Enzymatic Management of pH in White Wines
Abstract
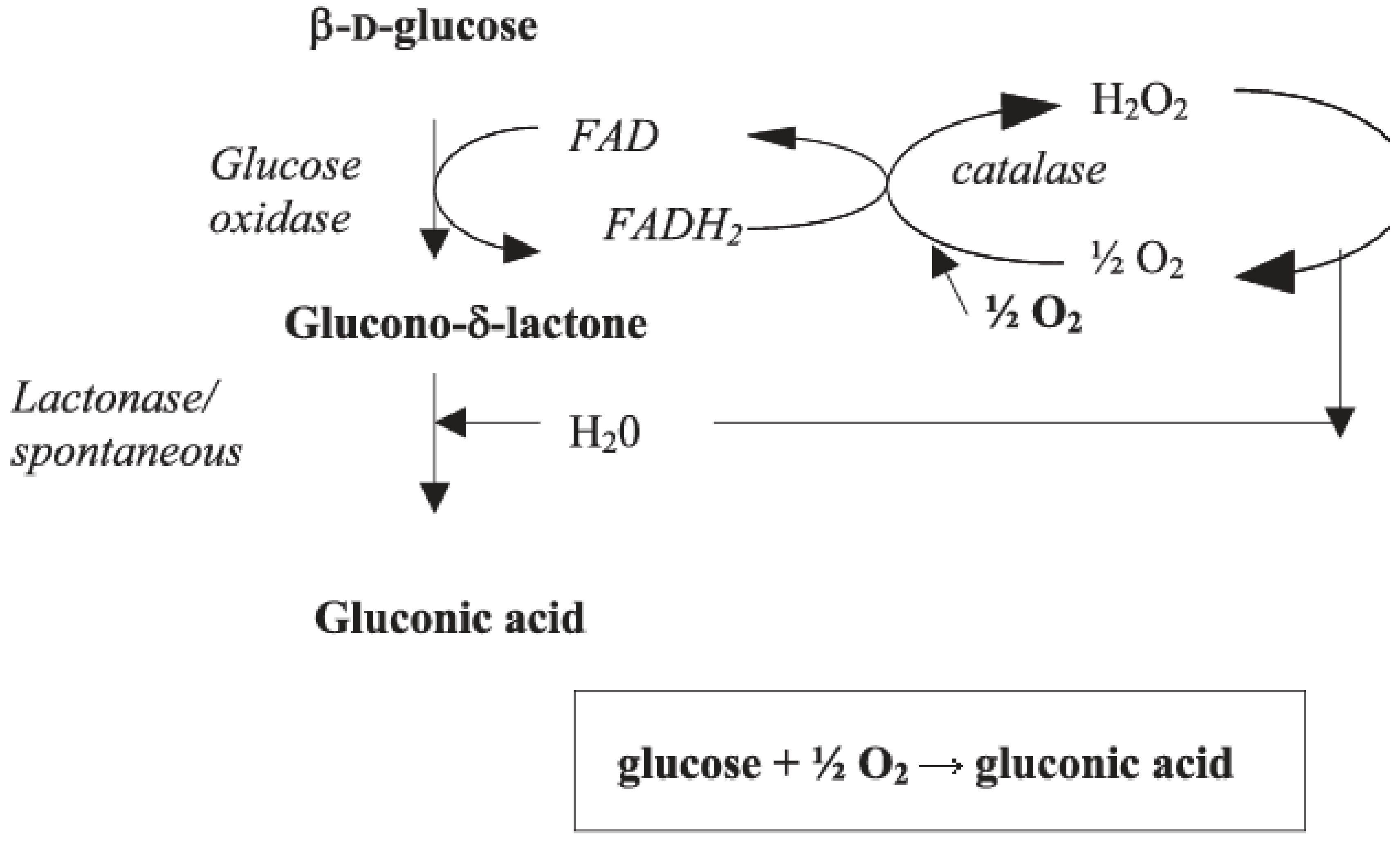
1. Introduction
2. Results
2.1. Chemical Analysis
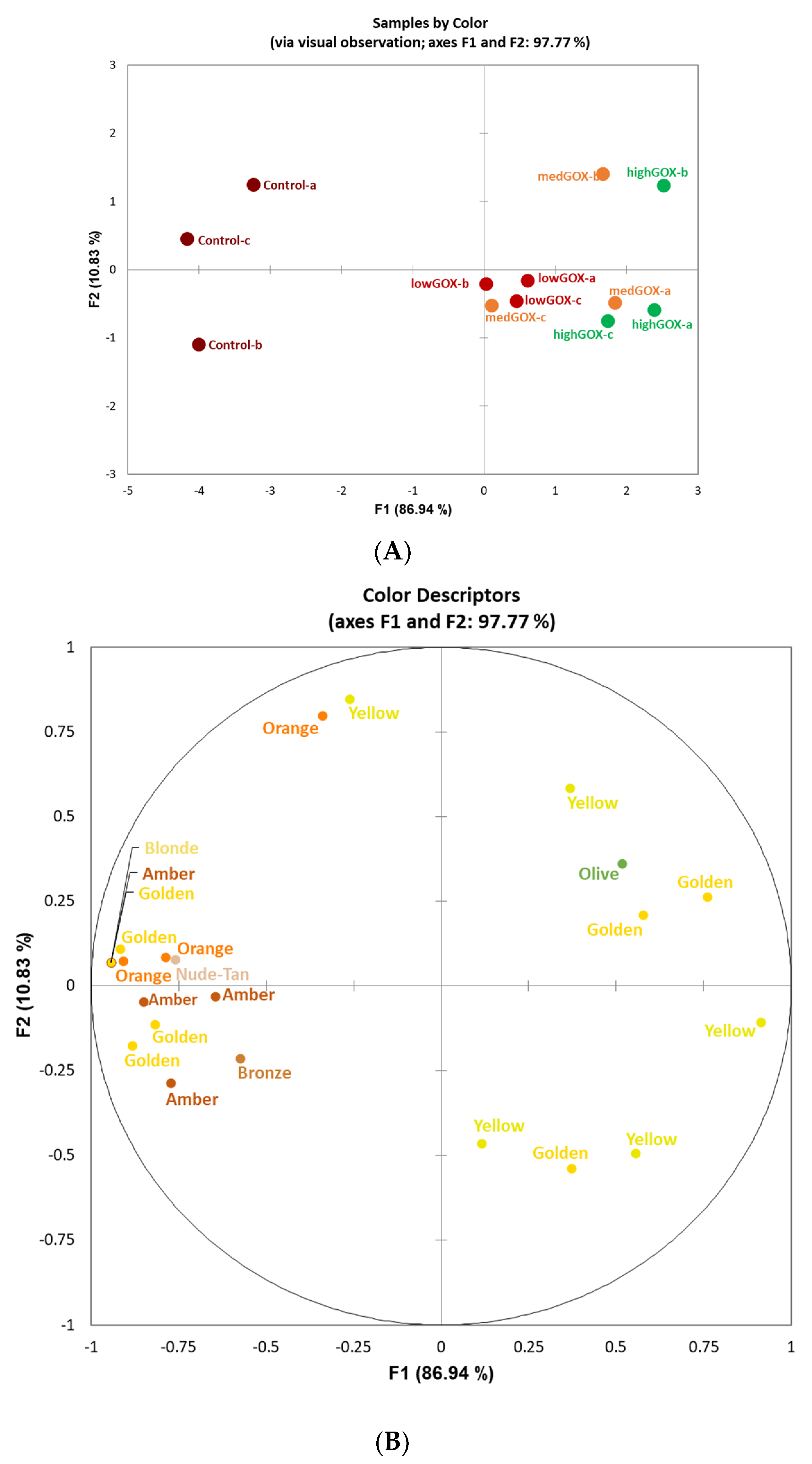
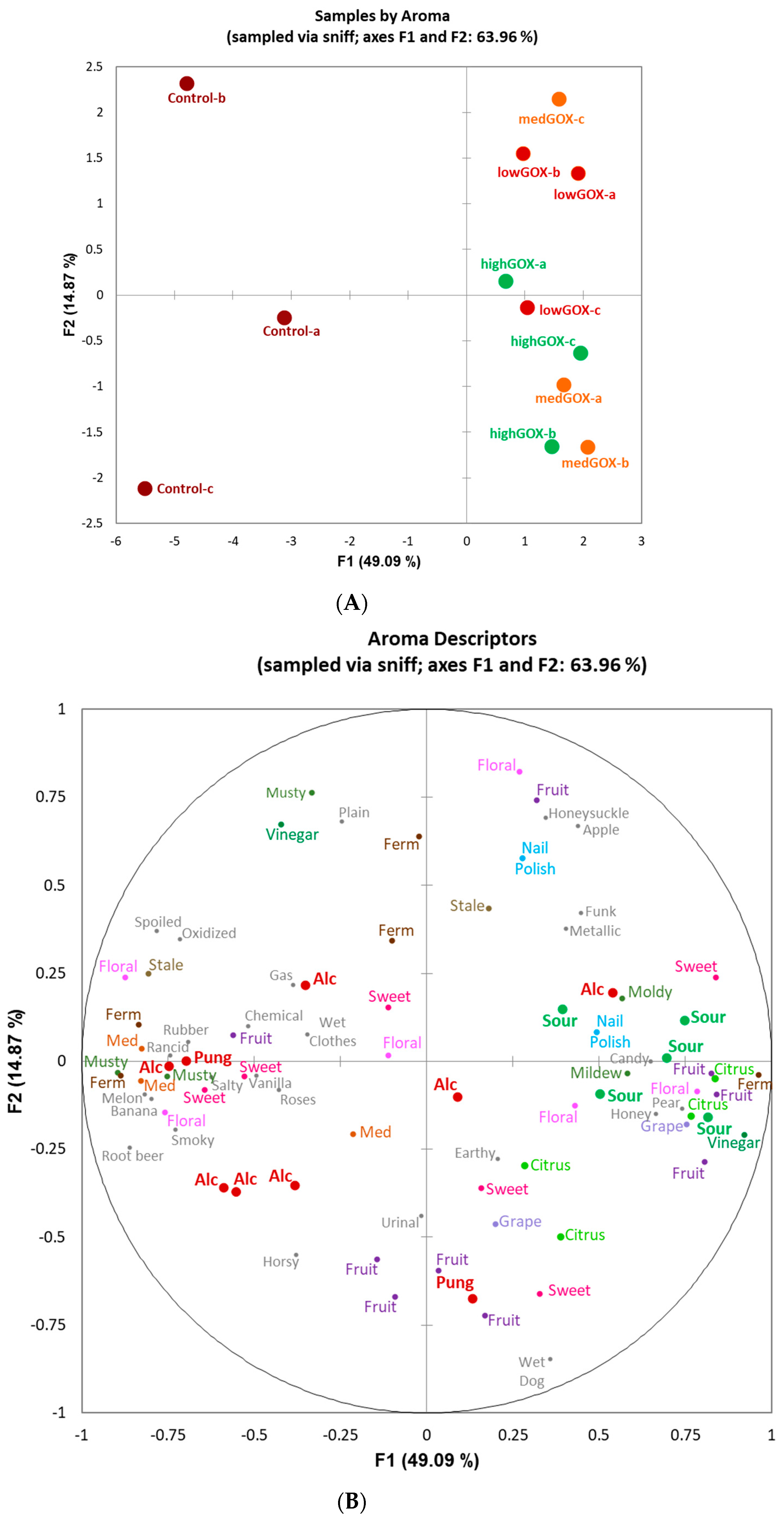
2.2. Sensory Analysis-Flash Profiling
3. Discussion
4. Materials and Methods
4.1. Enzyme
4.2. Grape Selection and Wine Production
4.3. Wine Production
4.4. Sensory Evaluation
Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jackson, R.S. Wine Science Principles and Applications, 3rd ed.; Elsevier: San Diego, CA, USA, 2008; pp. 419–420. [Google Scholar]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. Optimising glucose conversion in the production of reduced alcohol wine using glucose oxidase. Food Res. Int. 1998, 31, 685–692. [Google Scholar] [CrossRef]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. The production of reduced-alcohol wine using glucose oxidase treated juice. Part I. Composition. Am. J. Enol. Vitic. 1999, 50, 291–298. [Google Scholar]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. The production of reduced-alcohol wine using glucose oxidase-treated juice. Part III. Sensory. Am. J. Enol. Vitic. 1999, 50, 307–316. [Google Scholar]
