Medicinal Plants with Anti-Leukemic Effects: A Review
Abstract
:1. Introduction
2. The Anti-Leukemic Activity of Traditional Medicinal Plants
2.1. Medicinal Plants Clinically Used as Treatments for Leukemia
2.1.1. Cephalotaxus harringtonia
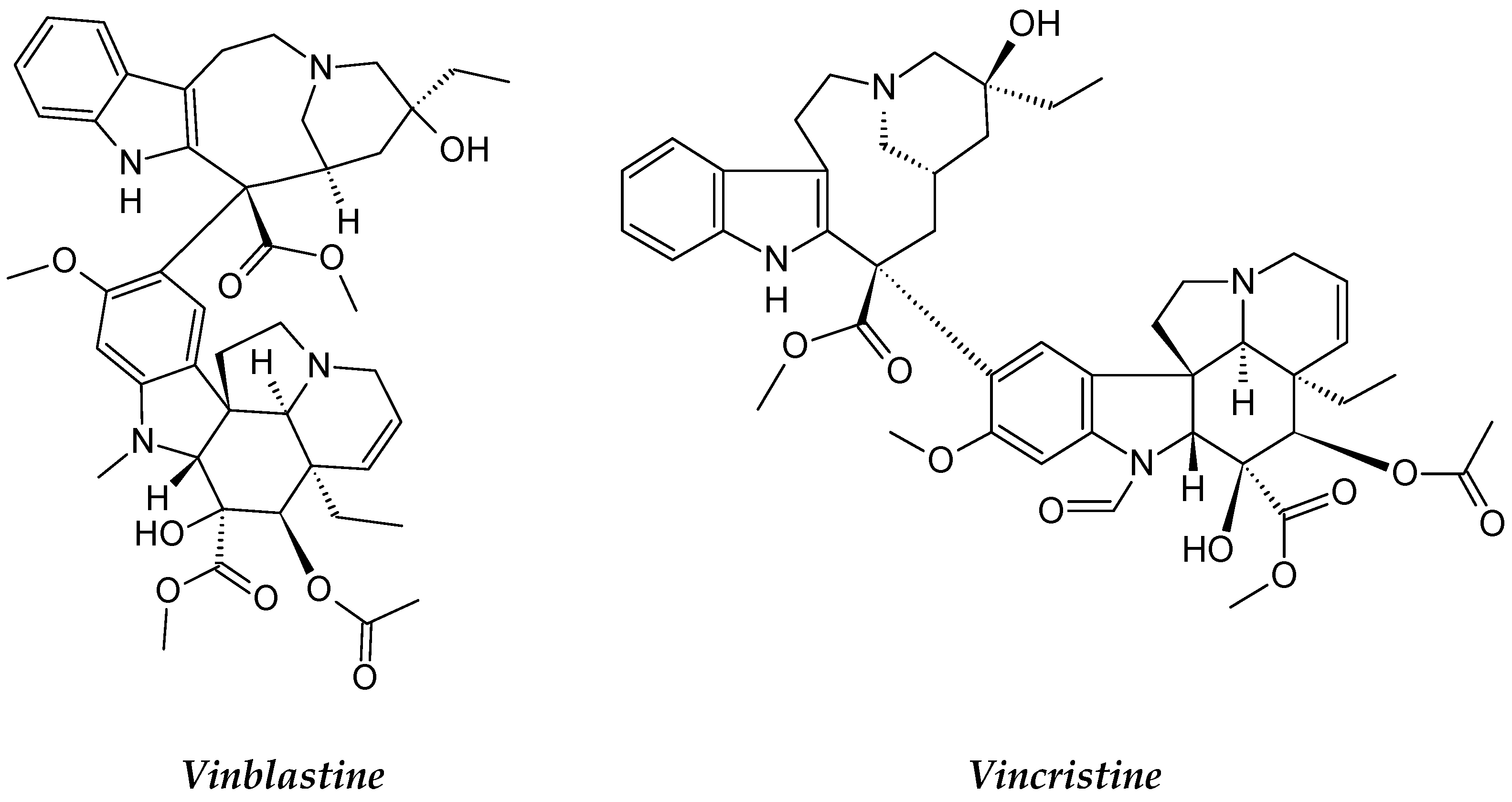
2.1.2. Catharanthus roseus
2.2. Medicinal Plants in Clinical Trials
2.2.1. Maytenus serrata
2.2.2. Dysoxylum binectariferum
2.3. Medicinal Plants in Pre-Clinical Investigations (In Vitro) for Leukemia
2.3.1. Salix Genus (Willows)
2.3.2. Vernonia amygdalina
2.3.3. Nyctanthes arbor-tristis
2.3.4. Annona glabra
2.3.5. Basella alba
2.3.6. Ferulago angulata
| Scientific Name | Family | Active Compound | Leukemia Cell Line | Mechanism of Action | References |
|---|---|---|---|---|---|
| Annona glabra | Annonaceae | Acetogenins Diterpenoid (Cunabic acid, Ent-kaur-en-oic acid) | Human drug-sensitive leukemia (CEM), Multidrug-resistant-derived (CEM/VLB) cell lines and HL-60 cell line | Inhibition of mitochondrial respiratory chain complex, Inhibition of proliferation and Induce apoptosis and necrosis | [62,63,64] |
| Basella alba | Basellaceae | β-Sitosterol Terpenoids (Lupeol). | U937 cell line and Jurkat cell lines | Anti-leukemic, and Growth Inhibition | [67] |
| Berberis amurensis | Berberidaceae | Alkaloids Bisbenzylisoquinoline group Berbamine,4-Chlorobenzoyl Berbamine and Berberine. | Leukemic NB4 cells, Leukemic cell line K562, Chronic myeloid Leukemia cell line KU812, Gleevec-sensitive and -resistant Ph+ Leukemia cells | Induce cell apoptosis, Apoptosis, K562-r cell growth arrest and Cell proliferation inhibition | [78,79,80] |
| Blumea lacera | Asteraceae | Alkaloids, tannins, steroids, gums. Terpenoids, diterpenoid glycoside | K562, L1210, P3HR1, Raji, U937 | Antiproliferation | [81] |
| Bidens Pilosa | Asteraceae | Flavonoids | L1210, U937, K562, Raji, P3HR1, | Antiproliferation | [82,83] |
| Catharanthus roseus | Apocynaceae | Alkaloids: vincristine, vinblastine, vindesine, vinorelbine. | L1210 and P1534 Leukemia cells. | Mitotic inhibitor and Arrests the cell division which causes the death of the cells | [84] |
| Coreopsis lanceolata | Asteraceae | Flavonoids: flavanone, chalcones, and aurones 4 Methoxylanceoletin. | Leukemia HL-60 cells | Antiproliferation and Apoptosis induction | [85,86,87] |
| Ferulago angulata | Apiaceae | Phenolic Flavonoid Monoterpenes: α-pinene β-pinene | Raji, U937, AML cell lines, PBL cell line, Leukemia, lymphoma cell lines, NHL, Raji, U937, KG-1A, PBMC cell lines and HL-60 cell line. | Antiproliferation, Apoptosis induction and Autophagy and necrosis. | [88,89] |
| Ficus deltoidea | Moraceae | Flavonoid, tannins, terpenoids, phenol, proanthocyanins, lignans, alkaloids and coumarins | HL60 cell line | Apoptosis induction | [90] |
| Houttuynia cordata Thunb | Saururaceae | Flavonoids | L1210, U937, K562, P3HR1, Jurkat Leukemia cell line, Acute T lymphoblastic leukemic Molt-4 cells and Human T-cell Leukemia | Antiproliferation, Apoptosis induction through an endoplasmic reticulum stress pathway. | [91,92] |
| Litchi chinensis Sonn | Sapindaceae | Phenols Flavonoids Polysaccharides Tannins: epicatechin, proanthocyanidin B2 and proanthocyanidin B4 | HL-60, U937 and K562 cell lines | Antiproliferation and Apoptosis induction | [93] |
| Nyctanthes arbor-tristis | Oleaceae | Phenols: phenol, (dimethylethyl), hydroxypyridine oxide Glycosides, tannins, phenols and steroids. | AML, CLL and Jurkat T cells, CML, K562 cell lines. | Antiproliferation andApoptosis induction | [57,58,59,60] |
| Olea europaea | Oleaceae | Phenols, oleuropeosides oleuropein/verbascoside, Hydroxytyrosol, Flavons Flavonols (rutin), catechin Phenols (vanillin, tyrosol hydroxytyrosol, caffeic acid and vanillic acid). | Jurkat, K562 cells line andHL60 cell line. | Antiproliferation, Apoptosis induction and Cell cycle arrest, apoptosis induction and differentiation | [94,95,96,97,98,99] |
| Scutellaria baicalensis | Lamiaceae | Flavonoid, Baicalein, baicalin, and wogonin. | HL-60, NB-4, THP-1, U937 cells (Blin-1, Nalm-6), lymphoma cell lines (Daudi, Raji, Ramos, NCEB1), NALM-6 cell line (human, peripheral blood, B-type human Leukemia), HL-60 cell line. | Growth inhibition induce apoptosis and cell cycle arrest, Induction of apoptosis and Dose-dependent reduction of mitochondrial metabolism. | [100,101] |
| Salvia officinalis | Lamiaceae | Flavonoids and rosmarinic acid | K-562, U937, KG-1A Cell line. | Antiproliferative, anti-migratory and antiangiogenic. | [102] |
| Tithonia diversifolia | Asteraceae | Sesquiterpene lactones (STLs) Chlorogenic acid derivatives (CAs) Flavonoids, phenolics, tannins and terpenoids | HL-60 cell line. Anti-K562, L1210, P3HR1, Raji and U937 Leukemia cells | Antiproliferation andCytotoxic. | [103,104] |
| Typhonium flagelliforme | Araceae | Hexadecanoic acid, gamma sitosterol, phytol¸ octadecadienoic acid, pentadecyne, squalene, eicosane, octacosane, and geranylgeraniol. Pheophorbide, oleic acid, campesterol, stigmasterol and sitosterol, Linoleic acid. | Murine Leukemia WEHI-cancer cell lines, P388 murine Leukemia cells, HL-60 Leukemia cells, and human T4-lymphoblastoid cell line CEMss. | Antiproliferation via apoptosis induction | [105,106,107,108,109] |
