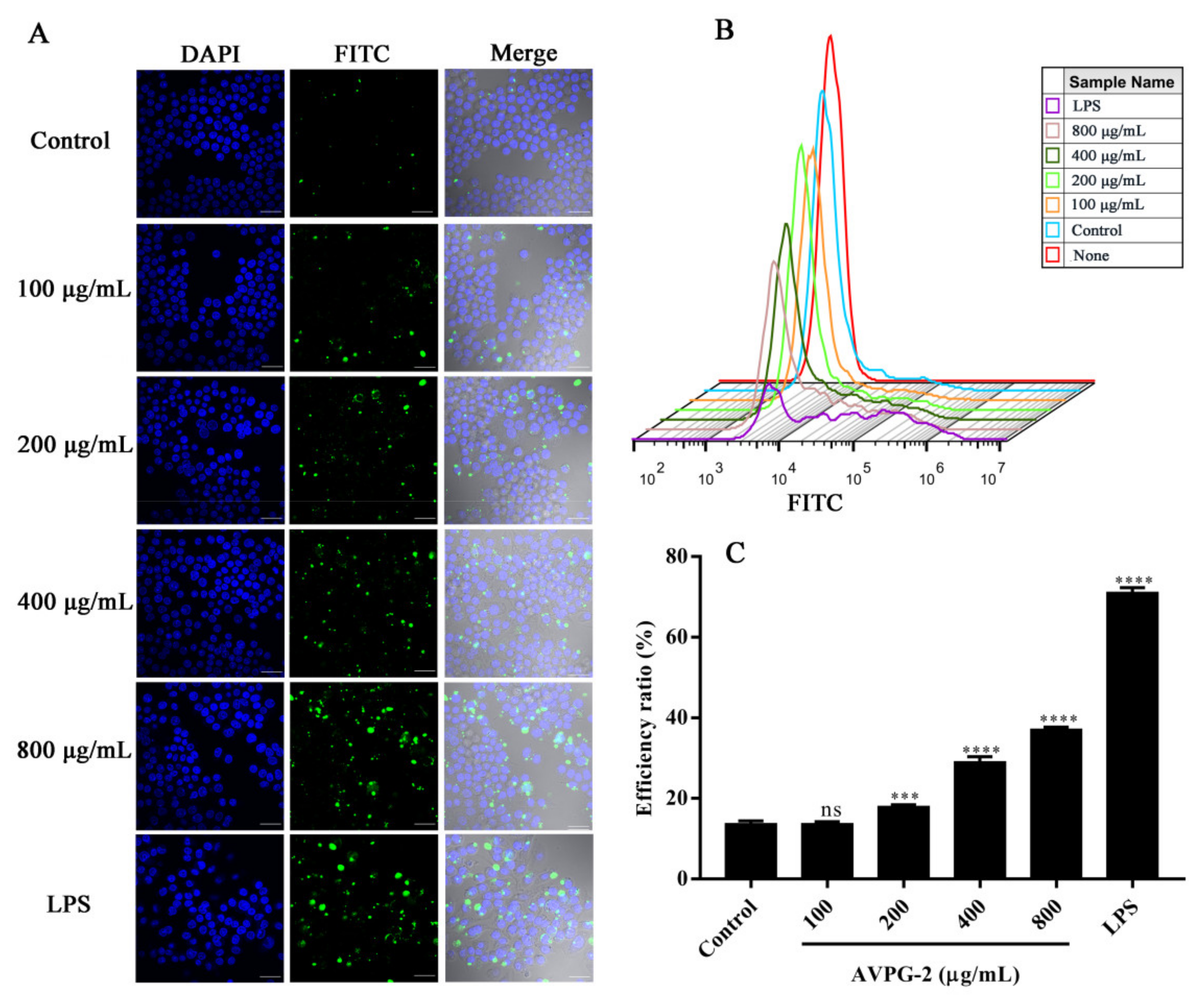
2.3.4. Effects of AVPG-2 on Phagocytic Capacity of Macrophages
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction and Purification of Polysaccharides
3.3. Preliminary Chemical Analysis
3.4. Determination of Homogeneity and Molecular Weight
3.5. Monosaccharide Composition Identification
3.6. UV Spectroscopy and FT-IR Spectroscopy Analysis
3.7. Determination of Triple-Helical Structure
3.8. Surface Microscopic Analysis
3.9. Methylation Analysis
3.10. NMR Spectroscopy Analysis
3.11. Immunomodulatory Activity
3.11.1. Cell Culture
3.11.2. Assay of Cell Viability
3.11.3. Assay of NO
3.11.4. Determination of Cytokines
3.11.5. Real Time (RT)-PCR
3.11.6. Assay of Macrophages Phagocytosis
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Suo, S.; Lai, Y.; Li, M.; Song, Q.; Cai, J.; Zhao, J.; Yang, Q.; Ung, C.O.L.; Hu, H. Phytochemicals, pharmacology, clinical application, patents, and products of Amomi fructus. Food Chem. Toxicol. 2018, 119, 31–36. [Google Scholar] [CrossRef]
- Tang, C.; Chen, J.; Zhou, Y.; Ding, P.; He, G.; Zhang, L.; Zhao, Z.; Yang, D. Exploring antimicrobial mechanism of essential oil of Amomum villosum Lour through metabolomics based on gas chromatography-mass spectrometry in methicillin-resistant Staphylococcus aureus. Microbiol. Res. 2021, 242, 126608. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Wang, J.; Chen, L.; Li, S.; Dai, C. Comparison of volatile oil between the fruits of Amomum villosum Lour. and Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen based on GC-MS and chemometric techniques. Molecules 2019, 24, 1663. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Dan, W.; Fan, B.; Guo, C.; Wu, K.; Li, D.; Xian, K.; Pescitelli, G.; Gao, J. Anti-inflammatory and α-glucosidase inhibitory activities of labdane and norlabdane diterpenoids from the rhizomes of Amomum Villosum. J. Nat. Prod. 2019, 82, 2963–2971. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, Z.; Li, Q.; Wang, D.; Xu, X.; Zhu, L.; Yang, D. Determination of flavonoids by solidification of floating organic drop liquid-phase microextraction and high-performance liquid chromatography. Anal. Lett. 2016, 2719, 2384–2396. [Google Scholar] [CrossRef]
- Huang, Y.; Yen, G.; Sheu, F.; Lin, J.; Chau, C. Dose effects of the food spice cardamom on aspects of hamster gut physiology. Mol. Nutr. Food Res. 2007, 51, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Choi, H.; Park, S.; Kim, H.; Yu, G.; Kim, J.; Park, W. Activation of the Nrf2/HO-1 pathway by Amomum villosum extract suppresses LPS-induced oxidative stress in vitro and ex vivo. Evid. Based. Complement. Alternat. Med. 2020, 2020, 2837853. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, S.H.; Bi, Q.; Liang, L.; Wang, Y.F.; Yang, X.X.; Gu, W.; Yu, J. Volatile Oil from Amomi Fructus attenuates 5-fluorouracil-induced intestinal mucositis. Front. Pharmacol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ni, W.; Yang, C.; Zhang, T.; Lu, S.; Zhao, R.; Mao, X.; Yu, J. Therapeutic effect of Amomum villosum on inflammatory bowel disease in rats. Front. Pharmacol. 