Easily Processable, Highly Transparent and Conducting Thiol-Functionalized Reduced Graphene Oxides Langmuir-Blodgett Films
Abstract
1. Introduction
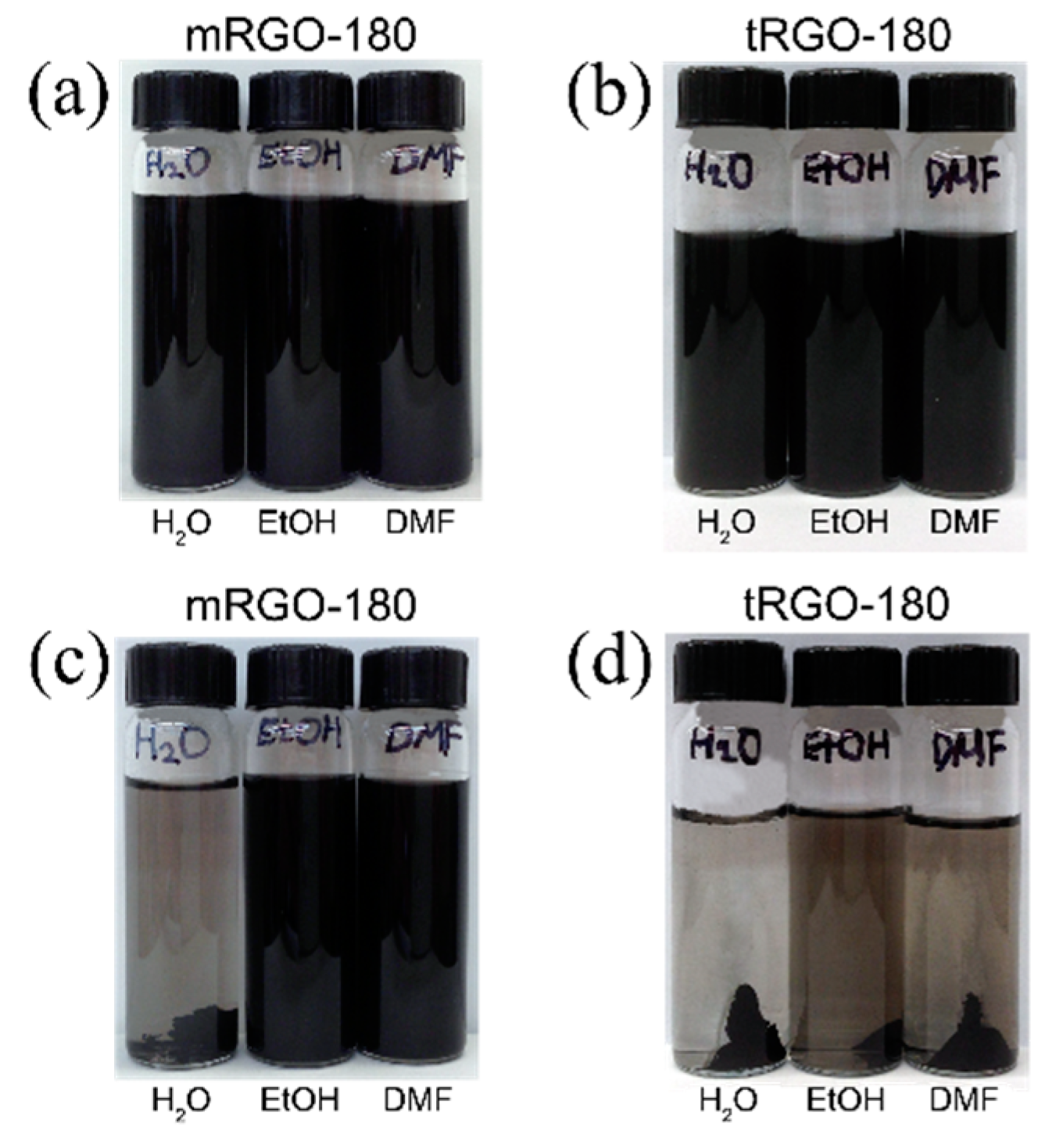
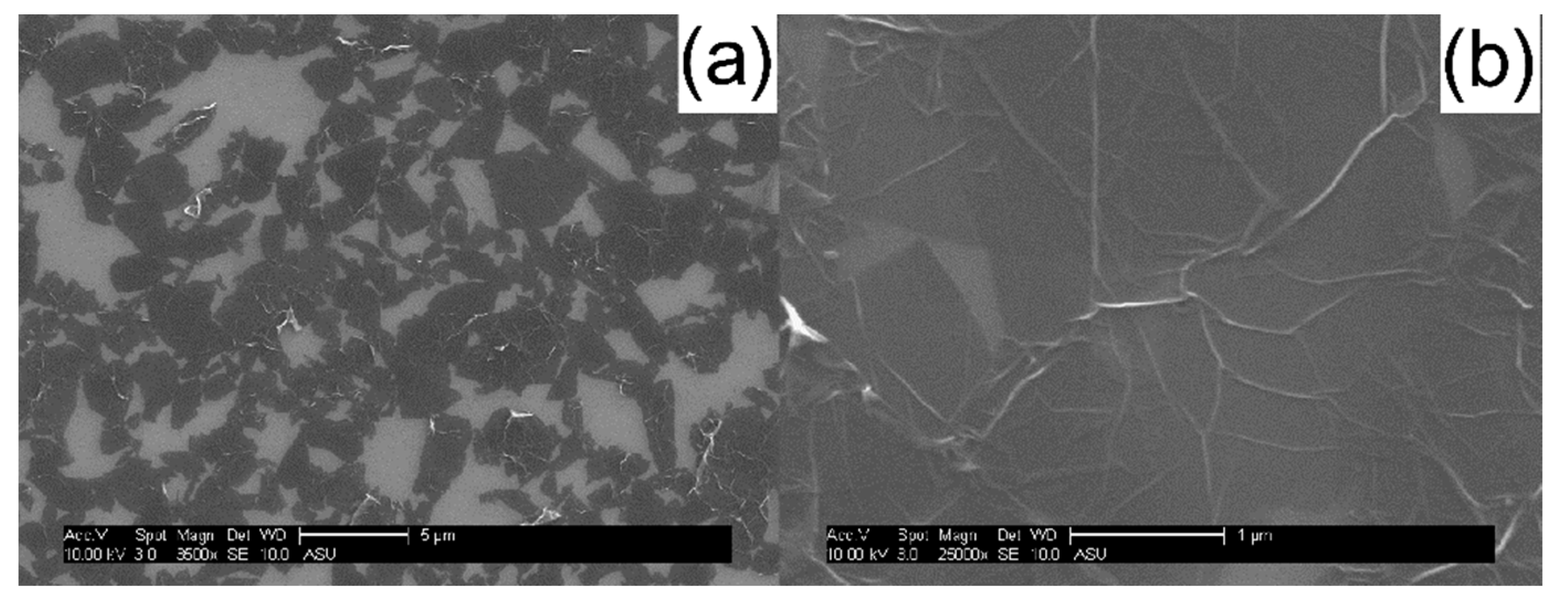
2. Results and Discussion
3. Materials and Methods
3.1. Thionation of GOs
3.2. Fabrication of mRGO Thin Films on Glass Substrate
3.3. Materials Characterization
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Neto, A.H.C.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Quintana, M.; Vazquez, E.; Prato, M. Organic functionalization of graphene in dispersions. Acc. Chem. Res. 2012, 46, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Liu, Z.L.; Zhang, X.; Wang, Y.; Tian, J.; Huang, Y.; Ma, Y.; Zhang, X.; Chen, Y. A graphene hybrid material covalently functionalized with porphyrin: Synthesis and optical limiting property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.B.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.H.; Ruoff, S.R.; Lee, H.Y. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73–79. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Liu, Y.; Chen, Z.; Pan, D.; Li, Z.; Jiao, Z.; Hu, P.; Shek, C.-H.; Wu, C.-M.L. Assembling tin dioxide quantum dots to graphene nanosheets by a facile ultrasonic route. Langmuir 2013, 29, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Choh, S.-Y.; Cross, D.; Wang, C. Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules 2011, 12, 1126–1136. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Poly. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Liu, J.; Jiang, G. Effective heavy metal removal from water aqueous systems by thiol functionalized magnetic mesoporous silica. J. Hazard. Mater. 2011, 192, 277–283. [Google Scholar]
