Robust Hydrogel Adhesive with Dual Hydrogen Bond Networks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Double-Layer Hydrogel
2.2. Hydrogen Bonds between DMAA and AAc Units
2.3. Hydrogen Bonds between UPy Units
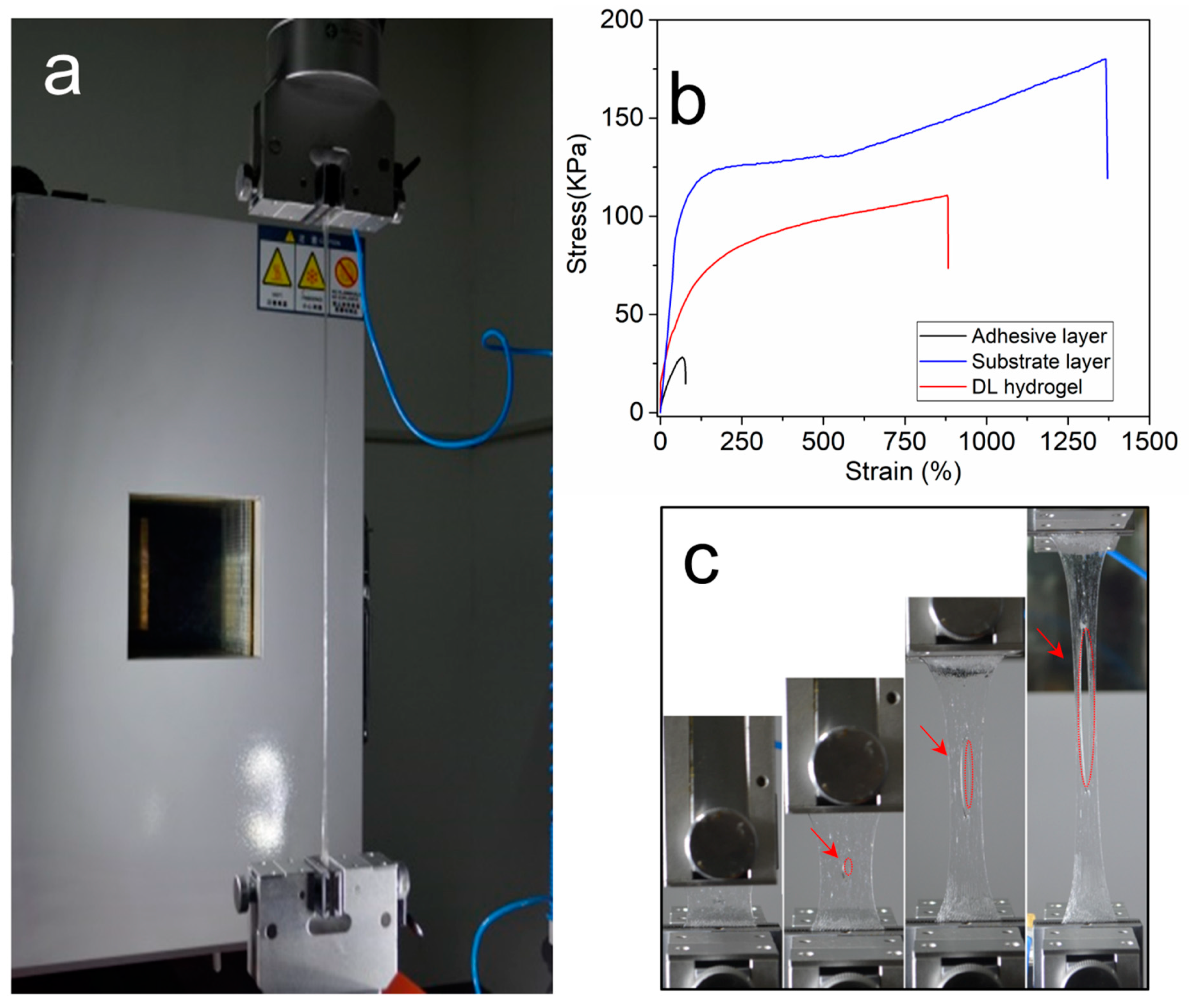
2.4. Mechanical Properties
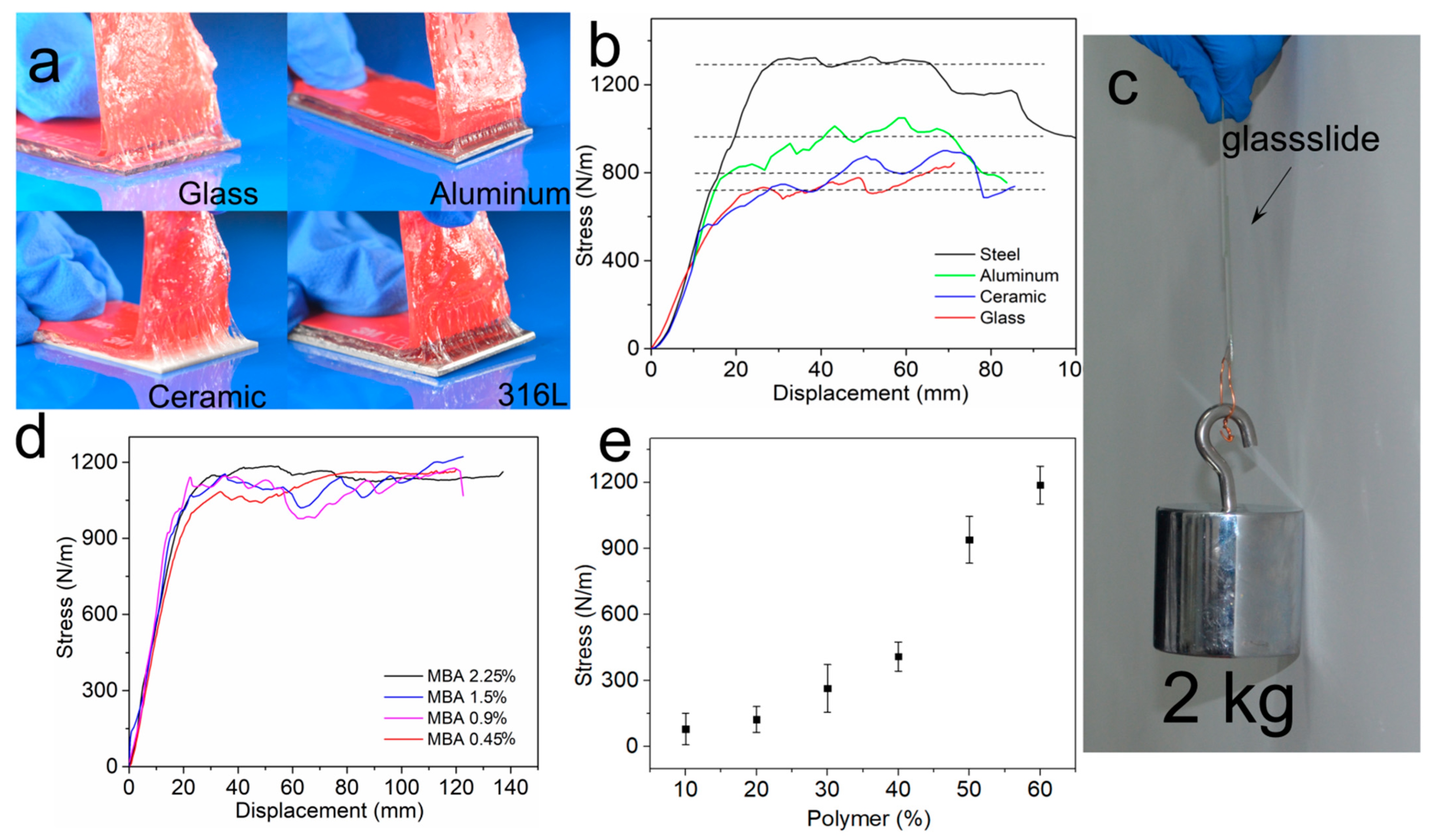
2.5. Adhesion Properties
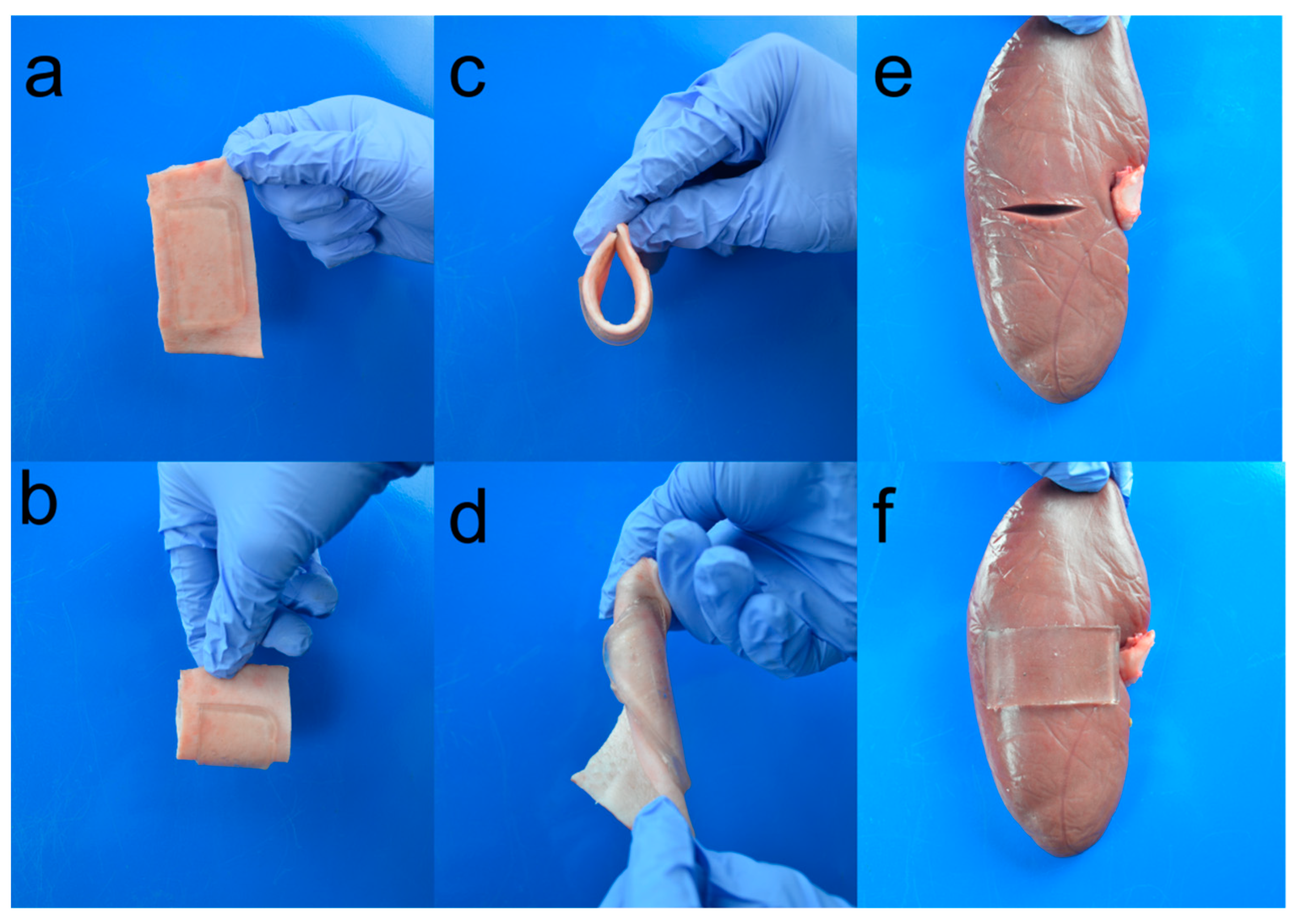
2.6. Attachment of Adhesive to Tissues
3. Experimental
3.1. Materials
3.2. Synthesis of DL Hydrogel
3.3. FT-IR
3.4. Mechanical Properties
3.5. Interfacial Toughness Measurement
3.6. Attachment of DL Adhesive to Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Keplinger, C.; Sun, J.-Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Roche, E.T.; Wohlfarth, R.; Overvelde, J.T.B.; Vasilyev, N.V.; Pigula, F.A.; Mooney, D.J.; Bertoldi, K.; Walsh, C.J. A Bioinspired Soft Actuated Material. Adv. Mat. 2014, 26, 1200–1206. [Google Scholar] [CrossRef]
- Feiner, R.; Engel, L.; Fleischer, S.; Malki, M.; Gal, I.; Shapira, A.; Shacham-Diamand, Y.; Dvir, T. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016, 15, 679–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, M.; Wendt, D.; Schaefer, D.; Jakob, M.; Hunziker, E.; Heberer, M.; Martin, I. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J. Biomech. 2005, 38, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E.; et al. Human Cartilage Repair with a Photoreactive Adhesive-Hydrogel Composite. Sci. Transl. Med. 2013, 5, 167ra6. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.T.; Shek, P.N. Novel wound sealants: Biomaterials and applications. Expert. Rev. Med. Devices 2010, 7, 639–659. [Google Scholar] [CrossRef]