2018, 9, 639. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Mi, W.; Cao, G.; Yang, R.; Chen, H.; Liu, Y.; Zou, H.; Yan, Y. The absorption characteristics of nonvolatile components in a water extraction from Amomi fructus as determined by in situ single-pass intestinal perfusion and high-performance liquid chromatography. Front. Pharmacol. 2020, 11, 711. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramberg, J.E.; Nelson, E.D.; Sinnott, R.A. Immunomodulatory dietary polysaccharides: A systematic review of the literature. Nutr. J. 2010, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ji, H.; Feng, Y.; Yu, J.; Liu, A. Structural characterization and antitumor activity of polysaccharides from Kaempferia galanga L. Oxid. Med. Cell. Longev. 2018, 2018, 9579262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.-F.; Ye, H.; Zhu, Y.-J.; Li, Y.-P.; Wang, J.-F.; Wang, P. Characterization and hypoglycemic activity of a rhamnan-type sulfated polysaccharide derivative. Mar. Drugs 2019, 17, 21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Li, S.; Xiong, Q.; Jiang, C.; Lai, X. Extraction, characterization and biological activities of polysaccharides from Amomum villosum. Carbohydr. Polym. 2013, 95, 114–122. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Q.; Li, S.; Li, X.; Chen, J.; Cao, M.; Lu, L.; Wan, M.; Zhang, D. A comparison study on polysaccharides from novel hybrids of Amomum villosum and its female parent. Int. J. Biol. Macromol. 2015, 81, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Wan, M.; Chen, J.; Li, S.; Cao, M.; Zhang, D. Effect of extraction methods on property and bioactivity of water-soluble polysaccharides from Amomum villosum. Carbohydr. Polym. 2015, 117, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-X.; Lai, Y.-F.; Zhang, W.-X.; Cai, J.; Hu, H.; Wang, Y.; Zhao, J.; Li, S.-P. Comparison of volatile compounds in different parts of fresh Amomum villosum Lour. from different geographical areas using cryogenic grinding combined HS-SPME-GC-MS. Chin. Med. 2020, 15, 97. [Google Scholar] [CrossRef]
- Lai, Y.-F.; Chen, L.-X.; Chen, Y.-N.; Zhao, J.; Leong, F.; Li, X.-W.; Yang, Q.; Li, P.; Hu, H. Sustainable development of Amomum villosum: A systematic inveatigation on three different production modes. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 97–104. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Miao, J.; Jing, S.; Li, X.; Huang, L.; Gao, W. The effect of different extraction techniques on property and bioactivity of polysaccharides from Dioscorea hemsleyi. Int. J. Biol. Macromol. 2017, 102, 847–856. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Ma, L.; Zhang, Z.; Cao, L.; Liu, J.; Zeng, X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Liu, G.; Yao, J.; Shan, T.; Sun, C.; Zhang, Q. The structure-activity relationship between polysaccharides from Sargassum thunbergii and anti-tumor activity. Int. J. Biol. Macromol. 2017, 105, 686–692. [Google Scholar] [CrossRef]