- Xu, S.; Han, X. A novel method to construct a third-generation biosensor: Self-assembling gold nanoparticles on thiol-functionalzied poly(styrene-co-acrylic acid) nanospheres. Biosens. Bioelectron. 2004, 19, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Potta, T.; Chun, C.; Song, S.-C. Chemically crosslinkable thermosensitive polyphosphazene gels as injectable materials for biomedical applications. Biomaterials 2009, 30, 6178–6192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Leem, G.; Srisombat, L.-O.; Lee, T.R. Rationally designed ligands that inhibit the aggregation of large gold nanoparticles in solution. J. Am. Chem. Soc. 2008, 130, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, B.A.; Fokina, N.A.; Chernish, L.V.; Dahl, J.E.; Liu, S.; Carlson, R.M.; Fokin, A.A.; Schreiner, P.R. Functionalized nanodiamonds part 3: Thiolation of tertiary/bridgehead alcohols. Org. Lett. 2006, 8, 1767–1770. [Google Scholar] [CrossRef]
- Thomas, H.R.; Marsden, A.J.; Walker, M.; Wilson, N.R.; Rourke, J.P. Sulfur-functionalized graphene oxide by epoxide ring-opening. Angew. Chem. Int. Ed. Engl. 2014, 53, 7613–7618. [Google Scholar] [CrossRef]
- Jeon, K.-W.; Seo, D.-K. Concomitant thionation and reduction of graphene oxide through solid/gas metathetical sulfidation reactions at high temperatures. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 721–737. [Google Scholar] [CrossRef]
- Pham, C.V.; Eck, M.; Krueger, M. Thiol functionalized reduced graphene oxide as a base material for novel graphene-nanoparticle hybrid composites. Chem. Eng. J. 2013, 231, 146–154. [Google Scholar] [CrossRef]
- Curran, S.A.; Cech, J.; Zhang, D.; Dewald, J.L.; Avadhanula, A.; Kandadai, M.; Roth, S. Thiolation of carbon nanotubes and sidewall functionalization. J. Mater. Res. 2006, 21, 1012–1018. [Google Scholar] [CrossRef]
