Biological Applications of Electron Paramagnetic Resonance Viscometry Using a 13C-Labeled Trityl Spin Probe
Abstract
:1. Introduction
2. Results
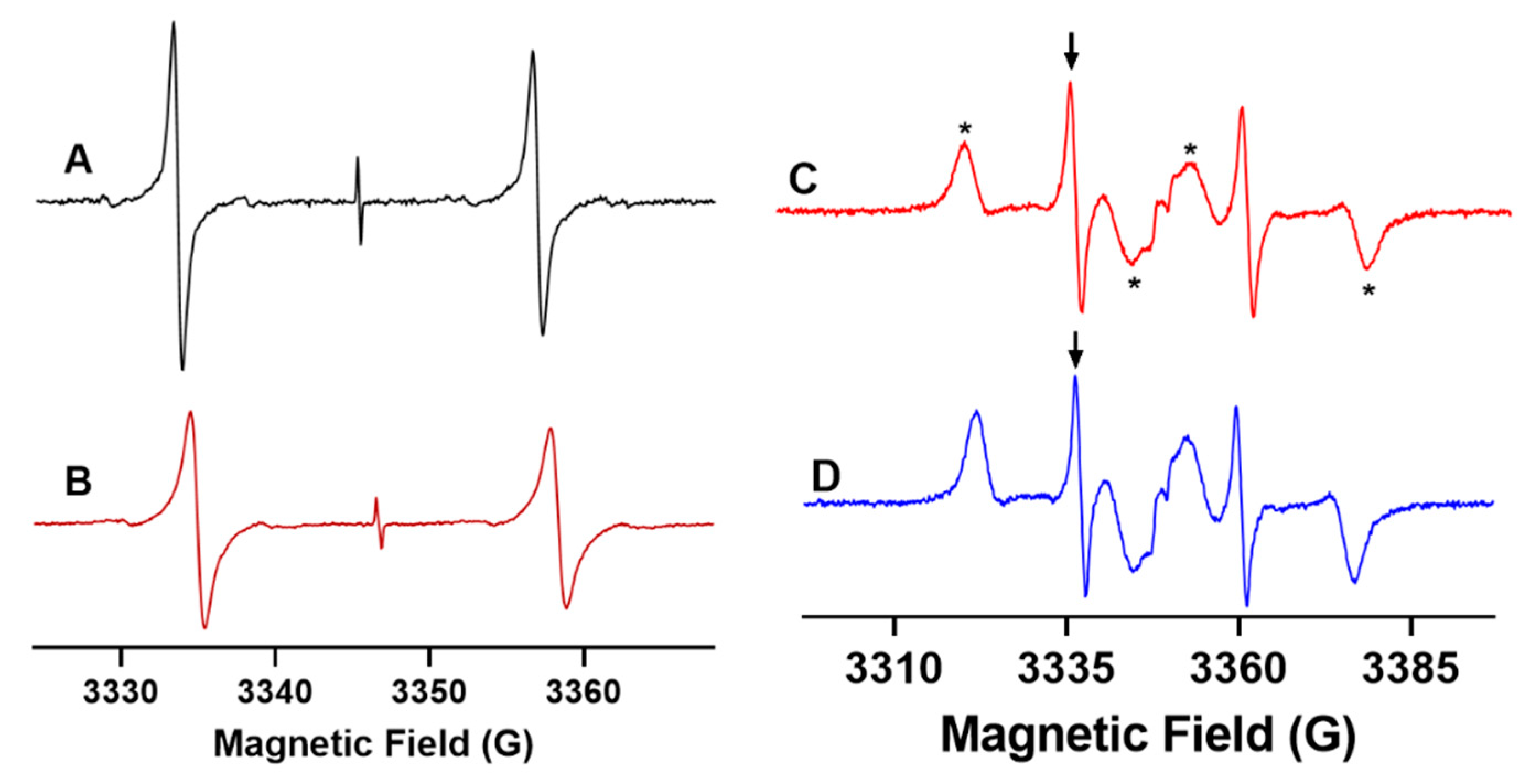
2.1. X-Band EPR Microviscometric Measurement of Human Blood
2.2. L-Band EPR Microviscometric Measurement in Isolated Organs and In Vivo
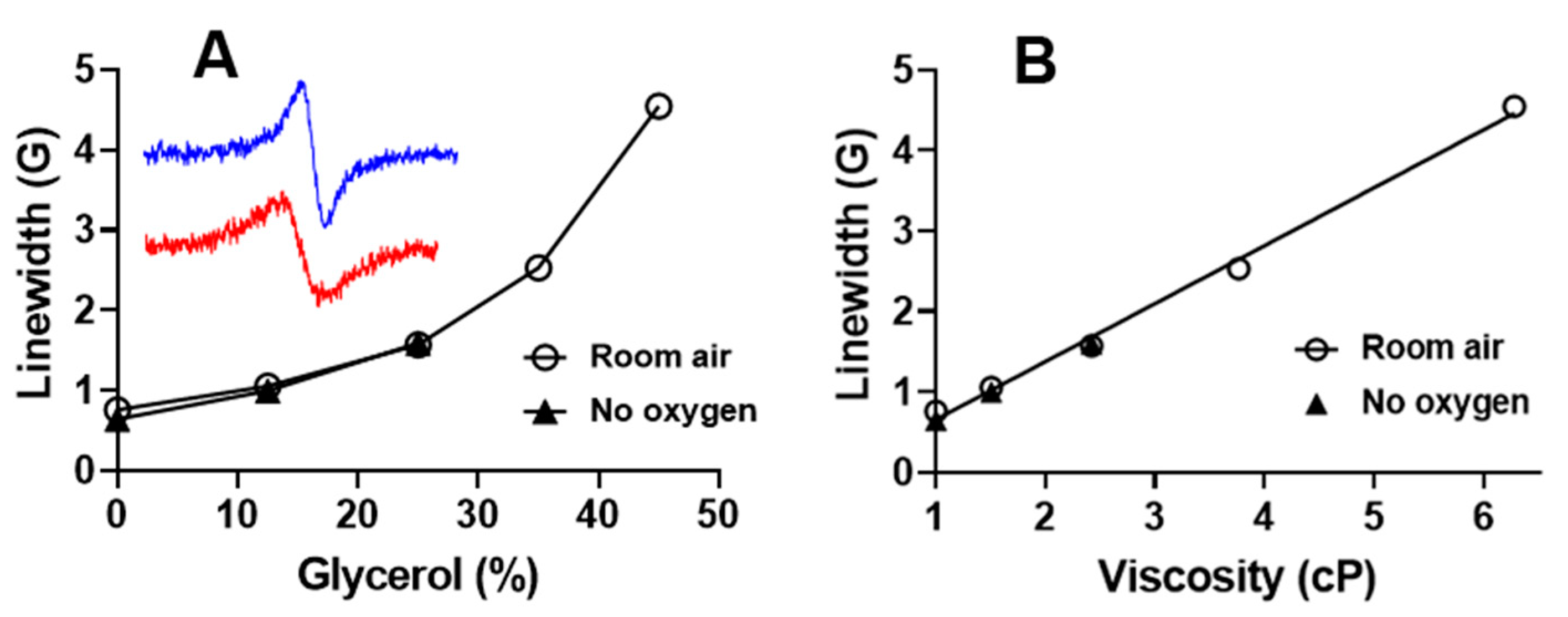
2.2.1. L-Band EPR Calibration of 13C-dFT Sensitivity to Viscosity
2.2.2. L-Band EPR Measurements of Viscosity of Isolated Organs from Mice
2.2.3. In Vivo EPR Measurements of the Microviscosity of Interstitial Fluids
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Synthesis of 13C1-Deuterated Finland Trityl Radical (13C-dFT)
4.3. Collection of Human Blood from Healthy Volunteers
4.4. Animals
4.5. Preparation of Organs for L-Band EPR Measurements
4.6. X-Band EPR Measurements
4.7. L-Band (1.2 GHz) EPR Measurements
4.8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 2020, 12, 571. [Google Scholar] [CrossRef]
- Jung, F.; Pindur, G.; Kiesewetter, H. Plasma viscosity dependence on proteins and lipoproteins-results of the Aachen study. Clin. Hemorheol. 1992, 12, 557–571. [Google Scholar]
- von Tempelhoff, G.F.; Schonmann, N.; Heilmann, L.; Pollow, K.; Hommel, G. Prognostic role of plasma viscosity in breast cancer. Clin. Hemorheol. Microcirc. 2002, 26, 55–61. [Google Scholar]
- Ho, C.H. White blood cell and platelet counts could affect whole blood viscosity. J. Chin. Med. Assoc. 2004, 67, 394–397. [Google Scholar] [PubMed]
- McMillan, D.E. Physical factors important in the development of atherosclerosis in diabetes. Diabetes 1981, 30 (Suppl. 2), 97–104. [Google Scholar] [CrossRef]
- Cakmak, G.; Alkan, F.A.; Korkmaz, K.; Saglam, Z.A.; Karis, D.; Yenigun, M.; Ercan, M. Blood viscosity as a forgotten factor and its effect on pulmonary flow. Transl. Respir. Med. 2013, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, W.; Sund, M.; Filipiak, B.; Doring, A.; Lowel, H.; Ernst, E. Plasma viscosity and the risk of coronary heart disease: Results from the MONICA-Augsburg Cohort Study, 1984 to 1992. Arter. Thromb. Vasc. Biol. 1998, 18, 768–772. [Google Scholar] [CrossRef] [Green Version]
- Lowe, G.D.O.; Barbenel, J.C. Plasma and blood viscosity. In Clinical Blood Rheology; Lowe, G.D.O., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 12–44. [Google Scholar]