- Gertsch, J.; Viveros-Paredes, J.M.; Taylor, P. Plant immunostimulants-scientific paradigm or myth? J. Ethnopharmacol. 2011, 136, 385–391. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, J.; Liu, Z.; Zhang, Y.; Li, J.; Li, Y.O. Biosynthesis of selenium rich exopolysaccharide (Se-EPS) by Pseudomonas PT-8 and characterization of its antioxidant activities. Carbohydr. Polym. 2016, 142, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Sachithanandan, N.; Graham, K.L.; Galic, S.; Honeyman, J.E.; Fynch, S.L.; Hewitt, K.A.; Steinberg, G.R.; Kay, T.W. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes 2011, 60, 2023–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Huang, Q.; Fu, X.; Yue, X.-J.; Liu, R.H.; You, L.-J. Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn. Int. J. Biol. Macromol. 2015, 75, 298–305. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Yu, P.; Mao, G.; Zhao, T.; Feng, W.; Yang, L.; et al. Structural elucidation and antioxidant activity a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Depolymerization of polysaccharides from Opuntia ficus indica: Antioxidant and antiglycated activities. Int. J. Biol. Macromol. 2015, 79, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, C.; Ran, C.; Tan, Y.; Yu, Q.; Kan, J. Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2019, 130, 903–914. [Google Scholar] [CrossRef]
- Jing, L.; Sheng, J.; Jiang, J.; Wang, Y.; Shen, X.; Liu, D.; Zhang, W.; Mao, S. Chemical characteristics and cytoprotective activities of polysaccharide fractions from Athyrium Multidentatum (Doll.) Ching. Int. J. Biol. Macromol. 2020, 158, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Regenstein, J.M.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, H.; Zhao, J.; Sun, J.; Li, M.; Sun, X. Microwave-assisted extraction, characterization and immunomodulatory activity on RAW264.7 cells of polysaccharides from Trichosanthes kirilowii Maxim seeds. Int. J. Biol. Macromol. 2020, 164, 2861–2872. [Google Scholar] [CrossRef]
- Guo, R.; Tian, S.; Li, X.; Wu, X.; Liu, X.; Li, D.; Liu, Y.; Ai, L.; Song, Z.; Wu, Y. Pectic polysaccharides from purple passion fruit peel: A comprehensive study in macromolecular and conformational characterizations. Carbohydr. Polym. 2020, 229, 115406. [Google Scholar] [CrossRef]
- He, B.-L.; Zheng, Q.-W.; Guo, L.-Q.; Huang, J.-Y.; Yun, F.; Huang, S.-S.; Lin, J.-F. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2020, 145, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, J.; Shang, H.; Guo, Y.; Chen, S. Extraction, purification, hypoglycemic and antioxidant activities of red clover (Trifolium pratense L.) polysaccharides. Int. J. Biol. Macromol. 2020, 148, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, S.; Wang, Q.; Lu, X.; Lin, L.; Tian, Y.; Xiao, J.; Zheng, B. Characterization and hypoglycemic activity of a β-pyran polysaccharides from bamboo shoot (Leleba oldhami Nakal) shells. Carbohydr. Polym. 2016, 144, 438–446. [Google Scholar] [CrossRef]