- Bergman, J.; Pettersson, B.; Hasimbegovic, V.; Svensson, P.H. Thionations using a P4S10-pyridine complex in solvents such as acetonitrile and dimethyl sulfone. J. Org. Chem. 2011, 76, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Mizuni, Y.; Ikehara, M.; Watanabe, K.A. Potential antimetabolites. I. selective thiation of uracil and 1,2,4-triazine-3,5(2H, 4H)-dione (6-azauracil). Chem. Pharm. Bull. 1962, 10, 647–652. [Google Scholar] [CrossRef][Green Version]
- Bourdauducq, P.; Demarcq, M.C. Kinetic study of the homogeneous methanolysis of tetraphosphorus decasulphide. J. Chem. Soc. Dalton Trans. 1987, 1897–1900. [Google Scholar] [CrossRef]
- Yang, H.-C.; Lin, S.-M.; Liu, Y.-H.; Wang, Y.; Chen, M.-M.; Sheu, H.-S.; Tsou, D.-L.; Lin, C.-H.; Luh, T.-Y. Ring opening metathesis polymerization of bisnorbornene derivatives linked by Cp2Ni2(μ-S)2 bridge. J. Organomet. Chem. 2006, 691, 3196–3200. [Google Scholar] [CrossRef]
- Compton, O.C.; Jain, B.; Dikin, D.A.; Abouimrane, A.; Amine, K.; Nguyen, S.T. Chemically active reduced graphene oxide with tunable C/O ratios. ACS Nano 2011, 5, 4380–4391. [Google Scholar] [CrossRef] [PubMed]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Jover, J.; Bosque, R.; Sales, J. Neural network based QSPR study for predicting pKa of phenols in different solvents. QSAR Comb. Sci. 2007, 26, 385–397. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Lin, S.F.; Chen, D.Q.; Chen, G.H. Study on the absorption coefficient of reduced graphene oxide dispersion. J. Phys. Chem. C 2014, 118, 12520–12525. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Feng, H.; Cheng, R.; Zhao, X.; Duan, X.; Li, J. A low-temperature method to produce highly reduced graphene oxide. Nat. Commun. 2013, 4, 1539–1548. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in glasses containing transition metal ions. J. Non Cryst. Solids 1968, 1, 1–17. [Google Scholar] [CrossRef]
