3.1. Immobilized Lipases and Media Tailoring for Sustainable Biocatalyzed Esterifications
To enhance the performance of the biocatalyst, the immobilization of enzymes and, more specifically, lipases are of high industrial interest, and it has become a requirement [
29]. Indeed, it was shown in numerous reports that through, e.g., adsorption or covalent binding with a solid carrier, some acyltransferases, such as the lipase B from
Candida antarctica (CaLB), could display improved activity, stability and reusability [
30,
31,
32]. This phenomenon can be understood
inter alia as a gain of rigidity for the enzyme’s tertiary structure, which limits protein unfolding and thus denaturation of the biocatalyst [
33]. This is in practice true in the case of the Novozym 435
® formulation that was recently qualified as the “perfect immobilized biocatalyst” in a review from Ortiz et al. [
34]. In parallel, the native CaLB has been demonstrated to be among the most stable commercially available lipases [
35,
36]. By binding it to a macroporous acrylic polymer resin (Lewatit VP OC 16,000), resulting in the now highly reported Novozym 435
®, it increased drastically the performances of the biocatalyst [
37].
In the current work, it might appear shocking that we use 50 g/L of the enzyme to reach the same value in titer of product. At least on the tube scale, it is likely that, in the case of Novozym 435
®, the costs associated with the immobilization might be fully compensated [
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48]. Indeed, the formulation can be recycled, using filtration, for sometimes up to 10 cycles, as a report from Liu et al. demonstrates [
49]. It is also important to remember that in such a bead-adsorbed enzyme formulation, the non-catalytic ballast of the carrier represents a tremendous portion of the biocatalyst’s mass (90 to >99 wt.%) [
37,
50]. Thus, it is remarkable that in our work, the immobilized versions of CaLB, such as Lipozyme 435
® or Novozym 435
®, rivalized with the buffered formulations as they should normally contain a higher enzyme concentration.
Interestingly, CLEA CA was, in our case, comparable to the other CaLB formulations. The Cross-Linked Enzyme Aggregates (CLEAs) first introduced by Sheldon et al. [
51], but also the Cross-Linked Enzyme Crystals (CLECs) [
52], represent very interesting and more elegant alternatives to the petrol-based polymer-carriers. CLEAs are a result of firsthand the precipitation and physical aggregation of the enzymes, then secondhand the cross-linking of these aggregates with a cross-linking agent, which can be typically glutaraldehyde. This gives several advantages to CLEAs over their non-covalently immobilized analogues, such as the quasi-nonexistent leaching of enzyme even under reportedly harsh conditions [
53]. Due to the covalent inter-molecular binding, CLEAs and CLECs afford complete removal of the carriers, thus resulting in carrier-free immobilized enzymes. However, they also present limitations and challenges for their industrial-scale production, notably for the control of the enzyme’s aggregation that can result in less active enzyme dimers [
54]; thus, they are, to this day, rather rarely produced in bulk. On a side note, it is important to mention that standard acrylic-bead-immobilized enzymes were easier to handle throughout the tube-scale synthesis and downstream processing, than their CLEA analogues.
Herein, we reached an optimal titer after 48 h of reaction, which is like other reports dealing with enzymatic production of sugar esters in DESs [
11] but much shorter than microbial fermentation to produce glycolipids. Indeed, as an example, to produce Mannosylerythritol Lipids (MELs), the average harvesting time is between 7 to 10 days for a titer ranging from 15 g/L to 165 g/L, to obtain, in the case of the highest yields, complex mixtures of MELs [
55,
56,
57,
58]. Additionally, and as we demonstrated, fewer factors must be investigated when selectively producing tailor-made glycolipids using either free or immobilized enzymes. On the other hand, microorganisms, such as yeasts or fungi, exhibit much more complex behaviors that require acute control of the reaction conditions. In this specific case, and despite MELs being well established on the market, mixtures of products are often obtained, thus adding a degree of complexity to the DSP. Our process using a “2-in-1” DES as media is rather more straightforward comparatively, but more time and investigation are needed to overcome challenges to make it relevant for industrial production. In regard to DSP, it has been shown in several studies that DESs can be recycled as well [
59,
60,
61]. In our case and for an efficient liquid–liquid extraction, the “2-in-1” DES was first disrupted via aqueous solvation. Thus, it could be foreseeable that choline chloride-rich wastes generated by such DES-mediated process could be re-valorized in feed additives [
62] or as agrochemical active ingredients [
63]. Unlike organic solvents, DESs do not have to undergo incineration; thus, their release in nature can be considered [
64].