- Biyela, B.N.; Du Toit, W.J.; Divol, B.; Malherbe, D.F.; van Rensburg, P. The production of reduced-alcohol wines using Gluzyme Mono® 10.000 BG-treated grape juice. S. Afr. J. Enol. Vitic. 2009, 30, 124–132. [Google Scholar] [CrossRef][Green Version]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The use of glucose oxidase and catalase for the enzymatic reduction of the potential ethanol content in wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.; Espinoza, K.; Ramirez, C.; Franco, W.; Urtubia, A. Technical feasibility of glucose oxidase as a prefermentation treatment for lowering the alcoholic degree of red wine. Am. J. Enol. Vitic. 2017, 68, 386–389. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- McLeod, R.; Ough, C.S. Some recent studies with glucose oxidase in wine. Am. J. Enol. Vitic. 1970, 21, 54–60. [Google Scholar]
- Ough, C.S. Further investigations with glucose oxidase-catalase enzyme systems for use with wine. Am. J. Enol. Vitic. 1975, 26, 30–36. [Google Scholar]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. GC-MS analysis of reduced-alcohol Müller-Thurgau wine produced using glucose oxidase-treated juice. LWT-Food Sci. Technol. 2001, 34, 89–94. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Villettaz, J.C. A new method for the production of low alcohol wines and better balanced wines. In Proceedings of the 6th Australian Wine Industry Technical Conference, Adelaide, Australia, 14–17 July 1986; Australian Industrial Publishers: Adelaide, Australia, 1987; pp. 125–128. [Google Scholar]
- Kitzberger, C.S.; Scholz, M.B.; da Silva, J.B. Free choice profiling sensory analysis to discriminate coffees. AIMS Agric. Food 2016, 1, 455–469. [Google Scholar] [CrossRef]
- Bredie, W.L.; Liu, J.; Dehlholm, C.; Heymann, H. Flash Profile Method. In Descriptive Analysis in Sensory Evaluation; Kemp, S.E., Hort, J., Hollowood, T., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 513–515. [Google Scholar] [CrossRef]
- Liu, J.; Grønbeck, M.S.; di Monaco, R.; Giacalone, D.; Bredie, W.L.P. Performance of Flash Profile and Napping with and without training for describing small sensory differences in a model wine. Food Qual. Prefer. 2016, 48, 41–49. [Google Scholar] [CrossRef]
- Liu, J.; Bredie, W.L.; Sherman, E.; Harbertson, J.F.; Heymann, H. Comparison of rapid descriptive sensory methodologies: Free-Choice Profiling, Flash Profile and modified Flash Profile. Food Res. Int. 2018, 106, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Škrobot, D.J.; Tomić, J.M.; Dapčević-Hadnađev, T.R.; Novaković, A.R.; Hadnađev, M.S.; Delić, J.D.; Mandra, M.J. Flash profile as a rapid descriptive analysis in sensory characterization of traditional dry fermented sausages. Food Feed Res. 2020, 47, 55–63. [Google Scholar] [CrossRef]
- Available online: https://www.law.cornell.edu/cfr/text/27/24.246 (accessed on 16 April 2021).
- Available online: https://www.oiv.int/public/medias/4310/f-coei-1-prenzy.pdf (accessed on 16 April 2021).

