Effect of Cryostructuring Treatment on Some Properties of Xanthan and Karaya Cryogels for Food Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM Analysis
2.2. MDSC Studies
2.3. Structural Order Degree
2.4. Activation Energies
2.5. FTIR-ATR Studies
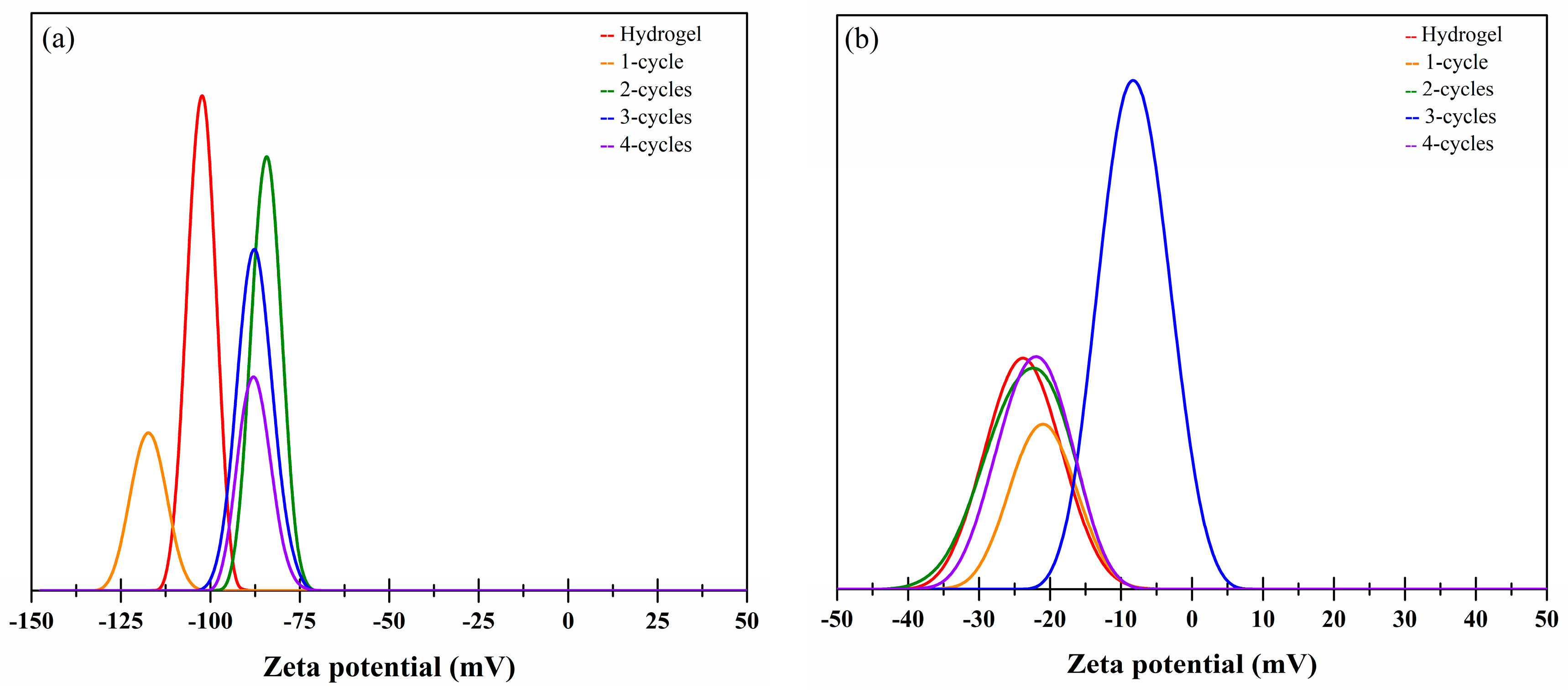
2.6. Zeta Potential Studies
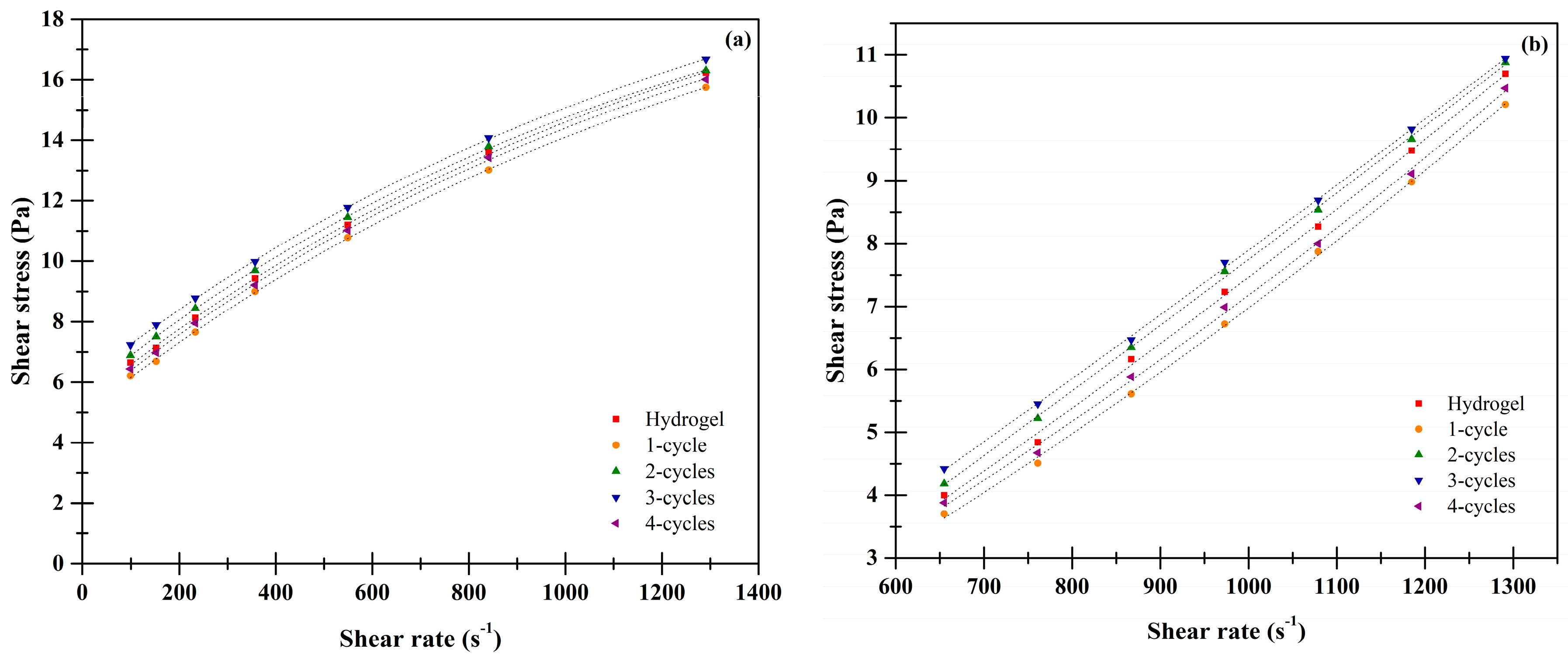
2.7. Rheological Analysis
3. Materials and Methods
3.1. Preparation of the Cryogels
3.2. Scanning Electron Microscopy (SEM)
3.3. Modulated Differential Scanning Calorimetry (MDSC) Analysis
3.4. Structural Order Degree
3.5. Activation Energies (Ea)
3.6. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflection (FTIR-ATR) Studies
3.7. Zeta Potential (ζ)
3.8. Rheological Analysis
3.9. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dickenson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2008, 23, 1473–1482. [Google Scholar] [CrossRef]
- Agoub, A.A.; Smith, A.M.; Giannouli, P.; Richardson, R.K.; Morris, E.R. “Melt-in-the-mouth” gels from mixtures of xanthan and konjac glucomannan under acidic conditions: A rheological and calorimetric study of the mechanism of synergistic gelation. Carbohydr. Polym. 2007, 69, 713–724. [Google Scholar] [CrossRef]