- Roy, C.K.; Guo, H.L.; Sun, T.L.; Bin Ihsan, A.; Kurokawa, T.; Takahata, M.; Nonoyama, T.; Nakajima, T.; Gong, J.P. Self-Adjustable Adhesion of Polyampholyte Hydrogels. Adv. Mater. 2015, 27, 7344–7348. [Google Scholar] [CrossRef]
- Faghihnejad, A.; Feldman, K.E.; Yu, J.; Tirrell, M.V.; Israelachvili, J.N.; Hawker, C.J.; Kramer, E.J.; Zeng, H. Adhesion and Surface Interactions of a Self-Healing Polymer with Multiple Hydrogen-Bonding Groups. Adv. Funct. Mater. 2014, 24, 2322–2333. [Google Scholar] [CrossRef]
- Kim, K.; Shin, M.; Koh, M.-Y.; Ryu, J.H.; Lee, M.S.; Hong, S.; Lee, H. TAPE: A Medical Adhesive Inspired by a Ubiquitous Compound in Plants. Adv. Funct. Mater. 2015, 25, 2402–2410. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Autumn, K. Gecko Adhesion: Structure, Function, and Applications. MRS Bull. 2007, 32, 473–478. [Google Scholar] [CrossRef]
- Cho, H.; Wu, G.; Jolly, J.C.; Fortoul, N.; He, Z.; Gao, Y.; Jagota, A.; Yang, S. Intrinsically reversible superglues via shape adaptation inspired by snail epiphragm. Proc. Natl. Acad. Sci. USA 2019, 116, 13774–13779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nat. Cell Biol. 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.H. Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications. J. Biomater. Appl. 1993, 7, 309–352. [Google Scholar] [CrossRef]
- Serban, M.A.; Panilaitis, B.; Kaplan, D.L. Silk fibroin and polyethylene glycol-based biocompatible tissue adhesives. J. Biomed. Mater. Res. Part A 2011, 98, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastjerdi, A.K.; Pagano, M.; Kaartinen, M.; McKee, M.; Barthelat, F. Cohesive behavior of soft biological adhesives: Experiments and modeling. Acta Biomater. 2012, 8, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tahir, M.N.; Kappl, M.; Tremel, W.; Metz, N.; Barz, M.; Theato, P.; Butt, H.-J. Influence of Binding-Site Density in Wet Bioadhesion. Adv. Mater. 2008, 20, 3872–3876. [Google Scholar] [CrossRef]