| Viscum album (Mistletoe) | Viscacea | Proteins such as lectins and polypeptides like viscotoxins | ALL, NALM-6 cell lines, Jurkat E6.1 and THP-1 cells | Apoptosis induction andG2/M cell cycle arrest. | [110] |
| Vernonia amygdalina (VA) | Compositae | Sesquiterpene lactones: vernodaline and vernolide | HL-60 cell line andALL and AML immature monocytes patients, Mononuclear cells. | Antiproliferation DNA damage, and apoptosis induction. | [1,111] |
| Willows tree | Salicaceae | Salicin and saligenin | ALL and AML cell lines b- HL-60 cell line | Apoptosis induction by causing DNA damage. Antiproliferation | [51] |
2.3.7. Litchi chinensis Sonn
2.3.8. Typhonium flagelliforme
2.3.9. Blumea lacera
2.3.10. Coreopsis lanceolata
2.3.11. Tithonia diversifolia
2.3.12. Bidens pilosa
2.3.13. Olea europaea
2.3.14. Houttuynia cordata Thunb
2.3.15. Viscum album
2.3.16. Salvia officinalis
2.3.17. Ficus deltoidea
2.3.18. Berberis amurensis Rupr
2.3.19. Scutellaria baicalensis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalafalla, M.M.; Abdellatef, E.; Daffalla, H.M.; Nassrallah, A.A.; Aboul-Enein, K.M.; Lightfoot, D.A.; El-Shemy, H.A. Antileukemia activity from root cultures of Vernonia amygdalina. J. Med. Plant Res. 2009, 3, 556–562. [Google Scholar]
- Sarkar, M.K.; Mahapatra, S.K.; Vadivel, V. Oxidative stress mediated cytotoxicity in leukemia cells induced by active phyto-constituents isolated from traditional herbal drugs of West Bengal. J. Ethnopharmacol. 2020, 251, 112527. [Google Scholar] [CrossRef]
- Davis, A.S.; Viera, A.J.; Mead, M.D. Leukemia: An overview for primary care. Am. Fam. Physician 2014, 89, 731–738. [Google Scholar]
- Berg, S.; Nand, S. Neurological Complications of the Leukemias Across the Ages. Curr. Neurol. Neurosci. Rep. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.L.A.; Limeira, R.R.T.; De Castro, R.D.; Bonan, P.R.F.; Valença, A.M.G. Oral Mucositis in Pediatric Patients in Treatment for Acute Lymphoblastic Leukemia. Int. J. Environ. Res. Public Health 2017, 14, 1468. [Google Scholar] [CrossRef] [Green Version]
- Zawitkowska, J.; Lejman, M.; Zaucha-Prażmo, A.; Drabko, K.; Płonowski, M.; Bulsa, J.; Romiszewski, M.; Mizia-Malarz, A.; Kołtan, A.; Derwich, K.; et al. Grade 3 and 4 Toxicity Profiles During Therapy of Childhood Acute Lymphoblastic Leukemia. In Vivo 2019, 33, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Fujita, H.; Akiyama, N.; Kimura, S.-I.; Hiramoto, N.; Hosono, N.; Takahashi, T.; Shigeno, K.; Minamiguchi, H.; Miyatake, J.; et al. Infectious complications in adults undergoing intensive chemotherapy for acute myeloid leukemia in 2001–2005 using the Japan Adult Leukemia Study Group AML201 protocols. Support. Care Cancer 2018, 26, 4187–4198. [Google Scholar] [CrossRef]
- Kaste, S.C.; Rai, S.N.; Bs, K.F.; McCammon, E.A.; Tylavsky, F.A.; Danish, R.K.; Rose, S.R.; Rt, C.D.S.; Pui, C.-H.; Hudson, M.M. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2005, 46, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.H.; Donohue, J.E.; Ness, K.K.; Dengel, D.R.; Baker, K.S.; Gurney, J.G. Bone mineral density in young adult survivors of acute lymphoblastic leukemia. Cancer 2008, 113, 3248–3256. [Google Scholar] [CrossRef] [Green Version]
- Lou, L.; Li, W.; Zhou, B.; Chen, L.; Weng, H.; Zou, Y.; Tang, G.; Bu, X.; Yin, S. (+)-Isobicyclogermacrenal and spathulenol from Aristolochia yunnanensis alleviate cardiac fibrosis by inhibiting transforming growth factor β/small mother against decapentaplegic signaling pathway. Phytotherapy Res. 2019, 33, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Bhaskar, M.; Hamzah, Z.; Podapati, S.K.; Reddy, L.V. Xanthine Oxidase Inhibitory Activity, Chemical Composition, Antioxidant Properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq). Molecules 2020, 25, 2658. [Google Scholar] [CrossRef]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Zakaria, S.; Hamzah, Z.; Reddy Pamuru, R.; Gundala, P.B.; Reduan, M.F.H. Micropropagation of Alocasia longiloba Miq and Comparative Antioxidant Properties of Ethanolic Extracts of the Field-Grown Plant, In Vitro Propagated and In Vitro-Derived Callus. Plants 2020, 9, 816. [Google Scholar] [CrossRef]
- Ferid, A.; Mohammed, A.; Khalivulla, S.I.; Korivi, M.; Razab, M.K.A.A. Plant Cell and Callus Cultures as an Alternative Source of Bioactive Compounds with Therapeutic Potential against Coronavirus Disease (COVID-19). IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012099. [Google Scholar] [CrossRef]
- Saedi, T.A.; Noor, S.M.; Ismail, P.; Othman, F. The Effects of Herbs and Fruits on Leukaemia. Evid. Based Complement. Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Cheng, H.-Y.; Chen, C.-C.; Lin, C.-C. In vitro Anti-leukemic and Antiviral Activities of Traditionally Used Medicinal Plants in Taiwan. Am. J. Chin. Med. 2004, 32, 695–704. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Daddiouaissa, D.; Amid, A. Anticancer Activity of Acetogenins from Annona Muricata Fruit. IIUM Med. J. Malays. 2018, 17, 103–112. [Google Scholar] [CrossRef]