Analogously, a eutectic mixture using organic solvents (NaOH/DMSO/2M2B) was investigated by Kim et al. for the synthesis of SL using lauric acid, reaching exceptionally high yields (97%) using ~500 g/L of the enzyme [
23]. Despite this remarkable achievement, we propose, in contrast, a method using a deep eutectic system made of ubiquitous, renewable and inexpensive compounds, such as choline chloride and sorbitol, using 10 times less biocatalyst. Concomitantly, they also reported an adverse effect of highly viscous mixtures on the reaction, which correlates to the decreased titer we obtained when the reaction’s water content was set to 2.5 wt.%. Overall, this might also explain our comparatively inferior conversion yields, as it seems that deep eutectic systems present challenges in terms of mass transfer limitations, meaning that our substrate hardly moves to the enzyme’s active site. Zhao et al. reported a bisolvent system containing either ILs or DESs in combination with 2M2B for glucose-based fatty acid esters production. Similar factors were investigated and gave results equivalent to ours, such as an optimal enzyme content of 20 g/L of Novozym 435
® at 60 °C to reach 46 % conversion yield from the 0.3 M of vinyl ester used [
65].
The ever-growing knowledge on enzyme immobilization and media-tailoring technologies have shown to be crucial tools to reach sustainability in biocatalysis. They are also of active interest for various industrial domains to develop greener and sustainable processes [
18,
66,
67,
68,
69]. Regarding this aim and as performance is of keen interest for industrial application, reactor technology and scalability represent two important pillars as well.
3.2. Reactor Technology: Toward Scaling-Up Lipase-Catalyzed Reactions
The development of suitable reactors and technologies that support lipase-catalyzed reactions is a topical subject for both academia and industry. Some parameters inherent to reactors have been shown to be of high importance for the performance of the process. For example, the speed of stirring can drastically influence the initial velocity of the reaction and the conversion yield. This was clearly demonstrated in a research article from Korupp et al. dealing with the enzymatic production of glycerol adipate using Novozym 435
® [
70]. In the latter, they observed the highest conversion rates at 100 rpm. The type of stirrer did not significantly influence the reaction; however, it was observed that only a helicon ribbon stirrer gave uniform convection of both substrate and catalyst. A similar observation was made in our case using a 3-bladed spiral propeller instead of a 4-bladed flat turbine (unpublished data), suggesting that homogeneity of highly viscous mixtures can be reached optimally in an STR with stirrers that induce vertical convection due to a rather axial flow. Indeed, studies from Wiemann et al. and Ansorge-Schumacher et al. are suggesting that viscous mixtures requiring high energy input might have a deleterious effect on the physical and mechanical properties of immobilized formulations [
71,
72]. To corroborate these affirmations, a study from Keng et al. used a Rushton turbine impeller, providing this time a radial flow that was seemingly more adapted to the relatively lower viscosities of their
n-hexane-based mixture [
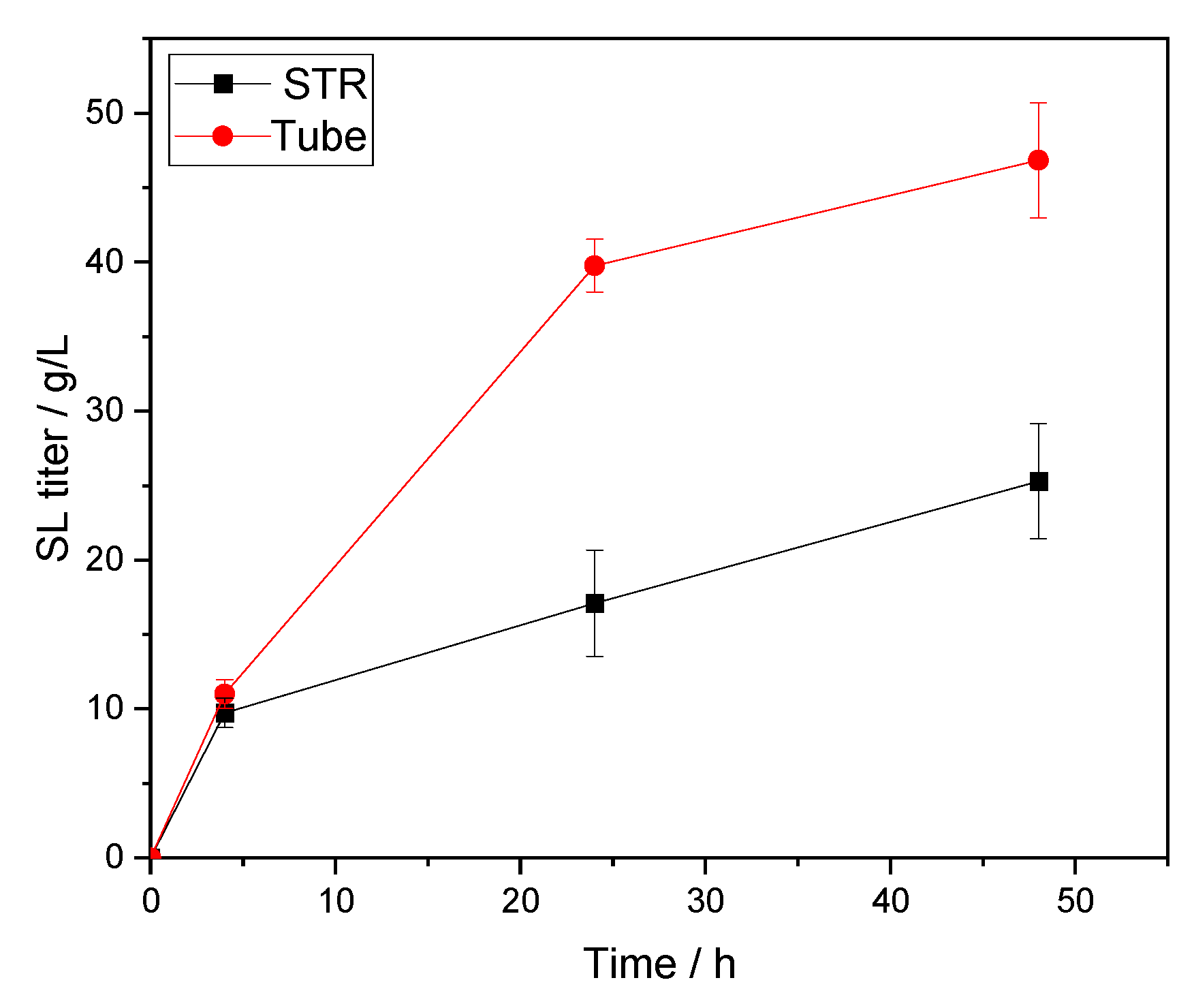