- Pu, X.; Ma, X.; Liu, L.; Ren, J.; Li, H.; Li, X.; Yu, S.; Zhang, W.; Fan, W. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr. Polym. 2016, 137, 154–164. [Google Scholar] [CrossRef]
- Cheng, J.; Song, J.; Wei, H.; Wang, Y.; Huang, X.; Liu, Y.; Lu, N.; He, L.; Lv, G.; Ding, H.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef]
- Patra, S.; Maity, P.; Chakraborty, I.; Sen, I.K.; Ghosh, D.; Rout, D.; Bhanja, S.K. Structural studies of immunomodulatory (1→3)-, (1→4)-α glucan from an edible mushroom Polyporus grammocephalus. Int. J. Biol. Macromol. 2020, 168, 649–655. [Google Scholar] [CrossRef]
- Guo, R.; Li, X.; Chen, X.; Kou, Y.; Hu, H.; Liu, X.; Li, D.; Liu, Y.; Ai, L.; Song, Z.; et al. An ultrasonic-extracted arabinoglucan from Tamarindus indica L. pulp: A study on molecular and structural characterizations. Int. J. Biol. Macromol. 2020, 164, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Molaei, H.; Jahanbin, K. Structural features of a new water-soluble polysaccharide from the gum exudates of Amygdalus scoparia Spach (Zedo gum). Carbohydr. Polym. 2018, 182, 98–105. [Google Scholar] [CrossRef]
- Guo, W.; Rao, G.; Wen, X. Arabinogalactan in banana: Chemical characterization and pharmaceutical effects. Int. J. Biol. Macromol. 2021, 167, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Sznaider, F.; Rojas, A.M.; Stortz, C.A.; Navarro, D.A. Chemical structure and rheological studies of arabinoglucuronoxylans from the Cercidium praecox exudate brea gum. Carbohydr. Polym. 2020, 228, 115388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, Z.; Mao, H.; Hu, P.; Li, X. Isolation and structure elucidation of polysaccharides from fruiting bodies of mushroom Coriolus versicolor and evaluation of their immunomodulatory effects. Int. J. Biol. Macromol. 2021, 166, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Makarova, E.N.; Shakhmatov, E.G. Structural characteristics of oxalate-soluble polysaccharides from Norway spruce (Picea abies) foliage. Carbohydr. Polym. 2020, 246, 116544. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.S.; Iacomini, M.; Cordeiro, L.M.C. β-L-Araf-containing arabinan and glucuronoxylan from guavira fruit pomace. Carbohydr. Res. 2019, 481, 16–22. [Google Scholar] [CrossRef]
- Lan, H.; Cheng, Y.; Mu, J.; Huang, Y.; Chen, H.; Zhao, L.; Wang, K.; Hu, Z. Glucose-rich polysaccharide from dried “Shixia” longan activates macrophages through Ca(2+) and CR3- mediated MAPKs and PI3K-AKT pathways. Int. J. Biol. Macromol. 2021, 167, 845–853. [Google Scholar] [CrossRef]
- Huo, J.; Wu, J.; Huang, M.; Zhao, M.; Sun, W.; Sun, X.; Zheng, F. Structural characterization and immuno-stimulating activities of a novel polysaccharide from Huangshui, a byproduct of Chinese Baijiu. Food Res. Int. 2020, 136, 109493. [Google Scholar] [CrossRef]