- Yakhni, M.; Briat, A.; El Guerrab, A.; Furtado, L.; Kwiatkowski, F.; Miot-Noirault, E.; Cachin, F.; Penault-Llorca, F.; Radosevic-Robin, N. Homoharringtonine, an approved anti-leukemia drug, suppresses triple negative breast cancer growth through a rapid reduction of anti-apoptotic protein abundance. Am. J. Cancer Res. 2019, 9, 1043–1060. [Google Scholar]
- Zhou, D.C.; Zittoun, R.; Marie, J.P. Homoharringtonine: An effective new natural product in cancer chemotherapy. Bull Cancer 1995, 82, 987–995. [Google Scholar] [PubMed]
- Xu, B.; Ding, J.; Chen, K.-X.; Miao, Z.-H.; Huang, H.; Liu, X.-Y.; Luo, X.-M. Advances in Cancer Chemotherapeutic Drug Research in China. In Recent Advances in Cancer Research and Therapy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 287–350. [Google Scholar]
- Chen, R.T.; Hua, Z.; Xu, B. Pharmacological study of harringtonine and homoharringtonine. Kexue Tongbao 1980, 25, 859–861. [Google Scholar]
- Takemura, Y.; Ohnuma, T.; Chou, T.C.; Okano, T.; Holland, J.F. Biologic and pharmacologic effects of harring-tonine on human leukemia-lymphoma cells. Cancer Chemother. Pharmacol. 1985, 14, 206–210. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Chen, D.L.; Shen, Z.S.; Koeffler, H.P. Effect of homoharringtonine on proliferation and differentiation of human leukemic cells in vitro. Cancer Res. 1990, 50, 2031–2035. [Google Scholar]
- Luo, C.Y.; Tang, J.Y.; Wang, Y.P. Homoharringtonine: A New Treatment Option for Myeloid Leukemia. Hematology 2004, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, B. Pharmacology of some natural products of China. Trends Pharmacol. Sci. 1981, 2, 271–272. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Kothari, A.; Hittelman, W.N.; Chambers, T.C. Cell cycle–dependent mechanisms underlie vincristine-induced death of primary acute lymphoblastic leukemia cells. Cancer Res. 2016, 76, 3553–3561. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.-C.; Chiu, Y.-J.; Tang, Y.-J.; Lin, K.-L.; Chiang, J.-H.; Jiang, Y.-L.; Jen, H.-F.; Kuo, Y.-H.; Agamaya, S.; Chung, J.-G.; et al. Houttuynia cordata Thunb extract inhibits cell growth and induces apoptosis in human primary colorectal cancer cells. Anticancer Res. 2010, 30, 3549–3556. [Google Scholar]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Vilpo, J.A.; Koski, T.; Vilpo, L.M. Selective toxicity of vincristine against chronic lymphocytic leukemia cells in vitro. Eur. J. Haematol. 2000, 65, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Varsha, K.; Sharma, A.; Kaur, A.; Madan, J.; Pandey, R.S.; Jain, U.K.; Chandra, R. Natural Plant-Derived Anticancer Drugs Nanotherapeutics: A Review on Preclinical to Clinical Success. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 775–809. [Google Scholar]
- Cassady, J.M.; Chan, K.K.; Floss, H.G.; Leistner, E. Recent Developments in the Maytansinoid Antitumor Agents. Chem. Pharm. Bull. 2004, 52, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Kusari, P.; Kusari, S.; Eckelmann, D.; Zühlke, S.; Kayser, O.; Spiteller, M. Cross-species biosynthesis of maytansine in Maytenus serrata. RSC Adv. 2016, 6, 10011–10016. [Google Scholar] [CrossRef] [Green Version]
- Kupchan, S.M.; Komoda, Y.; Thomas, G.J.; Hintz, H.P.J. Maytanprine and maytanbutine, new antileukaemic ansa macrolides from Maytenus buchananii. J. Chem. Soc., Chem. Commun. 1972, 19, 1065. [Google Scholar] [CrossRef]
- Lopus, M.; Oroudjev, E.; Wilson, L.; Wilhelm, S.; Widdison, W.; Chari, R.; Jordan, M.A. Maytansine and Cellular Metabolites of Antibody-Maytansinoid Conjugates Strongly Suppress Microtubule Dynamics by Binding to Microtubules. Mol. Cancer Ther. 2010, 9, 2689–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovtun, Y.; Noordhuis, P.; Whiteman, K.R.; Watkins, K.; Jones, G.E.; Harvey, L.; Lai, K.C.; Portwood, S.; Adams, S.; Sloss, C.M.; et al. IMGN779, a Novel CD33-Targeting Antibody–Drug Conjugate with DNA-Alkylating Activity, Exhibits Potent Antitumor Activity in Models of AML. Mol. Cancer Ther. 2018, 17, 1271–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, R.G.; Zhen, Y.S. Research and Development of Highly Potent Antibody-Based Drug Conjugates and Fusion Proteins for Cancer Therapy. In Recent Advances in Cancer Research and Therapy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 153–173. [Google Scholar]
- Lapusan, S.; Vidriales, M.B.; Thomas, X.; De Botton, S.; Vekhoff, A.; Tang, R.; Dumontet, C.; Morariu-Zamfir, R.; Lambert, J.M.; Ozoux, M.-L.; et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Investig. New Drugs 2011, 30, 1121–1131. [Google Scholar] [CrossRef]
- Arya, D.; Goel, S.; Shinde, P.; Joshi, G.C.; Sharma, O.R.; Sharma, S.K. Dysoxylum binacteriferum Hook. F.: A promising herbal drug used in folk medicine by Tharu community of Uttarakhand. World J. Pharmaceut. Res. 2017, 6, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.D.; Still, P.C.; Bueno Perez, L.; Grever, M.R.; Douglas Kinghorn, A. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr. Drug Targets 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.E.; Passaniti, A.; Gojo, I.; Kaufmann, S.; Bible, K.; Garimella, T.S.; Bauer, K.S. Phase I and pharmacokinetic study of flavopiridol followed by 1-β-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clinic. Cancer Res. 2005, 11, 8403–8412. [Google Scholar] [CrossRef] [Green Version]