73]. In their report dealing with the Lipozyme RM IM-catalyzed palm esters synthesis, it was clearly concluded as well that the shear effect of high impeller speed on the enzyme particles caused an adverse effect on reaction performances. Thus, potentially explaining our significant loss of titer (~2-fold decrease) and lower reaction velocity when scaling up from the tube to the STR (
Table 5 and
Figure 5). Altogether, it seems that a compromise needs to be found on the stirrer type and its speed to conserve the integrity of the biocatalyst. In this regard, more stable lipase formulations are commercialized to respond to this problem. A good example is the recently co-developed and industrially produced CalB immo Plus
® from c-Lecta and Purolite companies (
www.c-lecta.com, Leipzig, Germany/
www.purolite.com, King of Prussia, PA, USA). The highly hydrophobic carrier ECR1030M (DVB/acrylate copolymer) exhibits enhanced mechanical stability and offers a controlled size of spherical beads.
As a matter of fact, we mostly demonstrate what conditions were the most intrinsically influential on the reaction, but further studies would be needed to optimize the process at an even bigger scale. We essentially brought in the current work proof of the scalability of our process and, more generally, the scalability of the DES-mediated biocatalysis, which is scarcely represented in the literature [
74]. Despite a lack of in-depth understanding of how mechanistically DESs can activate and stabilize lipases, the room for improvement of this topic toward industrial application is rather wide. However, we were able to demonstrate that the preparative scale is reachable using a facile and straightforward method that requires the minimum necessary equipment, as shown in the flowsheet of
Figure 6. We removed, therein, the need for continuous pH and gas composition assessment that are standardly used in microbial fermentation, among other measurements that require probes combined with their respective computer software.
Although our discussion mainly revolved around the use of rather conventional reactors, such as STRs for batch heterogenous biocatalysis, innovative and commercially available alternatives have risen on the market. The Rotating Bed Reactors (RBRs), notably commercialized by the SpinChem company (
www.spinchem.com, Tvistevägen, Sweden), have proven to be promising alternatives to STRs and have shown good results when used for immobilized enzymatic reactions [
75]. Furthermore, their cooperation with Purolite (
www.purolite.com, King of Prussia, PA, USA) gave light to immobilized lipase cartridges, theoretically forming a rotating packed bed reactor, which removes the need for filtration during the DSP and simplifies the recovery of the biocatalyst. Remarkably, packed bed reactors have also been combined with DESs for lipase-catalyzed esterifications. In a relatively recent study from Guajardo et al., they managed the transition from a batch to a fed-batch and continuous process, enhancing simultaneously conversion yield and productivity, thus seemingly resolving the encountered issue of mass transfer limitations and biocatalyst inhibition [
76].
Recently as well, our research group published proof of a concept for the simultaneous extraction of lipids from yeast and the subsequent ester production in a “2-in-1” Sorbit DES, using microwave heating as an alternative to thermal heating [
25]. This simplified and fast method is only an example of the vast possibilities that DESs, immobilized enzymes and innovative reactor systems can offer not only to the field of biocatalysis but also to biotechnology in a broader scope.