Ferro- and Antiferromagnetic Interactions in Oxalato-Centered Inverse Hexanuclear and Chain Copper(II) Complexes with Pyrazole Derivatives
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparations of the Complexes
2.2.1. {[Cu(4-Hmpz)4][Cu(4-Hmpz)2(µ-ox-κ2O1,O2:κO2′:κO1′)(ClO4)2]}n (1)
2.2.2. {[Cu(3,4,5-Htmpz)4]2[Cu(3,4,5-Htmpz)2(µ3-ox-κ2O1,O2:κO2′:κO1′)(H2O)(ClO4)]2 [Cu2(3,4,5-Htmpz)4(µ-ox-κO1,O2:κ2O2′,O1′)]}(ClO4)4·6H2O (2)
2.3. Physical Techniques
2.4. Crystallographic Data Collection and Refinement
3. Results and Discussion
3.1. Synthesis and General Characterization
3.2. Description of the Structures
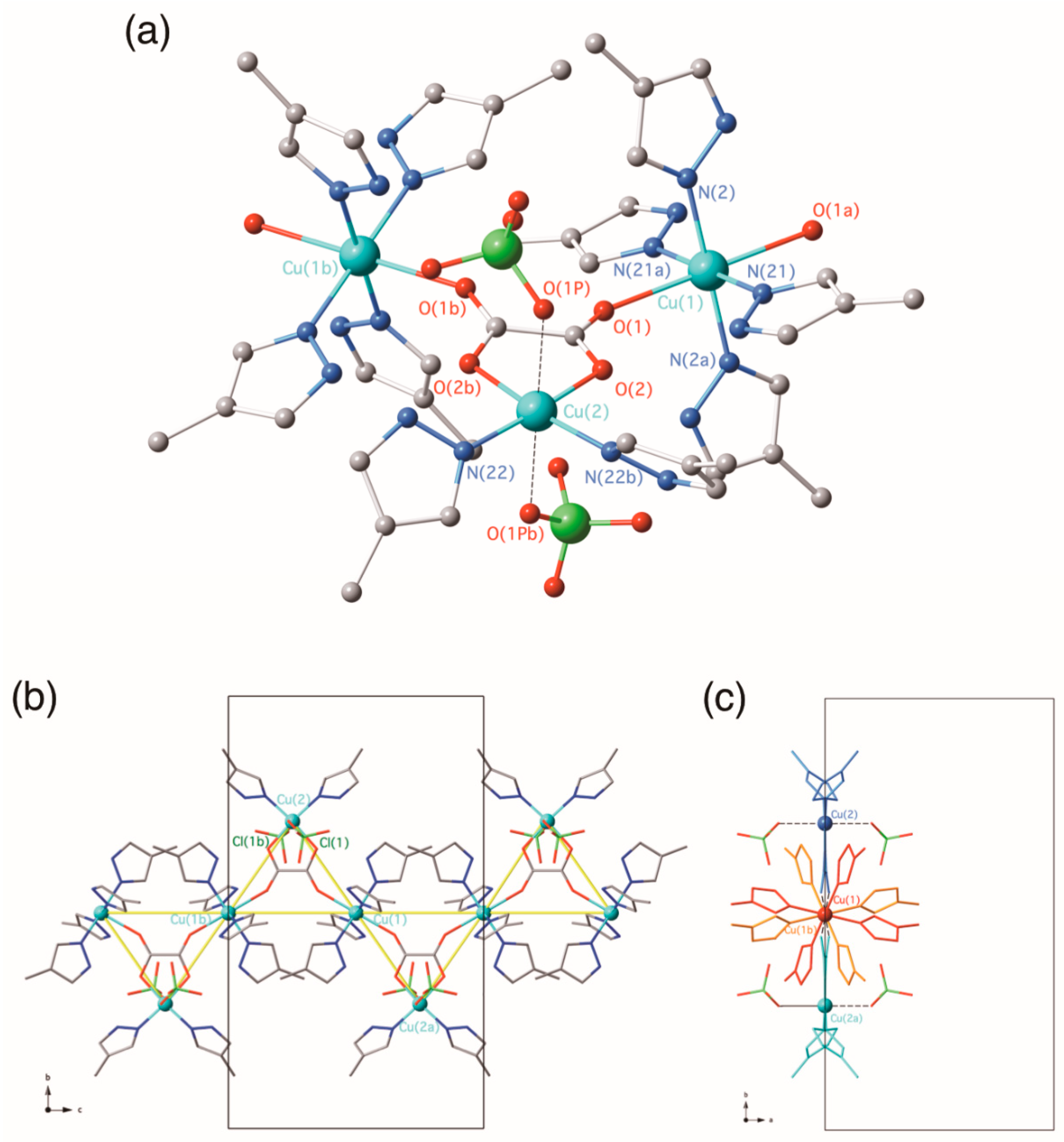
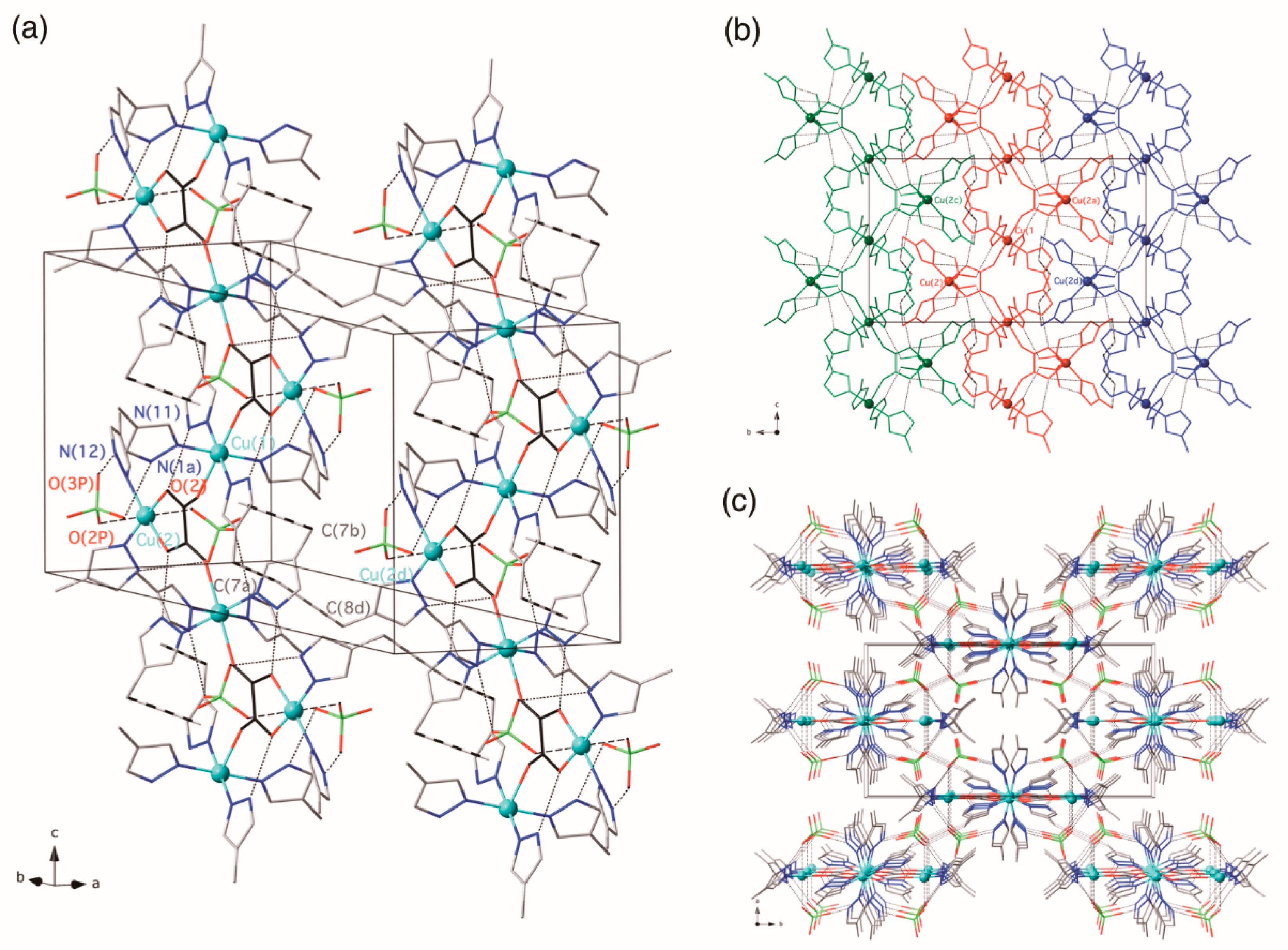
3.2.1. {[Cu(4-Hmpz)4][Cu(4-Hmpz)2(µ3-ox-κ2O1,O2:κO2′:κO1′)(ClO4)2]}n (1)
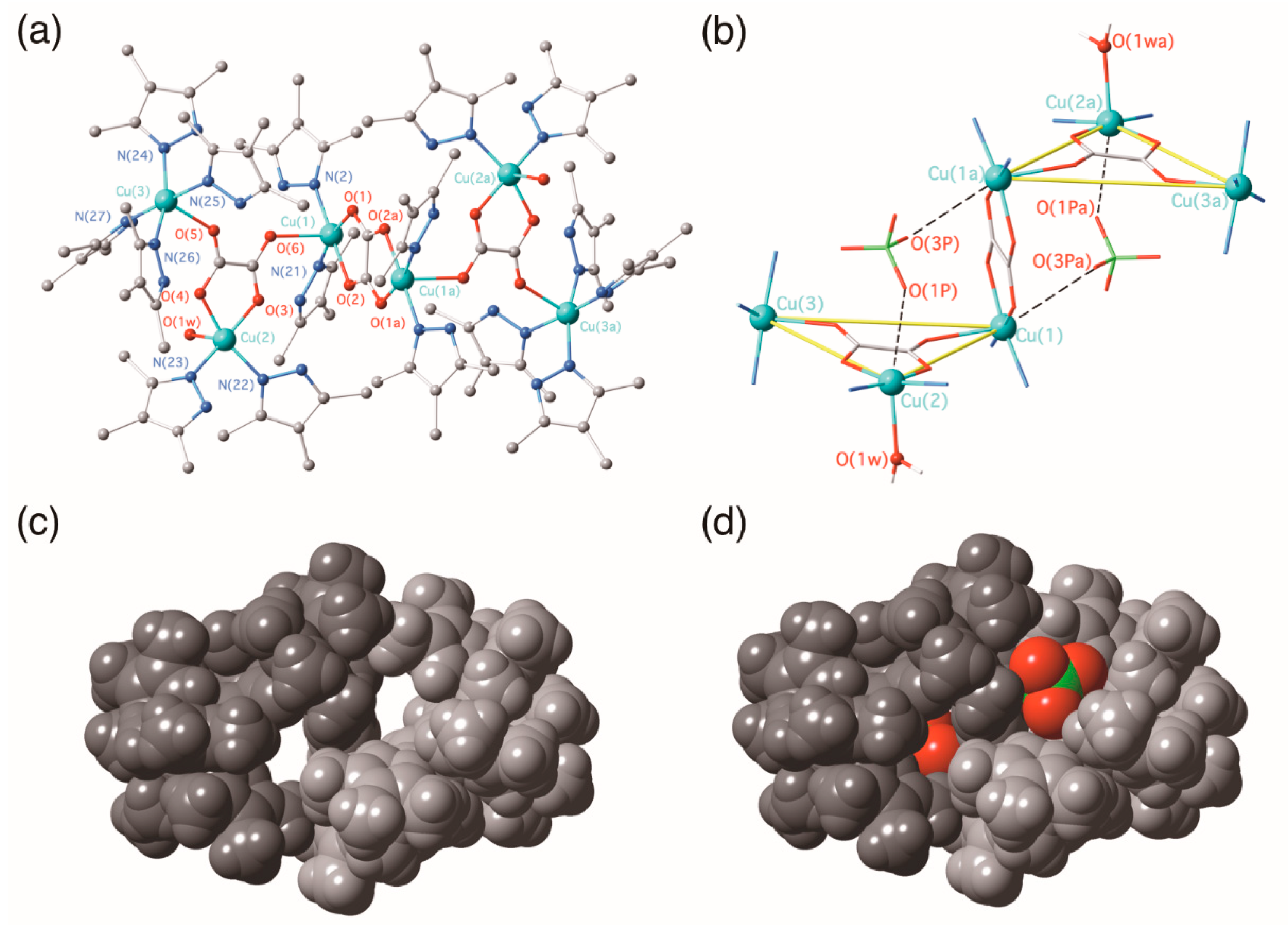
3.2.2. {[Cu(3,4,5-Htmpz)4]2[Cu(3,4,5-Htmpz)2(µ3-ox-κ2O1,O2:κO2′:κO1′)(H2O)(ClO4)]2 [Cu2(3,4,5-Htmpz)4(µ-ox-κO1,O2:κ2O2′,O1′)]}(ClO4)4·6H2O (2)