- Zeidner, J.F.; Karp, J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 2015, 39, 1312–1318. [Google Scholar] [CrossRef]
- Karp, J.E.; Donehower, R.C.; Enterline, J.P.; Dole, G.B.; Fox, M.G.; Burke, P.J. In vivo cell growth and pharmacologic determinants of clinical response in acute myelogenous leukemia. Blood 1989, 73, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Karp, J.E.; Ross, D.D.; Yang, W.; Tidwell, M.L.; Wei, Y.; Greer, J.; Colevas, A.D. Timed sequential therapy of acute leukemia with flavopiridol: In vitro model for a phase I clinical trial. Clin. Cancer Res. 2003, 9, 307–315. [Google Scholar]
- Chen, K.T.; Militao, G.G.; Anantha, M.; Witzigmann, D.; Leung, A.W.; Bally, M.B. Development and characterization of a novel flavopiridol formulation for treatment of acute myeloid leukemia. J. Control. Release 2021, 333, 246–257. [Google Scholar] [CrossRef]
- Huryn, D.M.; Wipf, P. Natural Product Chemistry and Anticancer Drug Discovery. In Cancer Drug Design and Discovery; Elsevier: Amsterdam, The Netherlands, 2008; pp. 107–130. [Google Scholar]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic Content, Antioxidant, Antimicrobial and Cytotoxic Activities of Ethanolic Extract of Salix Alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P. Salicin—A natural analgesic. Ethnobot. Leafl. 2005, 2003, 8. [Google Scholar]
- El-Shemy, H.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Fujita, K. Willow Leaves’ Extracts Contain Anti-Tumor Agents Effective against Three Cell Types. PLoS ONE 2007, 2, e178. [Google Scholar] [CrossRef] [Green Version]
- Hostanska, K.; Jürgenliemk, G.; Abel, G.; Nahrstedt, A.; Saller, R. Willow bark extract (BNO1455) and its fractions suppress growth and induce apoptosis in human colon and lung cancer cells. Cancer Detect. Prev. 2007, 31, 129–139. [Google Scholar] [CrossRef]
- Khalafalla, M.M.; Elgaali, E.I.; Ahmed, M.M. In vitro multiple shoot regeneration from nodal explants of Vernonia amygdalina—An important medicinal plant. In Proceedings of the 8th African Crop Science Society Conference, El-Minia, Egypt, 27–31 October 2007. [Google Scholar]
- Erasto, P.; Grierson, D.; Afolayan, A. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J. Ethnopharmacol. 2006, 106, 117–120. [Google Scholar] [CrossRef]
- Kumari, G.K.; Masilamani, S.; Ganesh, M.; Aravind, S.; Sridhar, S. Zaluzanin D: A fungistatic sesquiterpene from Vernonia arborea. Fitoterapia 2003, 74, 479–482. [Google Scholar] [CrossRef]
- Soni, A.; Krishnamurthy, R. Plants—The next generation treatment of leukemia. Indian J. Plant Sci. 2013, 2, 117–125. [Google Scholar]
- Rathee, J.S.; Hassarajani, S.A.; Chattopadhyay, S. Antioxidant activity of Nyctanthes arbor-tristis leaf extract. Food Chem. 2007, 103, 1350–1357. [Google Scholar] [CrossRef]
- Heendeniya, S.N.; Keerthirathna, L.R.; Manawadu, C.K.; Dissanayake, I.H.; Ali, R.; Mashhour, A.; Alzahrani, H.; Godakumbura, P.; Boudjelal, M.; Peiris, D.C. Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites. Biomolecules 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Khanapur, M.; Avadhanula, R.K.; Setty, O.H. In Vitro Antioxidant, Antiproliferative, and Phytochemical Study in Different Extracts of Nyctanthes arbor-tristis Flowers. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.K.; Pandey, A. The wonder of Ayurvedic medicine—Nyctanthes arbortristis. Int. J. Herb. Med. Plants 2016, 4, 9–17. [Google Scholar]
- Parekh, S.; Soni, A. Nyctanthes arbor-tristis: Comprehensive review on its pharmacological, antioxidant, and anticancer activities. J. Appl. Biol. Biotechnol. 2020, 8, 95–104. [Google Scholar]
- Pandeti, S.; Sharma, K.; Bathula, S.R.; Tadigoppula, N. Synthesis of novel anticancer iridoid derivatives and their cell cycle arrest and caspase dependent apoptosis. Phytomedicine 2014, 21, 333–339. [Google Scholar] [CrossRef]
- Cochrane, C.B.; Nair, P.K.R.; Melnick, S.J.; Resek, A.P.; Ramachandran, C. Anticancer effects of Annona glabra plant extracts in human leukemia cell lines. Anticancer Res. 2008, 28, 965–971. [Google Scholar]
- Hien, N.T.T.; Nhiem, N.X.; Yen, D.T.H.; Hang, D.T.T.; Tai, B.H.; Quang, T.H.; Kim, E.J. Chemical constituents of the Annona glabra fruit and their cytotoxic activity. Pharm. Biol. 2015, 53, 1602–1607. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, D.; Wan, W.; Zhang, H. In vitro mitochondria-mediated anticancer and antiproliferative effects of Annona glabra leaf extract against human leukemia cells. J. Photochem. Photobiol. B Biol. 2018, 189, 29–35. [Google Scholar] [CrossRef]
- Sreeja, P. Phtomorphological and Ecological Study of the Invasive Annona glabra. J. Plant Pest. Sci. 2014, 1, 60–65. [Google Scholar]