- Abugazleh, M.K.; Rougeau, B.; Ali, H. Adsorption of catechol and hydroquinone on titanium oxide and iron (III) oxide. J. Environ. Chem. Eng. 2020, 8, 104180. [Google Scholar] [CrossRef]
- Seo, S.; Lee, D.W.; Ahn, J.S.; Cunha, K.; Filippidi, E.; Ju, S.W.; Shin, E.; Kim, B.-S.; Levine, Z.A.; Lins, R.D.; et al. Significant Performance Enhancement of Polymer Resins by Bioinspired Dynamic Bonding. Adv. Mater. 2017, 29, 1703026. [Google Scholar] [CrossRef] [Green Version]
- Gebbie, M.A.; Wei, W.; Schrader, A.M.; Cristiani, T.R.; Dobbs, H.A.; Idso, M.; Chmelka, B.F.; Waite, J.H.; Israelachvili, J.N. Tuning underwater adhesion with cation–π interactions. Nat. Chem. 2017, 9, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Hwang, A.J.; Deming, T.J. Role ofl-3,4-Dihydroxyphenylalanine in Mussel Adhesive Proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Braun, M.; Menges, M.; Opoku, F.; Smith, A.M. The relative contribution of calcium, zinc and oxidation-based cross-links to the stiffness of Arion subfuscus glue. J. Exp. Biol. 2013, 216, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.-J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.-H.; Chung, H.-M.; et al. Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv. Funct. Mat. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Kan, Y.; Danner, E.W.; Israelachvili, J.N.; Chen, Y.; Waite, J.H. Boronate Complex Formation with Dopa Containing Mussel Adhesive Protein Retards pH-Induced Oxidation and Enables Adhesion to Mica. PLoS ONE 