- Vogel, D.Y.S.; Glim, J.E.; Stavenuiter, A.W.D.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H.J. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Cui, Y.-S.; Li, Y.-X.; Jiang, S.-L.; Song, A.-N.; Fu, Z.; Dong, C.-X.; Yao, Z.; Qiao, W. Isolation, purification, and structural characterization of polysaccharides from Atractylodis Macrocephalae Rhizoma and their immunostimulatory activity in RAW264.7 cells. Int. J. Biol. Macromol. 2020, 163, 270–278. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Effects of the molecular weight and protein and sulfate content of Chlorella ellipsoidea polysaccharides on their immunomodulatory activity. Int. J. Biol. Macromol. 2018, 107, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Hu, C.; Zou, X.; Lin, Y.; Xia, Y.; You, L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019, 128, 119–128. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xiao, X.; Liu, J.; Wang, J.; Zhang, N.; Bing, T.; Liu, X.; Zhang, Z.; Shangguan, D. Immunomodulatory effects of Lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules 2020, 25, 1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daskalaki, M.G.; Bafiti, P.; Kikionis, S.; Laskou, M.; Roussis, V.; Ioannou, E.; Kampranis, S.C.; Tsatsanis, C. Disulfides from the brown alga dictyopteris membranacea suppress M1 macrophage activation by inducing AKT and suppressing MAPK/ERK signaling pathways. Mar. Drugs 2020, 18, 527. [Google Scholar] [CrossRef] [PubMed]
- Zajd, C.M.; Ziemba, A.M.; Miralles, G.M.; Nguyen, T.; Feustel, P.J.; Dunn, S.M.; Gilbert, R.J.; Lennartz, M.R. Bone marrow-derived and elicited peritoneal macrophages are not created equal: The questions asked dictate the cell type used. Front. Immunol. 2020, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Hartenstein, V.; Martinez, P. Phagocytosis in cellular defense and nutrition: A food-centered approach to the evolution of macrophages. Cell Tissue Res. 2019, 377, 527–547. [Google Scholar] [CrossRef]
- Martinez, J.; Cunha, L.D.; Park, S.; Yang, M.; Lu, Q.; Orchard, R.; Li, Q.-Z.; Yan, M.; Janke, L.; Guy, C.; et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533, 115–119. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, G.; Zhang, P.; Zhu, L.; Zhang, L.; Chen, K. Rhizopus nigricans polysaccharide activated macrophages and suppressed tumor growth in CT26 tumor-bearing mice. Carbohydr. Polym. 2018, 198, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Bhunia, D.; Muller, S.; Nerella, S.G.; Alvala, M.; Halmuthur Mahabalarao, S.K. Novel trisaccharide based phospholipids as immunomodulators. Int. Immunopharmacol. 2019, 74, 105684. [Google Scholar] [CrossRef]
- Sandeep, A.; Reddy, B.S.; Hyder, I.; Halmuthur, M.S.K. Synthesis of a new class of glycolipids and the evaluation of their immunogenicity using murine splenocytes. J. Carbohydr. Chem. 2016, 35, 326–343. [Google Scholar] [CrossRef]