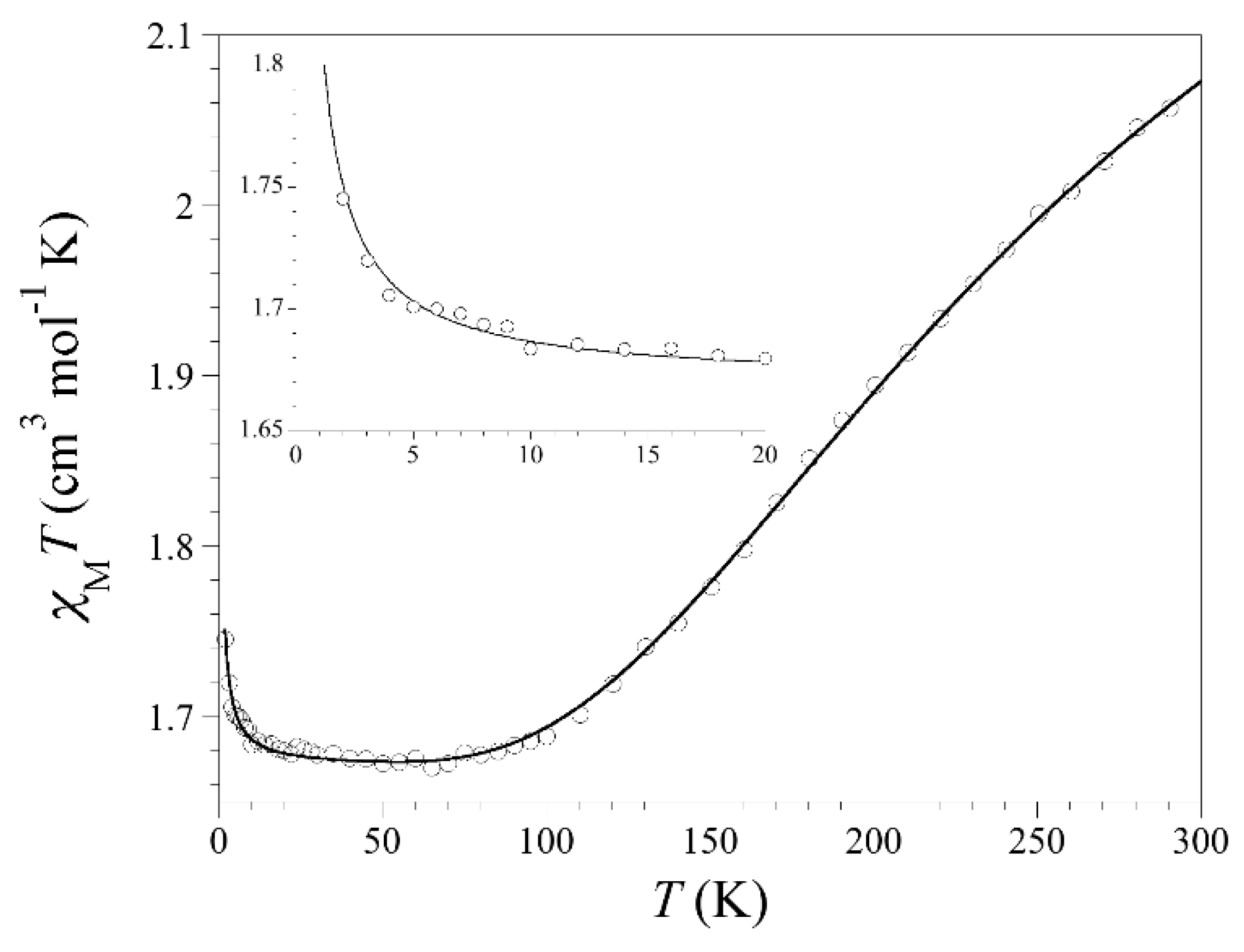
3.3. Magnetic Properties
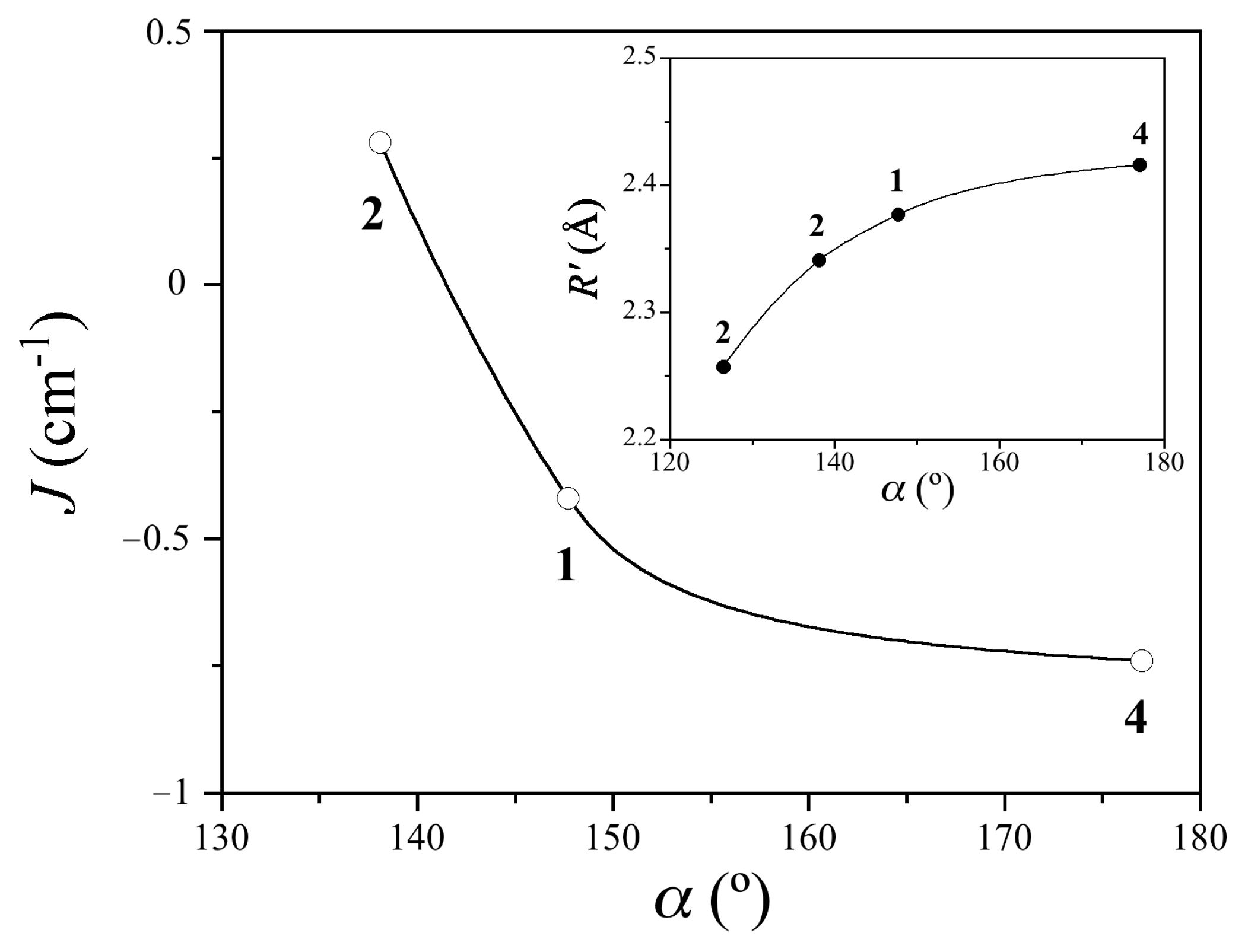
3.4. Magneto-Structural Correlations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Haiduc, I.; Tiekink, E.R.T. Inverse Coordination. A Novel Chemical Concept; Sunway University Press: Sunway City, Selangor, Malaysia, 2020. [Google Scholar]
- Haiduc, I. Inverse coordination—An emerging new chemical concept. Oxygen and other calchogens as coordination centers. Coord. Chem. Rev. 2017, 338, 1–26. [Google Scholar] [CrossRef]
- Haiduc, I. Inverse coordination—An emerging new chemical concept. II. Halogens as coordination centers. Coord. Chem. Rev. 2017, 348, 71–91. [Google Scholar] [CrossRef]
- Haiduc, I. Nitrogen centered inverse coordination complexes. A survey of molecular topologies. J. Coord. Chem. 2018, 71, 3139–3179. [Google Scholar] [CrossRef]