- Reinhart, W.H.; Danoff, S.J.; Usami, S.; Chien, S. Rheologic measurements on small samples with a new capillary viscometer. J. Lab. Clin. Med. 1984, 104, 921–931. [Google Scholar]
- Haidekker, M.A.; Tsai, A.G.; Brady, T.; Stevens, H.Y.; Frangos, J.A.; Theodorakis, E.; Intaglietta, M. A novel approach to blood plasma viscosity measurement using fluorescent molecular rotors. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1609–H1614. [Google Scholar] [CrossRef] [Green Version]
- Vysniauskas, A.; Lopez-Duarte, I.; Duchemin, N.; Vu, T.T.; Wu, Y.; Budynina, E.M.; Volkova, Y.A.; Pena Cabrera, E.; Ramirez-Ornelas, D.E.; Kuimova, M.K. Exploring viscosity, polarity and temperature sensitivity of BODIPY-based molecular rotors. Phys. Chem. Chem. Phys. 2017, 19, 25252–25259. [Google Scholar] [CrossRef] [Green Version]
- Hazlewood, C.F.; Nichols, B.L.; Chamberlain, N.F. Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature 1969, 222, 747–750. [Google Scholar] [CrossRef]
- Cooke, R.; Kuntz, I.D. The properties of water in biological systems. Annu. Rev. Biophys. Bioeng. 1974, 3, 95–126. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, A.M.; Luquita, A.; Rasia, M. Comparison between internal microviscosity of low-density erythrocytes and the microviscosity of hemoglobin solutions: An electron paramagnetic resonance study. Biophys. J. 1996, 71, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Morse, P.D., 2nd; Lusczakoski, D.M.; Simpson, D.A. Internal microviscosity of red blood cells and hemoglobin-free resealed ghosts: A spin-label study. Biochemistry 1979, 18, 5021–5029. [Google Scholar] [CrossRef]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Clark, A.; Sedhom, J.; Elajaili, H.; Eaton, G.R.; Eaton, S.S. Dependence of electron paramagnetic resonance spectral lineshapes on molecular tumbling: Nitroxide radical in water:glycerol mixtures. Concepts Magn. Reson. Part A 2016, 45A, e21423. [Google Scholar] [CrossRef]
- Moore, W.; McPeak, J.E.; Poncelet, M.; Driesschaert, B.; Eaton, S.S.; Eaton, G.R. (13)C isotope enrichment of the central trityl carbon decreases fluid solution electron spin relaxation times. J. Magn. Reson. 2020, 318, 106797. [Google Scholar] [CrossRef] [PubMed]
- Halpern, H.J.; Chandramouli, G.V.; Barth, E.D.; Yu, C.; Peric, M.; Grdina, D.J.; Teicher, B.A. Diminished aqueous microviscosity of tumors in murine models measured with in vivo radiofrequency electron paramagnetic resonance. Cancer Res. 1999, 59, 5836–5841. [Google Scholar]