- Efros, A.L.; Shklovskii, B.I. Coulomb gap and low temperature conductivity of disordered systems. J. Phys. C 1975, 8, L49–L51. [Google Scholar] [CrossRef]
- Joung, D.; Khondaker, S.I. Efros-shklovskii variable-range hopping in reduced graphene oxide sheets of varying carbon sp2 fraction. Phys. Rev. B 2012, 86, 235423–235425. [Google Scholar] [CrossRef]
- Zhang, H.; Jeon, K.W.; Seo, D.K. Equipment-free deposition of graphene-based molybdenum oxide nanohybrid Langmuir-blodgett films for flexible electrochromic panel application. ACS Appl. Mater. Interfaces 2016, 8, 21539–21544. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Lahaye, R.J.W.E.; Park, M.H.; An, K.H.; Kim, I.J.; Yang, C.W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of graphitic AB stacking order of graphite oxide. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Khattak, G.D.; Salim, M.A.; Wenger, L.E.; Gilani, A.H. X-ray photoelectron spectroscopy (XPS) and magnetic susceptibility studies of copper-vanadium phosphate glasses. J. Non Cryst. Solids 2000, 262, 66–79. [Google Scholar] [CrossRef]
- Whelan, C.M.; Smyth, M.R.; Barnes, C.J.; Brown, N.; Anderson, C.A. An XPS study of heterocyclic thiol self-assembly on Au (111). Appl. Surf. Sci. 1998, 134, 144–158. [Google Scholar] [CrossRef]
- Hutchison, J.E.; Postlethwaite, T.A.; Murray, R.W. Molecular films of thiol-derivatized tetraphenylporphyrins on gold: Film formation and electrocatalytic dioxygen reduction. Langmuir 1993, 9, 3277–3283. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Mielczarski, E.; Mielczarski, J.; Castillo, N.C.; Kiwi, J.; Pulgarin, C. Escherichia coli inactivation by N, S co-doped commercial TiO2 powders under UV and visible light. Appl. Catal. B 2008, 84, 448–456. [Google Scholar] [CrossRef]