- Islam, M.; Rahi, M.; Jahangir, C.A.; Rahman, M.H.; Jerin, I.; Amin, R.; Reza, M.A. In Vivo Anticancer Activity of Basella alba Leaf and Seed Extracts against Ehrlich’s Ascites Carcinoma (EAC) Cell Line. Evid. Based. Complement. Alternat. Med. 2018, 2018, 1537896. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Prasad, A.; Iyer, S.; Vaidya, S. Systematic pharmacognostical, phytochemical and pharmacological review on an ethno medicinal plant, Basella alba L. J. Pharm. Phytother. 2013, 5, 53–58. [Google Scholar]
- Deshmukh, S.; Gaikwad, D. A review of the taxonomy, ethnobotany, phytochemistry and pharmacology of Basella alba (Basellaceae). J. Appl. Pharm. Sci. 2014, 4, 153–165. [Google Scholar]
- Adhikari, R.; Kumar, H.N.; Shruthi, S. A review on medicinal importance of Basella alba L. Int. J. Pharm. Sci. Drug Res. 2012, 4, 110–114. [Google Scholar]
- Balachandran, P.; Govindarajan, R. Cancer—An ayurvedic perspective. Pharmacol. Res. 2005, 51, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Khanom, B.; Nahida, U.; Shapla, S.S.; Akter, M.S.; Farzana, H.; Zaman, F.; Islam, M.T. Phytochemical and pharmacological review on Basella alba L. Pharmacol. Online 2019, 1, 40–48. [Google Scholar]
- Sushila, R.; Deepti, A.; Permender, R.; Madhavi, T.; Dharmender, R.; Rathee, D. Cytotoxic and antibacterial activity of Basella alba whole plant: A relatively unexplored plant. Pharmacol. Online 2010, 3, 651–658. [Google Scholar]
- Pal, K. Cell growth inhibition and apoptosis by extract of Basella alba plant on U937 cells. World J. Pharm. Pharm. Sci. 2015, 5, 1251–1261. [Google Scholar]
- Shahneh, F.Z.; Valiyari, S.; Azadmehr, A.; Hajiaghaee, R.; Ali, B.; Baradaran, B. Cytotoxic activities of Ferulago angulata extract on human leukemia and lymphoma cells by induction of apoptosis. J. Med. Plant Res. 2013, 7, 677–682. [Google Scholar]
- Filiz, B.; Songül, K.; Bostanlık, D.; Gül, F.; Sibel, K.C. Anticancer effect of Ferulago mughlea Peşmen (Apiaceae) on cancer cell proliferation. Iran. J. Pharm. Sci. 2016, 15, 501–504. [Google Scholar]
- Karimi, P.; Pourgheysari, B.; Soltani, A. The anti-proliferative effects of Ferulago angulata on human promyelocytic leukemia cell line (HL-60). Trends Pharmacol. Sci. 2019, 5, 123–130. [Google Scholar]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Wei, Y.-L.; Xu, L.; Liang, Y.; Xu, X.-H.; Zhao, X.-Y. Berbamine exhibits potent antitumor effects on imatinib-resistant CML cells in vitro and in vivo. Acta Pharmacol. Sin. 2009, 30, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Qiu, X.; Xu, R.-Z.; Zhao, X.-Y. Berbamine inhibits proliferation and induces apoptosis of KU812 cells by increasing Smad3 activity. J. Zhejiang Univ. Sci. B 2011, 12, 568–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Dong, Q.; Yu, Y.; Zhao, X.; Gan, X.; Wu, D.; Lu, Q.; Xu, X.; Yu, X.-F. Berbamine: A novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk. Res. 2006, 30, 17–23. [Google Scholar] [CrossRef]
- Akter, R.; Uddin, S.J.; Tiralongo, J.; Grice, I.D.; Tiralongo, E. A new cytotoxic diterpenoid glycoside from the leaves of Blumea lacera and its effects on apoptosis and cell cycle. Nat. Prod. Res. 2016, 30, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-S.; Chiang, L.-C.; Chen, C.-C.; Liu, L.-T.; Wang, K.-C.; Lin, C.-C. Antileukemic Activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am. J. Chin. Med. 2001, 29, 303–312. [Google Scholar] [CrossRef]
- Yi, J.; Wu, J.-G.; Peng, W. Antioxidant and Anti-proliferative Activities of Flavonoids from Bidens pilosa L var radiata Sch Bip. Trop. J. Pharm. Res. 2016, 15, 341. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.N.; Verma, N.K. A brief study on Catharanthus roseus: A review. Intern. J. Res. Pharm. Pharm. Sci. 2017, 2, 20–23. [Google Scholar]
- Pardede, A.; Mashita, K.; Ninomiya, M.; Tanaka, K.; Koketsu, M. Flavonoid profile and antileukemic activity of Coreopsis lanceolata flowers. Bioorganic Med. Chem. Lett. 2016, 26, 2784–2787. [Google Scholar] [CrossRef]
- Pardede, A.; Adfa, M.; Kusnanda, A.J.; Ninomiya, M.; Koketsu, M. Chemical Constituents of Coreopsis lanceolata Stems and Their Antitermitic Activity against the Subterranean Termite Coptotermes curvignathus. J. Econ. Entomol. 2018, 111, 803–807. [Google Scholar] [CrossRef]
- Amirghofran, Z.; Bahmani, M.; Azadmehr, A.; Javidnia, K.; Miri, R. Immunomodulatory Activities of Various Medicinal Plant Extracts: Effects on Human Lymphocytes Apoptosis. Immunol. Investig. 2009, 38, 181–192. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Koravand, M.; Haddadi, H.; Rafieian-Kopaei, M.; Shirmardi, H.A.; Hosseini, Z. A review of botany, phyto-chemical and pharmacological properties of Ferulago angulata. Toxin Rev. 2019, 38, 13–20. [Google Scholar] [CrossRef]