- Haiduc, I. Review. Inverse coordination. Inorganic open and cyclic nitrogen heteroatom molecules as coordination centers. A survey of molecular topologies. J. Coord. Chem. 2019, 72, 35–52. [Google Scholar] [CrossRef]
- Haiduc, I. Review. Inverse coordination. Organic nitrogen heterocycles as coordination centers. A survey of molecular topologies and systematization. Part 1. Five-membered and smaller rings. J. Coord. Chem. 2019, 72, 2127–2159. [Google Scholar] [CrossRef]
- Haiduc, I. Review. Inverse coordination. Organic nitrogen heterocycles as coordination centers. A survey of molecular topologies and systematization. Part 2. Six-membered rings. J. Coord. Chem. 2019, 72, 2805–2903. [Google Scholar] [CrossRef]
- Haiduc, I. Inverse coordination metal complexes with oxalate and sulfur, selenium and nitrogen analogues as coordination centers. Topology and systematization. J. Coord. Chem. 2020, 73, 1619–1700. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Brechin, E.K.; Aromí, G. Synthesis of 3d metallic single-molecule magnets. Struct. Bond. 2006, 122, 1–69. [Google Scholar]
- Pedersen, K.S.; Bendix, J.; Clérac, R. Single-molecule magnet engineering: Building-block approaches. Chem. Commun. 2014, 50, 4396–4415. [Google Scholar] [CrossRef] [Green Version]
- Milios, C.J.; Winpenny, R.E.P. Cluster-based single-molecule magnets. Struct. Bond. 2015, 164, 1–109. [Google Scholar]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical ligand-containing single-molecule magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef] [Green Version]
- Papatriantafyllopoulou, C.; Moushi, E.E.; Christou, G.; Tasiopoulos, A.J. Filling the gap between the quantum and classical worlds of nanoscale magnetism: Giant molecular aggregates based on paramagnetic 3d metal ion. Chem. Soc. Rev. 2016, 45, 1597–1628. [Google Scholar] [CrossRef]