- Fernández, P.P.; Martino, M.N.; Zaritzky, N.E.; Guignon, B.; Sanz, P.D. Effects of locust bean, xanthan and guar gums on the ice crystals of sucrose solution frozen at high pressure. Food Hydrocoll. 2007, 21, 507–515. [Google Scholar] [CrossRef]
- Geetanjali, C.; Amit, V.; Amolina, D.; Keka, O. Rheological and breaking characteristics of Zr-crosslinked gum karaya gels for high-temperature hydraulic fracturing application. J. Pet. Sci. Eng. 2019, 172, 327–339. [Google Scholar]
- Yan, K.; Panyu, L.; Xiaotong, Z.; Xi, C.; Yi, X.; Yu, Z.; Yongkui, Z.; Tonghui, X. Biosynthesis, structure and antioxidant activities of xanthan gum from Xanthomonas campestris with additional furfural. Carbohydr. Polym. 2019, 216, 369–375. [Google Scholar]
- Truesdell, C. The meaning of viscometry in fluid dynamics. Annu. Rev. Fluid Mech. 1974, 6, 111–146. [Google Scholar] [CrossRef]
- Coria, H.J.; Méndez, A.A.; Meléndez, P.R.; Rosas, M.M.E.; Arjona, R.J.L. Thermal, Structural, and Rheological Characterization of Waxy Starch as a Cryogel for Its Application in Food Processing. Polymers 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Usp. Khim. 2002, 71, 559–585. [Google Scholar] [CrossRef]
- Alzhan, B.; Dmitriy, A.B.; Stavros, G.P.; Vassilis, J.I. A review of cryogels synthesis, characterization and applications on the removal of heavy metal from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar]
- Haiyan, Z.; Chunjie, L.; Liang, C.; Bin, D. Control of ice crystal growth and its effect on porous structure of chitosan cryogels. Chem. Eng. Sci. 2019, 201, 50–57. [Google Scholar]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selin, S.S.; Sahin, D.; Berkant, Y.; Rawil, F.; Ekaterina, N.; Oguz, O.; Ramesh, S.A.; Nurettin, S. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar]
- Giannouli, P.; Morris, E.R. Cryogelation of xanthan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Zubov, A.L.; Titova, E.F. Poly (vinyl alcohol) cryogels employed as matrices for cell immobilization. Entrapped cells resemble porous fillers in their effects on the properties of PVA-cryogel carrier. Enzym. Microb. Technol. 1997, 20, 182–190. [Google Scholar] [CrossRef]