- Norrizah, J.; Norizan, A.; Ruzaina, S.S.; Dzulsuhaim, D.; Hida, M.N. Cytotoxicity Activity and Reproductive Profiles of Male Rats Treated with Methanolic extracts of Ficus deltoidea. Res. J. Med. Plant 2012, 6, 197–202. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Prommaban, A.; Kodchakorn, K.; Kongtawelert, P.; Banjerdpongchai, R. Houttuynia cordata Thunb fraction induces human leukemic Molt-4 cell apoptosis through the endoplasmic reticulum stress pathway. Asian Pac. J. Cancer Prev. 2012, 13, 1977–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumnerdkhonkaen, P.; Saenglee, S.; Asgar, M.A.; Senawong, G.; Khongsukwiwat, K.; Senawong, T. Antiproliferative activities and phenolic acid content of water and ethanolic extracts of the powdered formula of Houttuynia cordata Thunb. fermented broth and Phyllanthus emblica Linn. fruit. Complement. Altern. Med. Plants. 2018, 18, 130. [Google Scholar] [CrossRef] [Green Version]
- Sutapa, B.M.; Vansh, K.; Vaibhav, A.; Gopa, R.B.; Majee, S.B.; Khattry, V.; Agarwal, V.; Biswas, G.R. Polyphenols derived from four indigenous Indian fruits for cancer chemoprevention and chemotherapy. J. Med. Plants Res. 2016, 10, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Fares, R.; Bazzi, S.; Baydoun, S.E.; Abdel-Massih, R.M. The Antioxidant and Anti-proliferative Activity of the Lebanese Olea europaea Extract. Plant Foods Hum. Nutr. 2011, 66, 58–63. [Google Scholar] [CrossRef]
- Samet, I.; Han, J.; Jlaiel, L.; Sayadi, S.; Isoda, H. Olive (Olea europaea) Leaf Extract Induces Apoptosis and Monocyte/Macrophage Differentiation in Human Chronic Myelogenous Leukemia K562 Cells: Insight into the Underlying Mechanism. Oxidative Med. Cell. Longev. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA Damage Is Prevented by Extracts of Olive Oil, Hydroxytyrosol, and Other Olive Phenolic Compounds in Human Blood Mononuclear Cells and HL60 Cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar] [CrossRef] [Green Version]
- Salah, M.B.; Abdelmelek, H.; Abderraba, M. Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Med. Chem. 2012, 2, 107–111. [Google Scholar]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Kumagai, T.; Müller, C.I.; Desmond, J.C.; Imai, Y.; Heber, D.; Koeffler, H.P. Scutellaria baicalensis, a herbal medicine: Anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk. Res. 2007, 31, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitro Antitumor Mechanisms of Various Scutellaria Extracts and Constituent Flavonoids. Planta Med. 2008, 75, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-T.; Wang, C.-Y.; Yang, R.-C.; Chu, C.-J.; Wu, H.-T.; Pang, J.-H.S. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine 2010, 17, 47–54. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Gu, J.-Q.; Gills, J.J.; Park, E.J.; Mata-Greenwood, E.; Hawthorne, M.E.; Axelrod, F.; Chavez, P.I.; Fong, H.H.S.; Mehta, R.G.; Pezzuto, J.M.; et al. Sesquiterpenoids from Tithonia diversifolia with potential cancer chemopreventive activity. J. Nat. Prod. 2002, 65, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.M.G.; Enot, M.M.; Baltazar, G.J.D.; Alinapon, C.V.; Buncales, E.O.; Barbosa, G.B. Antioxidant and Cytotoxic Activity of the Leaf Ethanolic Extracts of Tithonia diversofilia and Gliricidia sepium from Bukidnon, Philippines. Asian J. Biol. Life Sci. 2019, 8, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Sukari, M.A.; Abdullah, R.; Taha, M.M.E.; Beng, N.K.; Isa, N.M. Typhonium flagelliforme inhibits the proliferation of murine leukemia WEHI-3 cells in vitro and induces apoptosis in vivo. Leuk. Res. 2010, 34, 1483–1492. [Google Scholar] [CrossRef]
- Choo, C.Y.; Chan, K.L.; Sam, T.W.; Hitotsuyanagi, Y.; Takeya, K. The cytotoxicity and chemical constituents of the hexane fraction of Typhonium flagelliforme (Araceace). J. Ethnopharmacol. 2001, 77, 129–131. [Google Scholar] [CrossRef]
- Sianipar, N.F.; Purnamaningsih, R. Enhancement of the contents of anticancer bioactive compounds in mutant clones of rodent tuber (Typhonium flagelliforme Lodd.) based on GC-MS analysis. Pertanika J. Trop. Agric. Sci. 2018, 41, 305–320. [Google Scholar]
- Mohan, S.; Bustamam, A.; Ibrahim, S.; Al-Zubairi, A.S.; Aspollah, M.; Abdullah, R.; Elhassan, M.M. In Vitro Ultramorphological Assessment of Apoptosis on CEMss Induced by Linoleic Acid-Rich Fraction from Typhonium flagelliforme Tuber. Evid. Based Complement. Altern. Med. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Seifert, G.; Jesse, P.; Laengler, A.; Reindl, T.; Lüth, M.; Lobitz, S.; Henze, G.; Prokop, A.; Lode, H.N. Molecular mechanisms of mistletoe plant extract-induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 2008, 264, 218–228. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, S.; Sharma, R.S.; Singh, S.; Sardesai, M.M.; Sharma, S.; Mishra, V. Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells. J. Ethnopharmacol. 2018, 219, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Sims, J.N.; Njiki, S.; Tsabang, N.; Ogungbe, I.V.; Tchounwou, P.B. Vernonia Amygdalina Delile Exhibits A Potential for the Treatment of Acute Promyelocytic Leukemia. Glob. J. Adv. Eng. Technol. Sci. 2018, 5, 1–9. [Google Scholar] [PubMed]