2014, 9, e108869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, M.A.; Del Grosso, C.A.; Wilker, J.J. High Strength Underwater Bonding with Polymer Mimics of Mussel Adhesive Proteins. ACS Appl. Mater. Interfaces 2017, 9, 7866–7872. [Google Scholar] [CrossRef]
- Lane, D.D.; Kaur, S.; Weerasakare, G.M.; Stewart, R.J. Toughened hydrogels inspired by aquatic caddisworm silk. Soft Matter 2015, 11, 6981–6990. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, C.; Jiang, Z.; Qin, S.; Zhang, A. Mussel-Inspired Adhesive Polydopamine-Functionalized Hyaluronic Acid Hydrogel with Potential Bacterial Inhibition. Glob. Chall. 2019, 4, 1900068. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.K.; Das, S.; Linstadt, R.T.H.; Kaufman, Y.; Martinez-Rodriguez, N.R.; Mirshafian, R.; Kesselman, E.; Talmon, Y.; Lipshutz, B.H.; Israelachvili, J.N.; et al. High-performance mussel-inspired adhesives of reduced complexity. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A Water-Borne Adhesive Modeled after the Sandcastle Glue of P. californica. Macromol. Biosci. 2008, 9, 464–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghobril, C.; Charoen, K.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. A Dendritic Thioester Hydrogel Based on Thiol–Thioester Exchange as a Dissolvable Sealant System for Wound Closure. Angew. Chem. 2013, 52, 14070–14074. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Ramanathan, D.; Highsmith, J.; Lavelle, W.; Gerszten, P.; Vale, F.; Wright, N. DuraSeal Exact Is a Safe Adjunctive Treatment for Durotomy in Spine: Postapproval Study. Glob. Spine J. 2019, 9, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Barrett, D.G.; Bushnell, G.G.; Messersmith, P.B. Mechanically Robust, Negative-Swelling, Mussel-Inspired Tissue Adhesives. Adv. Heal. Mater. 