- Halpern, H.J.; Chandramouli, G.V.R.; Yu, C.; Peric, M.; Barth, E.D.; Teicher, B.A.; Grdina, D. Pharmacological compartment viscosity and polarity measured with very low-frequency EPR in tumors of living animals. Magn. Res. Chem. 1995, 33, S147–S153. [Google Scholar] [CrossRef]
- Poncelet, M.; Driesschaert, B. A (13) C-Labeled Triarylmethyl Radical as an EPR Spin Probe Highly Sensitive to Molecular Tumbling. Angew. Chem. Int. Ed. Engl. 2020, 59, 16451–16454. [Google Scholar] [CrossRef]
- Ardenkjær-Larsen, J.H.; Laursen, H.; Leunbach, I.; Ehnholm, G.; Wistrand, L.-G.; Petersson, J.S.; Golman, K. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J. Magn. Reson. 1998, 133, 1–12. [Google Scholar] [CrossRef]
- Kocherginsky, N.; Swartz, H.M. Nitroxide Spin Labels. Reactions in Biology and Chemistry; CRC Press: Boca Raton, FL, USA; New York, NY, USA; London, UK; Tokyo, Japan, 1995; p. 270. [Google Scholar]
- Song, Y.; Liu, Y.; Liu, W.; Villamena, F.A.; Zweier, J.L. Characterization of the Binding of the Finland Trityl Radical with Bovine Serum Albumin. RSC Adv. 2014, 4, 47649–47656. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1996; p. 432. [Google Scholar]
- Choi, S.; Choi, E.Y.; Kim, D.J.; Kim, J.H.; Kim, T.S.; Oh, S.W. A rapid, simple measurement of human albumin in whole blood using a fluorescence immunoassay (I). Clin. Chim. Acta 2004, 339, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Monkos, K. Viscosity of bovine serum albumin aqueous solutions as a function of temperature and concentration. Int. J. Biol. Macromol. 1996, 18, 61–68. [Google Scholar] [CrossRef]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression. Sci. Rep. 2017, 7, 41233. [Google Scholar] [CrossRef]
- Gorodetskii, A.A.; Eubank, T.D.; Driesschaert, B.; Poncelet, M.; Ellis, E.; Khramtsov, V.V.; Bobko, A.A. Development of multifunctional Overhauser-enhanced magnetic resonance imaging for concurrent in vivo mapping of tumor interstitial oxygenation, acidosis and inorganic phosphate concentration. Sci. Rep. 2019, 9, 12093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khramtsov, V.V. In Vivo Molecular Electron Paramagnetic Resonance-Based Spectroscopy and Imaging of Tumor Microenvironment and Redox Using Functional Paramagnetic Probes. Antioxid. Redox Signal. 2018, 28, 1365–1377. [Google Scholar] [CrossRef]