- Wen, L.; You, L.; Yang, X.; Yang, J.; Chen, F.; Jiang, Y.; Yang, B. Identification of phenolics in litchi and evaluation of anticancer cell proliferation activity and intracellular antioxidant activity. Free Radic. Biol. Med. 2015, 84, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, B.; Wang, J.; Liu, Y.; Yu, L.; Jiang, Y. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int. Immunopharmacol. 2007, 7, 162–166. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Zhao, M.; Liu, Y.; Wang, W.; Jiang, Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr. Res. 2006, 341, 634–638. [Google Scholar] [CrossRef]
- Roy, S.; Besra, S.E.; De, T.; Banerjee, B.; Mukherjee, J.; Vedasiromoni, J.R. Induction of Apoptosis in Human Leukemic Cell Lines U937, K562 and HL-60 by Litchi chinensis Leaf Extract Via Activation of Mitochondria Mediated Caspase Cascades. Open Leuk. J. 2008, 1, 1–14. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, S.; Wen, L.; Jiang, Y.; Zhao, M.; Chen, F.; Prasad, K.N.; Duan, X.; Yang, B. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013, 136, 563–568. [Google Scholar] [CrossRef]
- Wen, L.; Wu, D.; Jiang, Y.; Prasad, K.N.; Lin, S.; Jiang, G.; He, J.; Zhao, M.; Luo, W.; Yang, B. Identification of flavonoids in litchi (Litchi chinensis Sonn.) leaf and evaluation of anticancer activities. J. Funct. Foods 2014, 6, 555–563. [Google Scholar] [CrossRef]
- Kaushik, P.; Pahwa, P.; Kaushik, D. A comprehensive review on medicinal plants with anticancer activity. Glob. J. Pharmaceu. Edu. Res. 2014, 3, 1–13. [Google Scholar]
- Farida, Y.; Irpan, K.; Fithriani, L. Antibacterial and Antioxidant Activity of Keladi Tikus Leaves Extract (Typhonium Flagelliforme) (Lodd) Blume. Procedia Chem. 2014, 13, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Bustamam, A.; Ibrahim, S.; Al-Zubairi, A.S.; Aspollah, M. Anticancerous Effect of Typhonium flagelliforme on Human T4-Lymphoblastoid Cell Line CEM-ss. J. Pharmacol. Toxicol. 2008, 3, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Sukari, M.A.; Abdullah, R.; Taha, M.M.E.; Ibrahim, M.Y.; Syam, S. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: Its activation coupled with G0/G1 phase cell cycle arrest. J. Ethnopharmacol. 2010, 131, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Mas, R.H.; Nair, N.; Majid, M.; Mansor, S.; Navaratnam, V. Typhonium flagelliforme inhibits cancer cell growth in vitro and induces apoptosis: An evaluation by the bioactivity guided approach. J. Ethnopharmacol. 2008, 118, 14–20. [Google Scholar] [CrossRef]
- Tomar, A. Folk medicinal use of Blumea lacera (BURM. F.) DC. To cure threadworms. J. Med. Plants Stud. 2017, 5, 336–337. [Google Scholar]
- Ragasa, C.Y.; Wong, J.; Rideout, J.A. Monoterpene glycoside and flavonoids from Blumea lacera. J. Nat. Med. 2007, 61, 474–475. [Google Scholar] [CrossRef]
- Jama, B.; Palm, C.A.; Buresh, R.J.; Niang, A.; Gachengo, C.; Nziguheba, G.; Amadalo, B. Tithonia diversifolia as a green manure for soil fertility improvement in western Kenya: A review. Agrofor. Syst. 2000, 49, 201–221. [Google Scholar] [CrossRef]
- Oliveira, F.; Andrade-Neto, V.; Krettli, A.; Brandão, M. New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. J. Ethnopharmacol. 2004, 93, 39–42. [Google Scholar] [CrossRef]
- Bartolome, A.P.; Villaseñor, I.M.; Yang, W.C. Bidens pilosa L. (Asteraceae): Botanical properties, traditional uses, phytochemistry, and pharmacology. Evid. Based. Complement. Alternat. Med. 2013, 2013, 340215. [Google Scholar] [CrossRef] [Green Version]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E.; Gerothanassis, I.P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Pawinwongchai, J.; Chanprasert, S. Antileukemic activity of Houttuynia cordata Thunb. extracts in Jurkat and U937 human leukemic cells. J. Chem. Pharm. Res. 2011, 3, 204–212. [Google Scholar]
- Nguyen, V.T.; Le, V.M.; Vo, T.S.; Bui, L.M.; Anh, H.L.T.; Danh, V.T. Preliminary phytochemical screening and determination of total polyphenols and flavonoids content in the leaves of Houttuynia cordata Thunb. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062013. [Google Scholar] [CrossRef]
- Okwu, D.E.; Nnamdi, F.U. Two novel flavonoids from Bryophyllum pinnatum and their antimircobal activity. J. Chem. Pharm. Res. 2011, 3, 1–10. [Google Scholar]
- Evans, M.; Bryant, S.; Huntley, A.L.; Feder, G. Cancer Patients’ Experiences of Using Mistletoe (Viscum album): A Qualitative Systematic Review and Synthesis. J. Altern. Complement. Med. 2016, 22, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Nazaruk, J.; Orlikowski, P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res. 2016, 30, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Eggenschwiler, J.; Von Balthazar, L.; Stritt, B.; Pruntsch, D.; Ramos, M.; Urech, K.; Rist, L.; Simões-Wüst, A.P.; Viviani, A. Mistletoe lectin is not the only cytotoxic component in fermented preparations of Viscum album from white fir (Abies pectinata). BMC Complement. Altern. Med. 2007, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Horneber, M.; Van Ackeren, G.; Linde, K.; Rostock, M. Mistletoe therapy in oncology. Cochrane Database Syst. Rev. 2008, 2008, CD003297. [Google Scholar] [CrossRef]
- Miraj, S.; Kiani, S. A review study of therapeutic effects of Salvia officinalis L. Pharm. Lett 2016, 8, 299–303. [Google Scholar]