2013, 2, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Maier, G.P.; Rapp, M.V.; Waite, J.H.; Israelachvili, J.N.; Butler, A. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 2015, 349, 628–632. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Lawrence, P.G.; Lapitsky, Y. Self-Assembly of Stiff, Adhesive and Self-Healing Gels from Common Polyelectrolytes. Langmuir 2014, 30, 7771–7777. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.G.; Lapitsky, Y. Ionically Cross-Linked Poly(allylamine) as a Stimulus-Responsive Underwater Adhesive: Ionic Strength and pH Effects. Langmuir 2015, 31, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Y.; Yang, J.; Zheng, Q.; Wu, N.; Lv, Z.; Jiang, Z. Ultra-stretchable hydrogels with hierarchical hydrogen bonds. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Hao, M.-H. Theoretical Calculation of Hydrogen-Bonding Strength for Drug Molecules. J. Chem. Theory Comput. 2006, 2, 863–872. [Google Scholar] [CrossRef]
- Beijer, F.H.; Sijbesma, R.P.; Kooijman, H.; Spek, A.L.; Meijer, E.B. Strong Dimerization of Ureidopyrimidones via Quadruple Hydrogen Bonding. J. Am. Chem. Soc. 1998, 120, 6761–6769. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Meijer, E.B. Quadruple hydrogen bonded systems. Chem. Commun. 2002, 1, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Söntjens, S.H.M.; Sijbesma, R.P.; van Genderen, A.M.H.P.; Meijer, E.W. Stability and Lifetime of Quadruply Hydrogen Bonded 2-Ureido-4[1H]-pyrimidinone Dimers. J. Am. Chem. Soc. 2000, 122, 7487–7493. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nat. Cell Biol. 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Derail, C.; Allal, A.; Marin, G.; Tordjeman, P. Relationship between Viscoelastic and Peeling Properties of Model Adhesives. Part 1. Cohesive Fracture. J. Adhes. 1997, 61, 123–157. [Google Scholar] [CrossRef]