- Edelstein, N.; Kwok, A.; Maki, A.H. Effects of hydrostatic pressure on linewidths of free radical in solutions. I. Anisotropic region. J. Chem. Phys. 1964, 41, 179–183. [Google Scholar] [CrossRef]
- Il’yasov, A.V. Solvent effects in the EPR spectra of certain free radicals. J. Struct. Chem. 1962, 3, 84–86. [Google Scholar] [CrossRef]
- Volodarsky, L.B.; Grigor’ev, I.A.; Sagdeev, R.Z. Stable Imidazoline Nitroxides. In Biological Magnetic Resonance; Berliner, L.J., Reuben, J., Eds.; Plenum Press: New York, NY, USA, 1980; Volume 2, pp. 169–241. [Google Scholar]
- Volodarsky, L.B. Imidazoline Nitroxides; CRC Press: Boca Raton, FL, USA, 1988; Volume 1, p. 232. [Google Scholar]
- Khramtsov, V.V.; Volodarsky, L.B. Use of imidazoline nitroxides in studies of chemical reactions. ESR measurements of the concentration and reactivity of protons, thiols and nitric oxide. In Spin Labeling. The next Millennium; Berliner, L.J., Ed.; Plenum Press: New York, NY, USA, 1998; Volume 14, pp. 109–180. [Google Scholar]
- Khramtsov, V.V.; Weiner, L.M.; Grigoriev, I.A.; Volodarsky, L.B. Proton-Exchange in Stable Nitroxyl Radicals-Electron-Paramagnetic-Res Study of the pH of Aqueous-Solutions. Chem. Phys. Lett. 1982, 91, 69–72. [Google Scholar] [CrossRef]
- Khramtsov, V.V.; Yelinova, V.I.; Weiner, L.M.; Berezina, T.A.; Martin, V.V.; Volodarsky, L.B. Quantitative-Determination of SH-Groups in Low-Molecular-Weight and High-Molecular-Weight Compounds by an Electron-Spin Resonance Method. Anal. Biochem. 1989, 182, 58–63. [Google Scholar] [CrossRef]
- Samouilov, A.; Efimoya, O.V.; Bobko, A.A.; Sun, Z.; Petryakov, S.; Eubank, T.D.; Trofimov, D.G.; Kirilyuk, I.A.; Grigor’ev, I.A.; Takahashi, W.; et al. In Vivo Proton-Electron Double-Resonance Imaging of Extracellular Tumor pH Using an Advanced Nitroxide Probe. Anal. Chem. 2014, 86, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roshchupkina, G.I.; Bobko, A.A.; Bratasz, A.; Reznikov, V.A.; Kuppusamy, P.; Khramtsov, V.V. In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical. Free Radic. Biol. Med. 2008, 45, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khramtsov, V.V.; Bobko, A.A.; Tseytlin, M.; Driesschaert, B. Exchange Phenomena in the Electron Paramagnetic Resonance Spectra of the Nitroxyl and Trityl Radicals: Multifunctional Spectroscopy and Imaging of Local Chemical Microenvironment. Anal. Chem. 2017, 89, 4758–4771. [Google Scholar] [CrossRef]