- Privitera, G.; Luca, T.; Castorina, S.; Passanisi, R.; Ruberto, G.; Napoli, E. Anticancer activity of Salvia officinalis essential oil and its principal constituents against hormone-dependent tumour cells. Asian Pac. J. Trop. Biomed. 2019, 9, 24. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia Fruticosa, Salvia Officinalis, and Rosmarinic Acid Induce Apoptosis and Inhibit Proliferation of Human Colorectal Cell Lines: The Role in MAPK/ERK Pathway. Nutr. Cancer 2009, 61, 564–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshavarz, M.; Bidmeshkipour, A.; Mostafaei, A.; Mansouri, K.; Mohammadi, M.H. Anti tumor activity of Salvia officinalis is due to its anti-angiogenic, anti-migratory and anti-proliferative effects. Cell J. 2011, 12, 477–482. [Google Scholar]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef]
- Wei, L.S.; Wee, W.; Siong, J.Y.F.; Syamsumir, D.F. Characterization of Antioxidant, Antimicrobial, Anticancer Property and Chemical Composition of Ficus deltoidei Jack. Leaf Extract. J. Biol. Act. Prod. Nat. 2011, 1, 1–6. [Google Scholar] [CrossRef]
- Starr, F.; Starr, K.; Loope, L. Mistletoe fig, Moraceae. Ficus Deltoidea 2003, 2, 1–5. [Google Scholar]
- A’attiyyah, A.; Ghazali, W.A.S.W.; Ali, N.A.M.; Ponnuraj, K.T.; Mohamad, S.; Azlina, A. Phytochemical properties and traditional uses of selected medicinal plants in Malaysia: A review. JBCR 2018, 2, 14–25. [Google Scholar]
- Choo, C.; Sulong, N.; Man, F.; Wong, T. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J. Ethnopharmacol. 2012, 142, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Manurung, H.; Kustiawan, W.; Kusuma, I.W. Marjenah Total flavonoid content and antioxidant activity in leaves and stems extract of cultivated and wild tabat barito (Ficus deltoidea Jack). AIP Conf. Proc. 2017, 1813, 20007. [Google Scholar]
- Akhir, N.A.M.; Chua, L.S.; Majid, F.A.A.; Sarmidi, M.R. Cytotoxicity of Aqueous and Ethanolic Extracts of Ficus deltoidea on Human Ovarian Carcinoma Cell Line. Br. J. Med. Med Res. 2011, 1, 397–409. [Google Scholar] [CrossRef]
- Hanafi, M.M.M.; Afzan, A.; Yaakob, H.; Aziz, R.; Sarmidi, M.R.; Wolfender, J.-L.; Prieto, J.M. In Vitro Pro-apoptotic and Anti-migratory Effects of Ficus deltoidea L. Plant Extracts on the Human Prostate Cancer Cell Lines PC3. Front. Pharmacol. 2017, 8, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abolmaesoomi, M.; Aziz, A.A.; Junit, S.M.; Ali, J.M. Ficus deltoidea: Effects of solvent polarity on antioxidant and anti-proliferative activities in breast and colon cancer cells. Eur. J. Integr. Med. 2019, 28, 57–67. [Google Scholar] [CrossRef]
- Xie, J.; Ma, T.; Gu, Y.; Zhang, X.; Qiu, X.; Zhang, L.; Yu, Y.; Xu, R. Berbamine derivatives: A novel class of compounds for anti-leukemia activity. Eur. J. Med. Chem. 2009, 44, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, D.; Sun, H.; Zhang, Y.; Zhang, W.; Meng, F.; Du, X. Optimizing the extraction of anti-tumor alkaloids from the stem of Berberis amurensis by response surface methodology. Ind. Crop. Prod. 2015, 69, 68–75. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhingra, D.; Kumar, V. Memory-Enhancing Activity of Palmatine in Mice Using Elevated Plus Maze and Morris Water Maze. Adv. Pharmacol. Sci. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wu, J.; Ye, F.; Xue, L.; Jiang, S.; Yi, J.; Zhang, W.; Wei, H.; Sung, M.; Wang, W.; et al. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003, 63, 4037–4043. [Google Scholar]
- Giessrigl, B. New Approaches in the Targeting of Cell Cycle, Cell Death and Cancer Progression. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2011. [Google Scholar]
- Orzechowska, B.; Chaber, R.; Wiśniewska, A.; Pajtasz-Piasecka, E.; Jatczak, B.; Siemieniec, I.; Gulanowski, B.; Chybicka, A.; Błach-Olszewska, Z. Baicalin from the extract of Scutellaria baicalensis affects the innate immunity and apoptosis in leukocytes of children with acute lymphocytic leukemia. Int. Immunopharmacol. 2014, 23, 558–567. [Google Scholar] [CrossRef]
- Jintao, X.; Quanwei, Y.; Chunyan, L.; Yun, J.; Shuangxi, W.; Mingxiang, Z.; Peng, L. Rapid and Simultaneous Determination of Three Active Components in Raw and Processed Root Samples of Scutellaria baicalensis by Near-infrared Spectroscopy. Planta Med. 2018, 85, 72–80. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maher, T.; Ahmad Raus, R.; Daddiouaissa, D.; Ahmad, F.; Adzhar, N.S.; Latif, E.S.; Abdulhafiz, F.; Mohammed, A. Medicinal Plants with Anti-Leukemic Effects: A Review. Molecules 2021, 26, 2741. https://doi.org/10.3390/molecules26092741
Maher T, Ahmad Raus R, Daddiouaissa D, Ahmad F, Adzhar NS, Latif ES, Abdulhafiz F, Mohammed A. Medicinal Plants with Anti-Leukemic Effects: A Review. Molecules. 2021; 26(9):2741. https://doi.org/10.3390/molecules26092741
Chicago/Turabian StyleMaher, Tahani, Raha Ahmad Raus, Djabir Daddiouaissa, Farah Ahmad, Noor Suhana Adzhar, Elda Surhaida Latif, Ferid Abdulhafiz, and Arifullah Mohammed. 2021. "Medicinal Plants with Anti-Leukemic Effects: A Review" Molecules 26, no. 9: 2741. https://doi.org/10.3390/molecules26092741