- Webber, R.E.; Creton, C.; Brown, H.R.; Gong, J.P. Large Strain Hysteresis and Mullins Effect of Tough Double-Network Hydrogels. Macromolecules 2007, 40, 2919–2927. [Google Scholar] [CrossRef]
- Beharaj, A.; Ekladious, I.; Grinstaff, M.W. Poly(Alkyl Glycidate Carbonate)s as Degradable Pressure-Sensitive Adhesives. Angew. Chem. 2019, 58, 1407–1411. [Google Scholar] [CrossRef]
- Keizer, H.H.; Van Kessel, R.; Sijbesma, R.R.; Meijer, E.B. Scale-up of the synthesis of ureidopyrimidinone functionalized telechelic poly(ethylenebutylene). Polymer 2003, 44, 5505–5511. [Google Scholar] [CrossRef]
- Bal-Ozturk, A.; Karal-Yilmaz, O.; Akguner, Z.P.; Aksu, S.; Tas, A.; Olmez, H. Sponge-like chitosan-based nanostructured antibacterial material as a topical hemostat. J. Appl. Polym. Sci. 2019, 136, 47522. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Li, Y.; Shen, Y.; Yang, J.; Zhang, Z.; You, Y.; Lv, Z.; Yao, L. Robust Hydrogel Adhesive with Dual Hydrogen Bond Networks. Molecules 2021, 26, 2688. https://doi.org/10.3390/molecules26092688
Jiang Z, Li Y, Shen Y, Yang J, Zhang Z, You Y, Lv Z, Yao L. Robust Hydrogel Adhesive with Dual Hydrogen Bond Networks. Molecules. 2021; 26(9):2688. https://doi.org/10.3390/molecules26092688
Chicago/Turabian StyleJiang, Zhiqiang, Ya Li, Yirui Shen, Jian Yang, Zongyong Zhang, Yujing You, Zhongda Lv, and Lihui Yao. 2021. "Robust Hydrogel Adhesive with Dual Hydrogen Bond Networks" Molecules 26, no. 9: 2688. https://doi.org/10.3390/molecules26092688