- Suárez, E.R.; Syvitski, R.; Kralovec, J.A.; Noseda, M.D.; Barrow, C.J.; Ewart, H.S.; Lumsden, M.D.; Grindley, T.B. Immunostimulatory polysaccharides from Chlorella pyrenoidosa. A new galactofuranan. measurement of molecular weight and molecular weight dispersion by DOSY NMR. Biomacromolecules 2006, 7, 2368–2376. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Zhou, S.; Yip, K.; Xu, J.; Chen, H. Laser microdissection hyphenated with high performance gel permeation chromatography-charged aerosol detector and ultra performance liquid chromatography-triple quadrupole mass spectrometry for histochemi. Int. J. Biol. Macromol. 2018, 107, 332–342. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, J.; Bi, E.; Zhao, Y.; Qin, T.; Wang, Y.; Wang, A.; Gao, S.; Yi, Q.; Wang, S. TNF-α enhances Th9 cell differentiation and antitumor immunity via TNFR2-dependent pathways. J. Immunother. cancer 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Frady, L.N.; Bash, R.E.; Cohen, S.M.; Schorzman, A.N.; Su, Y.-T.; Irvin, D.M.; Zamboni, W.C.; Wang, X.; Frye, S.V.; et al. MerTK as a therapeutic target in glioblastoma. Neuro. Oncol. 2018, 20, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Hang, J.-J.; Han, T.; Zhuo, M.; Jiao, F.; Wang, L.-W. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016, 37, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Z.; Fu, L.; Xu, T. Macrophage polarization in the development and progression of ovarian cancers: An overview. Front. Oncol. 2019, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Cheng, F.; Pan, X.; Zhou, T.; Liu, X.; Zheng, Z.; Luo, L.; Zhang, Y. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug Deliv. 2017, 24, 1360–1371. [Google Scholar] [CrossRef] [Green Version]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E. Inhibition of digestive enzyme activities by pectic polysaccharides in model solutions. Bioact. Carbohydrates Diet. Fibre 2014, 4, 27–38. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Z.; Huang, Z.; Wu, Z.; Xu, J.; Wang, K. A comparative study on the structures of Grifola frondosa polysaccharides obtained by different decolourization methods and their in vitro antioxidant activities. Food Funct. 2019, 10, 6720–6731. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, M.; Yi, P.; Yan, C. Structural characterization and osteoprotective effects of a novel oligo-glucomannan obtained from the rhizome of Cibotium barometz by alkali extraction. Ind. Crops Prod. 2018, 113, 202–209. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Qing, D.; Chen, J.; Zhang, Q.; Yan, C. Structural characterization and osteogenic bioactivities of a novel Humulus lupulus polysaccharide. Food Funct. 2020, 11, 1165–1175. [Google Scholar] [CrossRef] [PubMed]



| Methylated Sugars | Linkage | AVPG-1 (Molar Ratios) | AVPG-2 (Molar Ratios) |
|---|---|---|---|
| 2,3,6-Me3-Glcp | →4)-Glcp-(1→ | 0.65 | 0.36 |
| 2,6-Me2-Glcp | →3,4)-Glcp-(1→ | 0.16 | ND |
| 2,3,6-Me3-Galp | →4)-Galp-(1→ | 0.09 | 0.04 |
| 2,3,4-Me3-Xylp | Xylp-(1→ | 0.04 | ND |
| 2,3,5-Me3-Araf | Araf-(1→ | 0.06 | 0.09 |
| 1,2,3,4-Me4-Glcp | →6)-Glcp | ND | 0.09 |
| 2,4,6-Me3-Galp | →3)-Galp-(1→ | ND | 0.03 |
| 2,4-Me2-Galp | →3,6)-Galp-(1→ | ND | 0.17 |
| 2,3-Me2-Araf | →5)-Araf-(1→ | ND | 0.06 |
| 2,3-Me2-Xylp | →4)-Xylp-(1→ | ND | 0.10 |
| 2,3,4-Me3-Rhalp | Rhap-(1→ | ND | 0.02 |