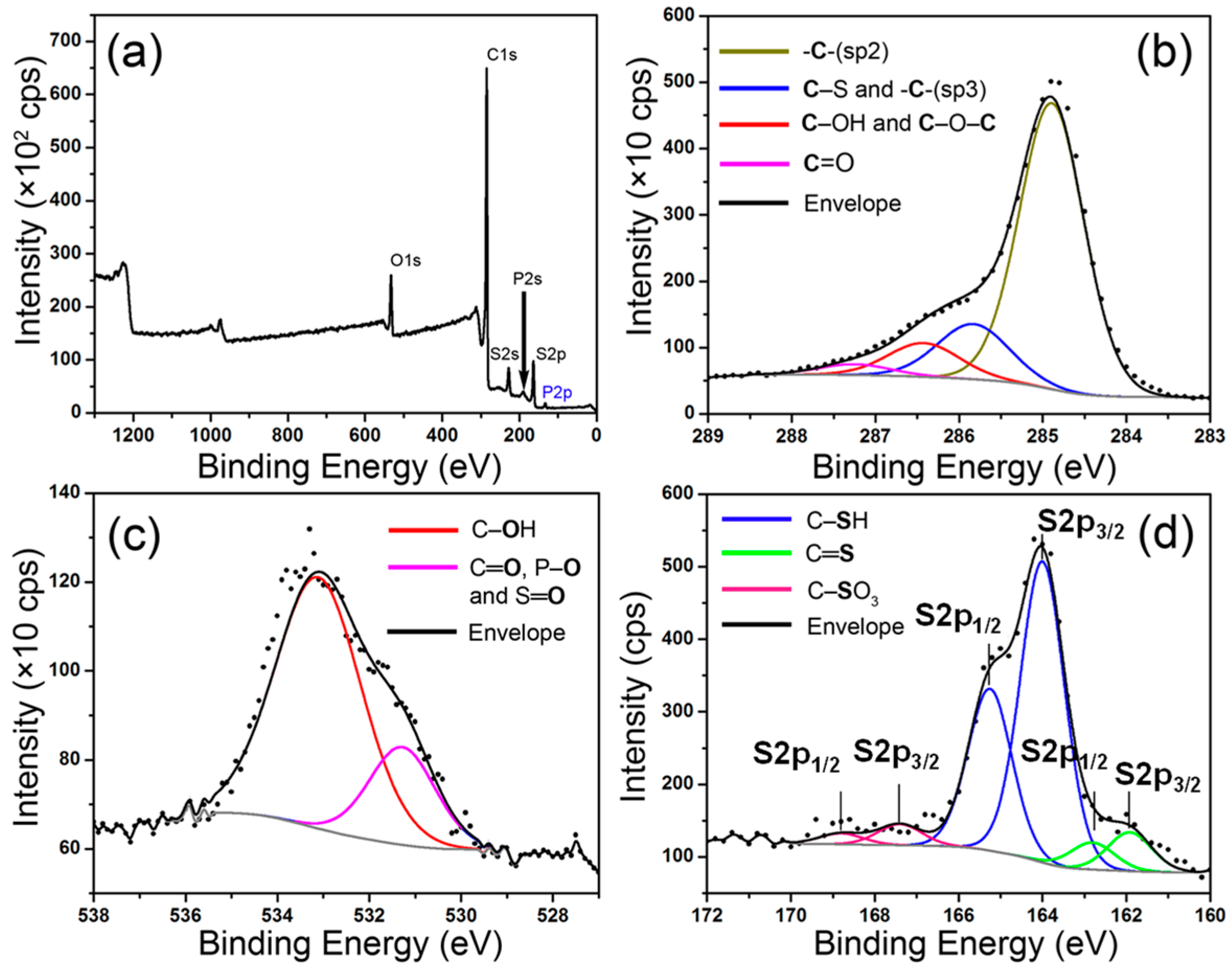


| Sample | Relative Atomic Ratios | % C atoms Attached with Different Functional Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C:O:S:P | C/(O+S) | Graphitic a | C-OH a | C=O b | COO a | C-O-C b | C-SH | C=S | C-SO3− | |
| GO | 2.1:1:0.07:0 | 1.96 | 41.4 | 24.3 | 8.5 | 3.6 | 18.8 | 0 | 0 | 3.3 |
| mRGO-120 | 9.7:1:0.68:0.12 | 5.77 | 82.7 | 7.8 | 2.5 | 0 | 0 | 6 | 0.6 | 0.3 |
| mRGO-150 | 11:1:0.91:0.18 | 5.76 | 82.6 | 6.5 | 2.5 | 0 | 0 | 6.7 | 1.0 | 0.6 |
| mRGO-180 | 13:1:1.2:0.8 | 5.90 | 83.1 | 5.9 | 1.8 | 0 | 0 | 7.7 | 1.1 | 0.5 |
| tRGO-120 | 4.9:1:0:0 | 4.90 | 79.6 | 10.2 | 10.0 | 0 | 0 | 0 | 0 | 0 |
| tRGO-150 | 5.8:1:0.03:0 | 5.63 | 82.2 | 9.6 | 7.6 | 0 | 0 | 0 | 0 | 0.5 |
| tRGO-180 | 6.6:1:0.06:0 | 6.23 | 83.1 | 10.3 | 4.8 | 0 | 0 | 0 | 0 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, K.-W. Easily Processable, Highly Transparent and Conducting Thiol-Functionalized Reduced Graphene Oxides Langmuir-Blodgett Films. Molecules 2021, 26, 2686. https://doi.org/10.3390/molecules26092686
Jeon K-W. Easily Processable, Highly Transparent and Conducting Thiol-Functionalized Reduced Graphene Oxides Langmuir-Blodgett Films. Molecules. 2021; 26(9):2686. https://doi.org/10.3390/molecules26092686
Chicago/Turabian StyleJeon, Ki-Wan. 2021. "Easily Processable, Highly Transparent and Conducting Thiol-Functionalized Reduced Graphene Oxides Langmuir-Blodgett Films" Molecules 26, no. 9: 2686. https://doi.org/10.3390/molecules26092686
APA StyleJeon, K.-W. (2021). Easily Processable, Highly Transparent and Conducting Thiol-Functionalized Reduced Graphene Oxides Langmuir-Blodgett Films. Molecules, 26(9), 2686. https://doi.org/10.3390/molecules26092686