- Coria, H.J.; Meléndez, P.R.; Rosas, M.M.E.; Llorente, B.A.; Arjona, R.J.L. Activation Energy for Protein Denaturation in Frozen and Freeze-Dried Pork Meat (Longissimus thoracis) by MDSC. Arct. J. 2017, 70, 16–30. [Google Scholar]
- Nur Hazirah, M.A.S.P.; Isa, M.I.N.; Sarbon, N.M. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, M.D. Gelatinization kinetics of rice starch studied by nonisothermal calorimetric technique: Influence of extraction method, water concentration and heating rate. J. Food Eng. 2004, 62, 337–344. [Google Scholar] [CrossRef]
- Calzetta, A.R.; Suarez, C. Gelatinization kinetics of amaranth starch. Int. J. Food Sci. Technol. 2001, 36, 441–448. [Google Scholar]
- Cornillon, P. Characterization of osmotic dehydrated Apple by NMR and DSC. LWT-Food Sci. Technol. 2000, 33, 261–267. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, R.; Xu, W.; Bai, Z.; Zhou, Y.; Zhao, S.; Xu, Y.; Yu, D. Rheological behavior and microstructure of release-controlled hydrogels based on xanthan gum crosslinked with sodium trimetaphosphate. Food Hydrocoll. 2016, 52, 923–933. [Google Scholar] [CrossRef]