| Peak | Glycosyl Residue | H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 |
|---|---|---|---|---|---|---|---|
| Chemical shifts of residues of AVPG-1 | |||||||
| A | -4)-α-d-Glcp-(1- | 5.38/99.70 | 3.62/71.01 | 3.95/72.92 | 3.64/73.25 | 3.76/72.26 | 3.41, 4.03 /68.98 |
| B | -3,4)-β-d-Glcp-(1- | 4.45/101.61 | 3.35/72.80 | 3.80/80.18 | 3.74/73.55 | 3.56/73.40 | 3.70, 3.94 /60.44 |
| C | β-d-Xylp-(1- | 4.71/103.10 | 3.76/75.83 | 4.03/71.18 | 3.41/71.67 | 3.70, 4.12/67.49 | |
| D | -4)-α-d-Galp-(1- | 5.22/108.35 | 3.56/78.60 | 3.26/72.21 | 3.69/79.54 | 4.11/76.60 | 3.80, 4.00 /67.54 |
| E | α-l-Araf-(1- | 5.20/109.20 | 4.19/80.85 | 3.92/75.86 | 4.11/83.77 | 3.36, 4.20/62.46 | |
| F | α-d-GlcpA-(1- | 5.26/97.60 | 4.14/80.37 | 3.69/79.68 | 3.82/70.89 | 4.04/61.64 | -/179.22 |
| Chemical shifts of residues of AVPG-2 | |||||||
| A | -6)-β-d-Glcp | 4.62/95.84 | 3.22/71.19 | 3.45/75.81 | 3.87/69.30 | 4.09/68.42 | 3.70, 3.94/61.23 |
| B | -4)-α-d-Glcp-(1- | 5.40/99.70 | 3.62/71.55 | 3.94/73.19 | 3.61/76.66 | 3.75/76.47 | 3.41, 3.98/68.70 |
| C | -3)-α-d-Galp-(1- | 5.39/101.63 | 3.62/71.43 | 3.70/79.94 | 3.40/69.51 | 3.93/73.11 | 4.12, 4.19/68.14 |
| D | -4)-α-d-Galp-(1- | 5.22/108.27 | 3.50/71.36 | 3.26/72.73 | 3.67/72.58 | 3.40/72.25 | 3.71, 3.82/60.94 |
| E | -3,6)-β-d-Galp-(1- | 4.41/103.09 | 3.50/71.63 | 3.70/80.03 | 3.53/73.81 | 3.36/69.57 | 3.71, 4.09/68.50 |
| F | α-l-Araf-(1- | 5.23/109.18 | 4.19/81.19 | 3.80/76.31 | 4.11/76.43 | 3.45, 3.80/60.36 | |
| G | α-l-Araf-(1- | 5.20/92.01 | 4.20/81.25 | 3.91/76.63 | 4.11/76.52 | 3.45, 3.80/60.36 | |
| H | -5)-α-l-Araf-(1- | 5.07/98.92 | 4.11/83.85 | 3.92/76.49 | 4.19/81.18 | 3.68, 3.91/76.67 | |
| I | -4)-β-d-Xylp-(1- | 4.49/102.72 | 3.34/73.17 | 3.55/73.97 | 3.94/76.49 | 3.39, 4.10/62.89 | |
| J | α-l-Rhap-(1- | 4.71/100.70 | 3.90/73.37 | 4.11/80.40 | 3.41/72.03 | 4.01/69.88 | 1.23/16.42 |
| K | α-d-GlcpA-(1- | 5.26/97.61 | 4.14/80.60 | 3.92/76.47 | 4.19/80.94 | 3.98/68.66 | 177.05 |
| L | -4)-α-d-GalpA | 5.06/107.43 | 3.74/79.84 | 3.63/71.49 | 4.10/83.89 | 3.92/76.46 | 175.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Qian, C.; Yang, D.; Tang, C.; Xu, X.; Liu, E.-H.; Zhong, J.; Zhu, L.; Zhao, Z. Purification, Structural Characterization and Immunomodulatory Effects of Polysaccharides from Amomumvillosum Lour. on RAW 264.7 Macrophages. Molecules 2021, 26, 2672. https://doi.org/10.3390/molecules26092672
Zhou Y, Qian C, Yang D, Tang C, Xu X, Liu E-H, Zhong J, Zhu L, Zhao Z. Purification, Structural Characterization and Immunomodulatory Effects of Polysaccharides from Amomumvillosum Lour. on RAW 264.7 Macrophages. Molecules. 2021; 26(9):2672. https://doi.org/10.3390/molecules26092672
Chicago/Turabian StyleZhou, Yang, Chunguo Qian, Depo Yang, Cailin Tang, Xinjun Xu, E-Hu Liu, Jingtang Zhong, Longping Zhu, and Zhimin Zhao. 2021. "Purification, Structural Characterization and Immunomodulatory Effects of Polysaccharides from Amomumvillosum Lour. on RAW 264.7 Macrophages" Molecules 26, no. 9: 2672. https://doi.org/10.3390/molecules26092672