| Treatment | |||||
|---|---|---|---|---|---|
| Hour | p-Value e | Control | 0.2 g/L | 0.6 g/L | 1 g/L |
| 0 | 0.81 | 4.0 ± 0.08 | 4.0 ± 0.01 | 3.9 ± 0.01 | 3.9 ± 0.03 |
| 1 | 0.11 | 3.9 ± 0.00 | 3.9 ± 0.01 | 3.9 ± 0.00 | 3.9 ± 0.00 |
| 2 | 0.03 | 4.0 ± 0.03 a | 3.9 ± 0.02 ab | 3.8 ± 0.01 b | 3.8 ± 0.01 b |
| 3 | 0.01 | 3.9 ± 0.00 a | 3.8 ± 0.01 ab | 3.7 ± 0.01 bc | 3.7 ± 0.01 c |
| 4 | 0.009 | 3.9 ± 0.00 a | 3.8 ± 0.00 b | 3.7 ± 0.01 c | 3.7 ± 0.00 c |
| 5 | 0.002 | 3.9 ± 0.01 a | 3.7 ± 0.00 b | 3.6 ± 0.01 c | 3.6 ± 0.00 c |
| 6 | 0.002 | 3.9 ± 0.01 a | 3.7 ± 0.00 b | 3.6 ± 0.01 c | 3.6 ± 0.00 c |
| 7 | 0.001 | 3.9 ± 0.01 a | 3.7 ± 0.00 b | 3.6 ± 0.00 c | 3.5 ± 0.00 c |
| 8 | 0.0005 | 3.9 ± 0.01 a | 3.6 ± 0.01 b | 3.5 ± 0.01 c | 3.5 ± 0.00 c |
| 9 | 0.0003 | 3.9 ± 0.01 a | 3.6 ± 0.00 b | 3.5 ± 0.00 c | 3.5 ± 0.00 c |
| 10 | 0.001 | 3.9 ± 0.01 a | 3.6 ± 0.01 b | 3.5 ± 0.01 c | 3.5 ± 0.00 c |
| 24 | <0.0001 | 3.9 ± 0.01 a | 3.5 ± 0.00 b | 3.3 ± 0.00 c | 3.2 ± 0.00 d |
| Hour | p-Value a | Control | 0.2 g/L (Low) | 0.6 g/L (Medium) | 1 g/L (High) |
|---|---|---|---|---|---|
| 0 | 0.72 | 108 ± 0.3 | 110 ± 0.3 | 111 ± 0.4 | 110 ± 0.6 |
| 1 | 0.76 | 108 ± 0.6 | 109 ± 0.3 | 110 ± 0.8 | 107 ± 0.6 |
| 2 | 0.86 | 109 ± 0.3 | 111 ± 0.5 | 110 ± 0.4 | 109 ± 0.2 |
| 3 | 0.42 | 110 ± 0.6 | 112 ± 0.7 | 112 ± 0.7 | 109 ± 0.2 |
| 4 | 0.47 | 113 ± 0.6 | 111 ± 0.6 | 110 ± 0.4 | 107 ± 0.2 |
| 5 | 0.54 | 113 ± 0.6 | 113 ± 0.5 | 112 ± 0.3 | 108 ± 0.6 |
| 6 | 0.67 | 105± 0.6 | 105 ± 0.8 | 103 ± 0.6 | 102 ± 0.3 |
| 7 | 0.34 | 106 ± 1.1 | 105 ± 1.0 | 103 ± 1.1 | 101 ± 1.0 |
| 8 | 0.2 | 107 ± 0.9 | 105 ± 0.4 | 101 ± 0.6 | 100 ± 1.5 |
| 9 | 0.3 | 105 ± 0.2 | 104 ± 0.3 | 102 ± 0.4 | 99 ± 0.3 |
| 10 | 0.13 | 107 ± 0.3 | 104 ± 0.8 | 101 ± 0.7 | 94 ± 0.9 |
| 24 | 0.09 | 103 ± 0.6 | 100 ± 0.3 | 95 ± 0.3 | 91 ± 0.4 |
| Treatment | |||||
|---|---|---|---|---|---|
| Hour | p-Value e | Control | 0.2 g/L (Low) | 0.6 g/L (Medium) | 1 g/L (High) |
| 0 | 0.25 | 627 ± 10.0 | 346 ± 18.9 | 263 ± 11.0 | 327 ± 35.8 |
| 1 | 0.008 | 682 ± 21.1 c | 1274 ± 11.3 c | 2126 ± 55.8 b | 3153 ± 62.6 a |
| 2 | 0.0008 | 709 ± 23.1 d | 2259 ± 35.5 c | 3717 ± 21.9 b | 4810 ± 43.4 a |
| 3 | 0.0002 | 809 ± 24.7 d | 3391 ± 15.8 c | 5328 ± 23.6 b | 7636 ± 101.9 a |
| 4 | 0.001 | 864 ± 7.3 d | 4255 ± 28.1 c | 6878 ± 60.8 b | 8356 ± 38.0 a |
| 5 | 0.0001 | 925 ± 29.6 d | 5003 ± 15.3 c | 8065 ± 189.7 b | 9547 ± 75.7 a |