- Bilanovic, D.; Starosvetsky, J.; Armon, R.H. Cross-linking xanthan and other compounds with glycerol. Food Hydrocoll. 2015, 44, 129–135. [Google Scholar] [CrossRef]
- Shalviri, A.; Liu, Q.; Abdekhodaie, M.J.; Wu, X.Y. Novel modified starch-xanthan gum hydrogels for controlled drug delivery: Synthesis and characterization. Carbohydr. Polym. 2010, 79, 898–907. [Google Scholar] [CrossRef]
- Le Cerf, D.; Irinei, F.; Muller, G. Solution Properties of Gum Exudates from Sterculia urens (Karaya Gum). Carbohydr. Polym. 1990, 13, 375–386. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Ray, S.S. Synthesis of co-polymer-grafted gum karaya and silica hybrid organic–inorganic hydrogel nanocomposite for the highly effective removal of methylene blue. Chem. Eng. J. 2015, 279, 166–179. [Google Scholar] [CrossRef]
- Xuran, C.; Yan, H.; Zhengbiao, G.; Yayuan, Z. The effect of electrostatic interactions on pasting properties of potato starch/xanthan gum combinations. Food Res. Int. 2011, 44, 3079–3086. [Google Scholar]
- Laha, B.; Sanjib, D.; Sabyasachi, M.; Kalyan, K.S. Novel propyl karaya gum nanogels for bosentan: In vitro and in vivo drug delivery performance. Colloids Surf. B 2019, 180, 263–272. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Cao, Y.; Liu, S.; Deng, D.; Zhang, J.; Huang, G. Effects of xanthan and konjac gums on pasting, rheology, microstructure, crystallinity and in vitro digestibility of mung bean resistant starch. Food Chem. 2021, 339, 128001. [Google Scholar] [CrossRef] [PubMed]
- Postulkova, H.; Chamradova, I.; Pavlinak, D.; Humpa, O.; Jancar, J.; Vojtova, L. Study of effects and conditions on the solubility of natural polysaccharide gum karaya. Food Hydrocoll. 2017, 67, 148–156. [Google Scholar] [CrossRef]
- Patel, A.K.; Bajpai, R.; Keller, J.M. On the crystallinity of PVA/palm leaf biocomposite using DSC and XDR techniques. Microsyst. Technol. 2014, 20, 41–49. [Google Scholar] [CrossRef]
- Pérez-De León, A.; Plasencia, J.; Vázquez, D.A.; Méndez, A.A. Comparison of the In Vitro Antifungal and Anti-fumonigenic Activities of Copper and Silver Nanoparticles Against Fusarium verticillioides. J. Clust. Sci. 2020, 31, 213–220. [Google Scholar] [CrossRef]