- Beaudoin, A.G.; Mizukami, H. Esr Correlation Times of 2,2,6,6-Tetramethyl Piperidone-N-Oxyl (Tempone) in Solutions of Hemoglobin-a and Hemoglobin-S. Biochim. Biophys. Acta 1978, 532, 41–47. [Google Scholar] [CrossRef]
- Siepe, S.; Herrmann, W.; Borchert, H.H.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Microenvironmental pH and microviscosity inside pH-controlled matrix tablets: An EPR imaging study. J. Control. Release 2006, 112, 72–78. [Google Scholar] [CrossRef]
- Kempe, S.; Metz, H.; Mader, K. Application of Electron Paramagnetic Resonance (EPR) spectroscopy and imaging in drug delivery research-Chances and challenges. Eur. J. Pharm. Biopharm. 2010, 74, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Minato, S.; Takenouchi, A.; Uchida, J.; Tsuboi, A.; Kurata, M.; Fukuo, K.; Kazumi, T. Association of Whole Blood Viscosity with Metabolic Syndrome in Type 2 Diabetic Patients: Independent Association With Post-Breakfast Triglyceridemia. J. Clin. Med. Res. 2017, 9, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Tutal, E.; Erkmen Uyar, M.; Uyanik, S.; Bal, Z.; Guliyev, O.; Toprak, S.K.; Ilhan, O.; Sezer, S.; Haberal, M. Hyperviscosity in renal transplant recipients. Transpl. Proc. 2015, 47, 1165–1169. [Google Scholar] [CrossRef]
- Krishna, M.C.; English, S.; Yamada, K.; Yoo, J.; Murugesan, R.; Devasahayam, N.; Cook, J.A.; Golman, K.; Ardenkjaer-Larsen, J.H.; Subramanian, S.; et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: Coregistration of tumor anatomy and tissue oxygen concentration. Proc. Natl. Acad. Sci. USA 2002, 99, 2216–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epel, B.; Halpern, H.J. In Vivo pO2 Imaging of Tumors: Oxymetry with Very Low-Frequency Electron Paramagnetic Resonance. Methods Enzym. 2015, 564, 501–527. [Google Scholar]
- Dhimitruka, I.; Bobko, A.A.; Eubank, T.D.; Komarov, D.A.; Khramtsov, V.V. Phosphonated trityl probes for concurrent in vivo tissue oxygen and pH monitoring using electron paramagnetic resonance-based techniques. J. Am. Chem. Soc. 2013, 135, 5904–5910. [Google Scholar] [CrossRef] [Green Version]