| 6 | <0.0001 | 723 ± 13.9 d | 4912 ± 35.8 c | 6932 ± 99.8 b | 7968 ± 56.4 a |
| 7 | 0.0004 | 742 ± 30.4 d | 5062 ± 85.2 c | 7418 ± 335.6 b | 8521 ± 296.8 a |
| 8 | 0.0004 | 697 ± 33.7 d | 5344 ± 47.6 c | 8238 ± 288.4 b | 9557 ± 289.8 a |
| 9 | <0.0001 | 718 ± 18.7 d | 5538 ± 47.0 c | 8784 ± 72.8 b | 10,081 ± 67.5 a |
| 10 | 0.0004 | 865 ± 7.9 d | 6094 ± 32.9 c | 9742 ± 48.7 b | 11,865 ± 59.9 a |
| 24 | 0.0001 | 846 ± 20.5 d | 8818 ± 75.2 c | 15,711 ± 92.0 b | 20,485 ± 112.1 a |
| Treatment | |||||
|---|---|---|---|---|---|
| Hour | p-Value d | Control | 0.2 g/L (Low) | 0.6 g/L (Medium) | 1 g/L (High) |
| 0 | 0.88 | 29 ± 0.2 | 28 ± 0.3 | 26 ± 0.2 | 28 ± 0.1 |
| 1 | 0.007 | 29 ± 0.2 b | 50 ± 0.5 a | 42 ± 0.7 ab | 39 ± 0.1 ab |
| 2 | 0.03 | 29 ± 0.1 b | 56 ± 0.3 a | 53 ± 0.2 a | 45 ± 0.3 a |
| 3 | 0.01 | 30 ± 0.3 b | 56 ± 0.2 a | 57 ± 0.4 a | 50 ± 0.3 a |
| 4 | 0.004 | 29 ± 0.3 b | 55 ± 0.2 a | 55 ± 0.2 a | 50 ± 0.5 a |
| 5 | 0.007 | 28 ± 0.2 b | 58 ± 0.6 a | 56 ± 0.2 a | 52 ± 0.4 a |
| 6 | 0.01 | 24 ± 0.2 b | 54 ± 0.1 a | 50 ± 0.3 a | 46 ± 0.3 a |
| 7 | 0.008 | 24 ± 0.2 c | 51 ± 1.8 a | 49 ± 0.2 ab | 41 ± 0.3 b |
| 8 | 0.008 | 22 ± 0.1 b | 53 ± 0.2 a | 48 ± 0.1 a | 42 ± 0.4 a |
| 9 | 0.006 | 21 ± 0.1 c | 45 ± 0.1 a | 39 ± 0.1 ab | 33 ± 0.2 b |
| 10 | 0.01 | 22 ± 0.1 b | 51 ± 0.5 a | 47 ± 0.2 a | 45 ± 1.3 a |
| 24 | 0.3 | 20 ± 0.1 | 34 ± 0.2 | 32 ± 0.1 | 38 ± 0.3 |
| Alcohol (% by vol.) | pH | TA (g/L) | |
|---|---|---|---|
| Control | 13.5 ± 0.09 a | 3.9 ± 0.04 a | 5 ± 0.01 d |
| Low (0.2 g/L) | 13.0 ± 0.07 b | 3.6 ± 0.07 b | 9 ± 0.04 c |
| Medium (0.6 g/L) | 12.7 ± 0.04 b | 3.4 ± 0.04 c | 11 ± 0.04 b |
| High (1 g/L) | 12.4 ± 0.04 c | 3.3 ± 0.07 d | 13 ± 0.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botezatu, A.; Elizondo, C.; Bajec, M.; Miller, R. Enzymatic Management of pH in White Wines. Molecules 2021, 26, 2730. https://doi.org/10.3390/molecules26092730
Botezatu A, Elizondo C, Bajec M, Miller R. Enzymatic Management of pH in White Wines. Molecules. 2021; 26(9):2730. https://doi.org/10.3390/molecules26092730
Chicago/Turabian StyleBotezatu, Andreea, Carlos Elizondo, Martha Bajec, and Rhonda Miller. 2021. "Enzymatic Management of pH in White Wines" Molecules 26, no. 9: 2730. https://doi.org/10.3390/molecules26092730
APA StyleBotezatu, A., Elizondo, C., Bajec, M., & Miller, R. (2021). Enzymatic Management of pH in White Wines. Molecules, 26(9), 2730. https://doi.org/10.3390/molecules26092730