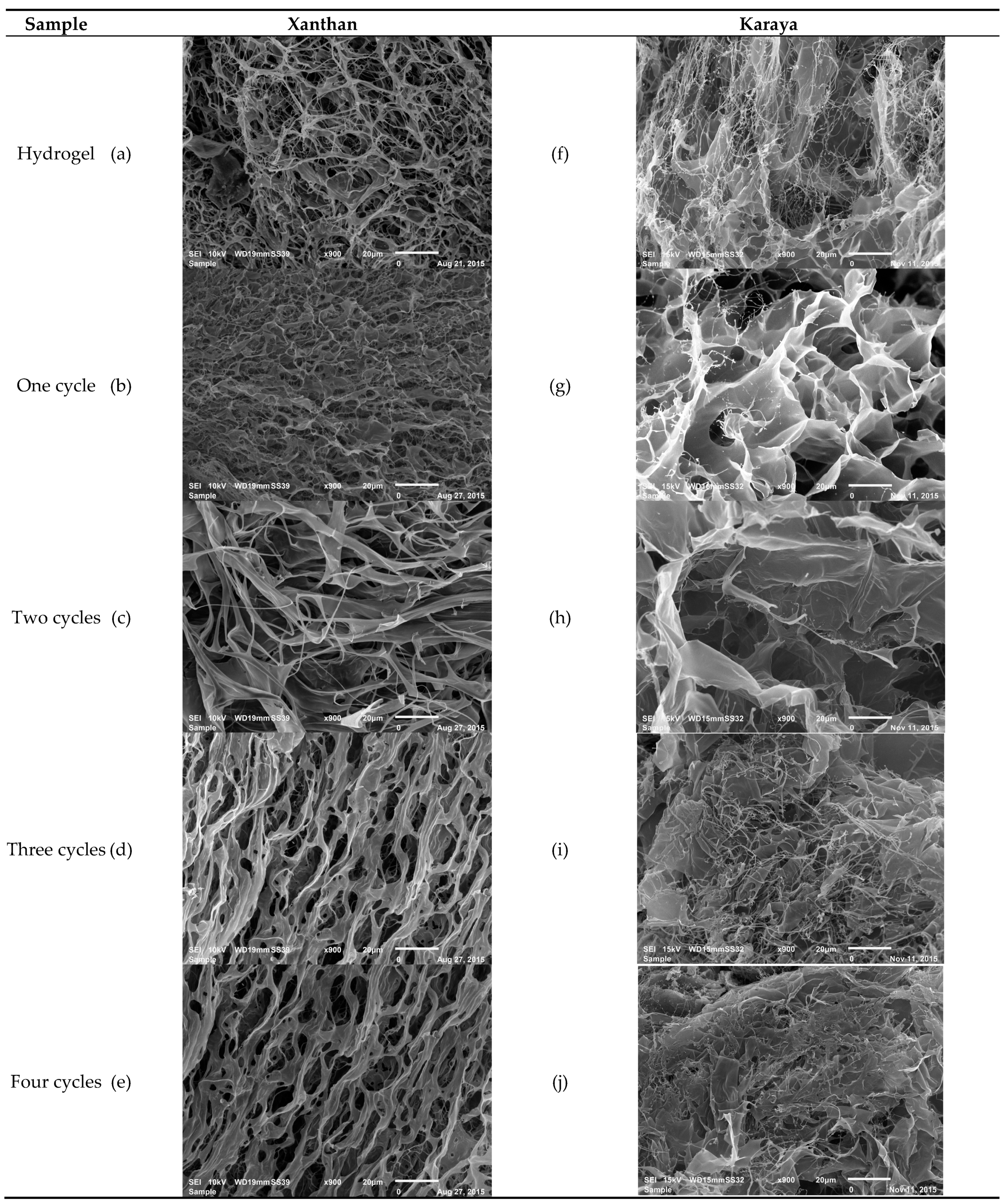
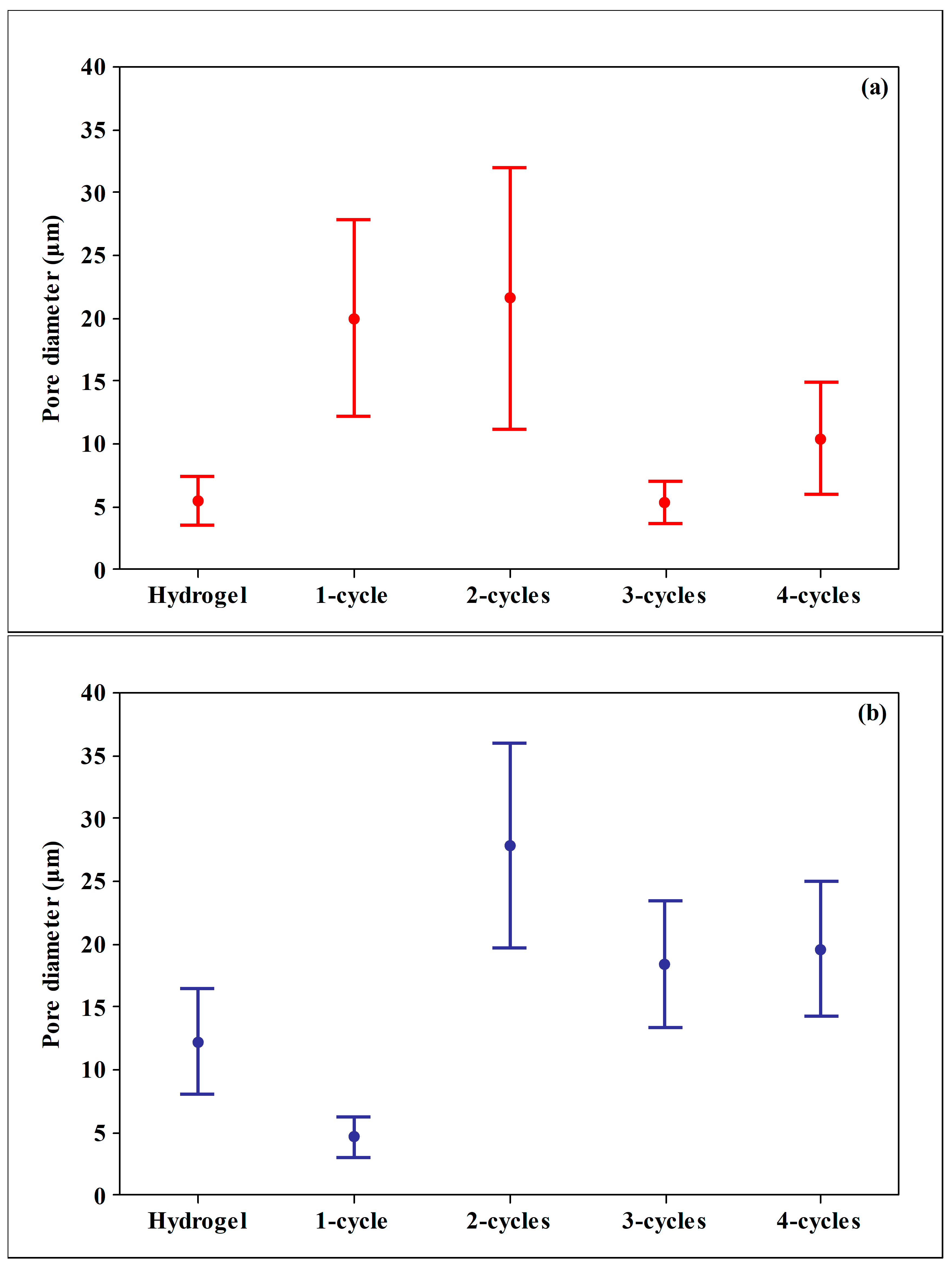


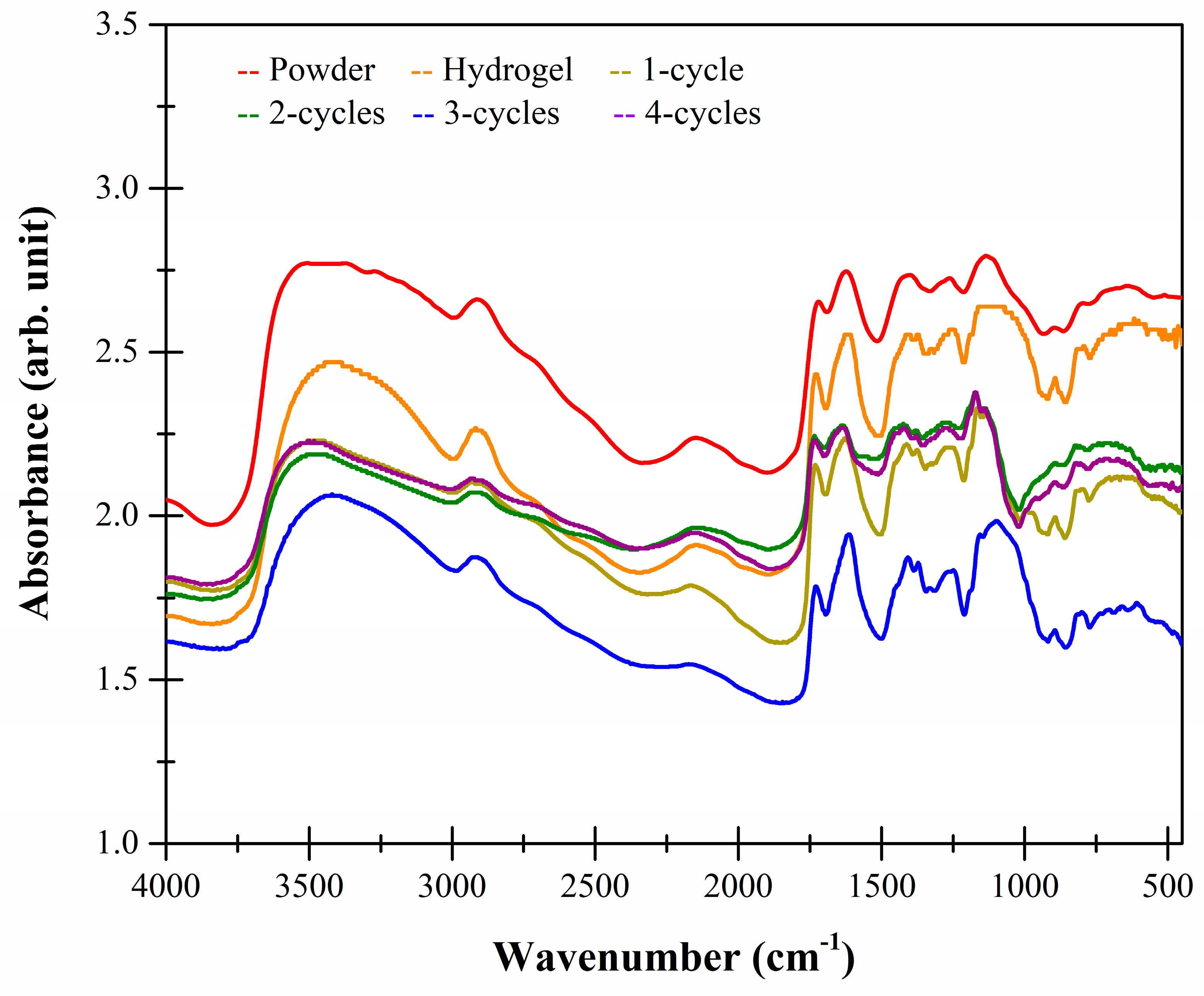
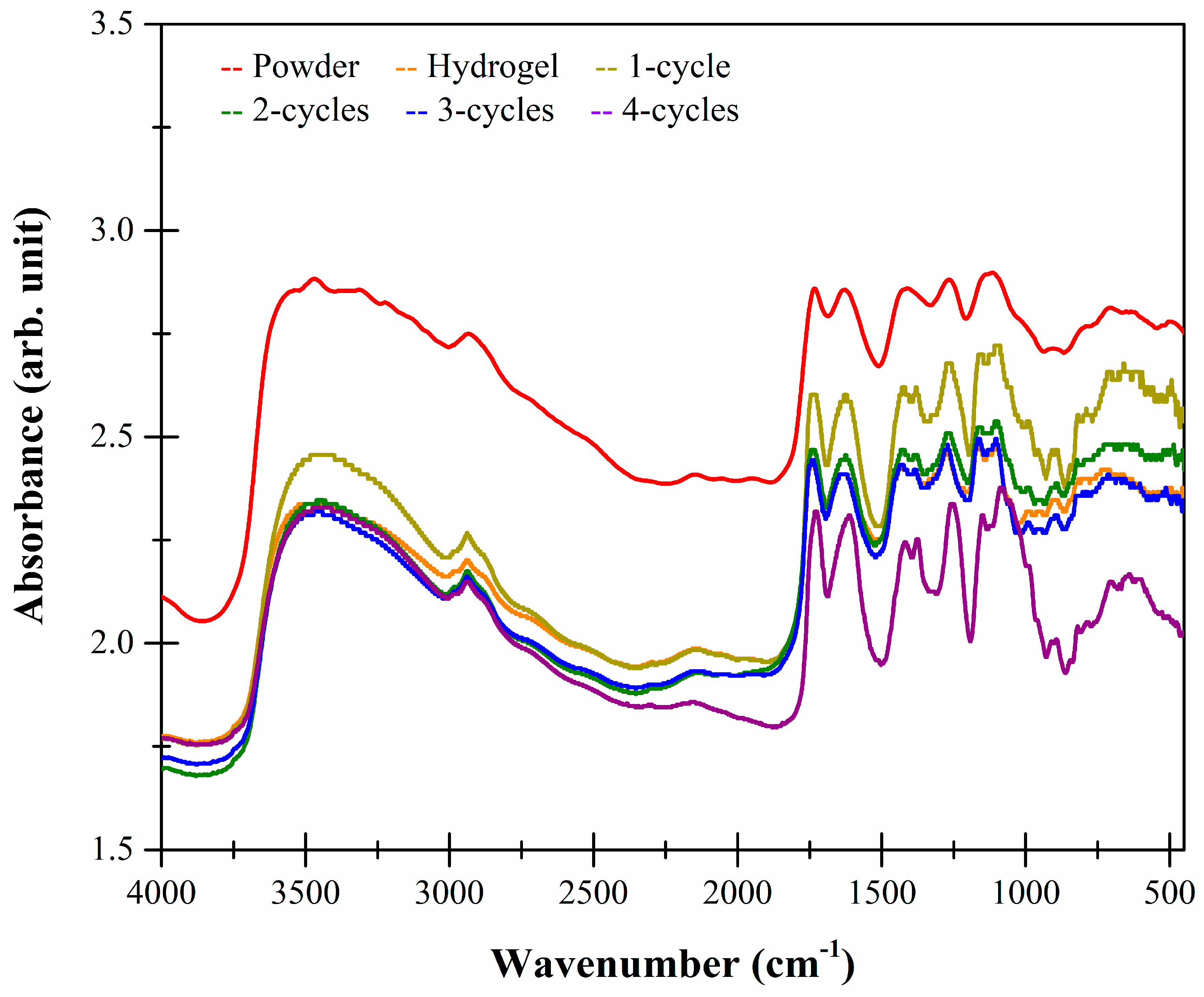
| Sample | Xanthan | Karaya |
|---|---|---|
| Hydrogel | 93.43 ± 0.48 a | 87.16 ± 0.68 c |
| One cycle | 92.34 ± 0.55 a | 79.89 ± 1.21 a |
| Two cycles | 94.36 ± 0.71 b | 80.40 ± 1.38 a |
| Three cycles | 95.55 ± 0.92 c | 84.48 ± 1.74 b |
| Four cycles | 92.17 ± 0.34 a | 78.28 ± 1.91 a |
| Sample | Xantana | Karaya |
|---|---|---|
| Hydrogel | 196.48 ± 1.24 b | 174.83 ± 0.99 a |
| One cycle | 250.28 ± 1.12 d | 376.12 ± 1.85 c |
| Two cycles | 200.74 ± 1.57 b | 388.10 ± 1.33 c |
| Three cycles | 154.79 ± 0.95 a | 244.17 ± 1.52 b |
| Four cycles | 212.12 ± 1.03 bc | 377.90 ± 1.74 c |
| Sample | Xanthan | Karaya | ||
|---|---|---|---|---|
| n | k | n | k | |
| Hydrogel | 0.3580 ± 0.0002 b | 1.2001 ± 0.0010 a | 1.3375 ± 0.0031 a | 0.0008 ± 0.0003 b |
| One cycle | 0.3728 ± 0.0024 c | 1.0466 ± 0.0009 a | 1.4048 ± 0.0052 b | 0.0005 ± 0.0001 b |
| Two cycles | 0.3427 ± 0.0010 a | 1.3496 ± 0.0011 b | 1.4646 ± 0.0033 c | 0.0003 ± 0.0001 a |