- Driesschaert, B.; Marchand, V.; Leveque, P.; Gallez, B.; Marchand-Brynaert, J. A phosphonated triarylmethyl radical as a probe for measurement of pH by EPR. Chem. Commun. 2012, 48, 4049–4051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Song, Y.G.; Rockenbauer, A.; Sun, J.; Hemann, C.; Villamena, F.A.; Zweier, J.L. Synthesis of Trityl Radical-Conjugated Disulfide Biradicals for Measurement of Thiol Concentration. J. Org. Chem. 2011, 76, 3853–3860. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.P.; Villamena, F.A.; Rockenbauer, A.; Zweier, J.L. Trityl-nitroxide biradicals as unique molecular probes for the simultaneous measurement of redox status and oxygenation. Chem. Commun. 2010, 46, 628–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.L.; Ji, K.Y.; Wang, X.; Yao, R.; Han, G.F.; Villamena, F.A.; Zweier, J.L.; Song, Y.G.; Rockenbauer, A.; Liu, Y.P. Discriminative Detection of Biothiols by Electron Paramagnetic Resonance Spectroscopy using a Methanethiosulfonate Trityl Probe. Angew. Chem. Int. Ed. 2020, 59, 928–934. [Google Scholar] [CrossRef]
- Tormyshev, V.M.; Chubarov, A.S.; Krumkacheva, O.A.; Trukhin, D.V.; Rogozhnikova, O.Y.; Spitsyna, A.S.; Kuzhelev, A.A.; Koval, V.V.; Fedin, M.V.; Godovikova, T.S.; et al. Methanethiosulfonate Derivative of OX063 Trityl: A Promising and Efficient Reagent for Side-Directed Spin Labeling of Proteins. Chem. Eur. J. 2020, 26, 2705–2712. [Google Scholar] [CrossRef]
- Poncelet, M.; Huffman, J.L.; Khramtsov, V.V.; Dhimitruka, I.; Driesschaert, B. Synthesis of hydroxyethyl tetrathiatriarylmethyl radicals OX063 and OX071. RSC Adv. 2019, 9, 35073–35076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velayutham, M.; Poncelet, M.; Eubank, T.D.; Driesschaert, B.; Khramtsov, V.V. Biological Applications of Electron Paramagnetic Resonance Viscometry Using a 13C-Labeled Trityl Spin Probe. Molecules 2021, 26, 2781. https://doi.org/10.3390/molecules26092781
Velayutham M, Poncelet M, Eubank TD, Driesschaert B, Khramtsov VV. Biological Applications of Electron Paramagnetic Resonance Viscometry Using a 13C-Labeled Trityl Spin Probe. Molecules. 2021; 26(9):2781. https://doi.org/10.3390/molecules26092781
Chicago/Turabian StyleVelayutham, Murugesan, Martin Poncelet, Timothy D. Eubank, Benoit Driesschaert, and Valery V. Khramtsov. 2021. "Biological Applications of Electron Paramagnetic Resonance Viscometry Using a 13C-Labeled Trityl Spin Probe" Molecules 26, no. 9: 2781. https://doi.org/10.3390/molecules26092781