| Three cycles | 0.3303 ± 0.0021 a | 1.5022 ± 0.0024 c | 1.4724 ± 0.0049 c | 0.0003 ± 0.0000 a |
| Four cycles | 0.3645 ± 0.0028 bc | 1.1322 ± 0.0008 a | 1.5163 ± 0.0041 d | 0.0002 ± 0.0000 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coria-Hernández, J.; Meléndez-Pérez, R.; Méndez-Albores, A.; Arjona-Román, J.L. Effect of Cryostructuring Treatment on Some Properties of Xanthan and Karaya Cryogels for Food Applications. Molecules 2021, 26, 2788. https://doi.org/10.3390/molecules26092788
Coria-Hernández J, Meléndez-Pérez R, Méndez-Albores A, Arjona-Román JL. Effect of Cryostructuring Treatment on Some Properties of Xanthan and Karaya Cryogels for Food Applications. Molecules. 2021; 26(9):2788. https://doi.org/10.3390/molecules26092788
Chicago/Turabian StyleCoria-Hernández, Jonathan, Rosalía Meléndez-Pérez, Abraham Méndez-Albores, and José Luis Arjona-Román. 2021. "Effect of Cryostructuring Treatment on Some Properties of Xanthan and Karaya Cryogels for Food Applications" Molecules 26, no. 9: 2788. https://doi.org/10.3390/molecules26092788