- Schnack, J. Large magnetic molecules and what we learn from them. Contemp. Phys. 2019, 60, 127–144. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Kuzniak, E.; Liberka, M.; Chorazy, S.; Sieklucka, B.; Podgajny, R. Modular approach towards functional multimetallic coordination clusters. Coord. Chem. Rev. 2020, 419, 213394. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, H.; Nam, D.; Lah, M.S.; Choe, W. The rise of metal-organic polyhedra. Chem. Soc. Rev. 2021, 50, 528–555. [Google Scholar] [CrossRef]
- Linert, W.; Verdaguer, M. Molecular Magnets. Recent Highlights; Springer: Berlin, Germany, 2003. [Google Scholar]
- Bartolomé, J.; Luis, F.; Fernández, J.F. (Eds.) Molecular Magnets. Physics and Applications; Springer: Berlin, Germany, 2014. [Google Scholar]
- Miller, J.S. Magnetically ordered molecule-based materials. Chem. Soc. Rev. 2011, 40, 3266–3296. [Google Scholar] [CrossRef]
- Wan, X.-Y.; Avendaño, C.; Dunbar, K. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar]
- Coronado, E.; Mínguez-Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clérac, R. Single-chain magnets and related systems. Struct. Bond. 2015, 164, 143–184. [Google Scholar]
- Thorarinsdottir, A.E.; Harris, T.D. Metal-organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Timco, G.A.; Faust, T.B.; Tuna, F.; Winpenny, R.E.P. Linking heterometallic rings for quantum information processing and amusement. Chem. Soc. Rev. 2011, 40, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Timco, G.A.; McInnes, E.J.L.; Winpenny, R.E.P. Physical studies of heterometallic rings: An ideal system for studying magnetically-coupled systems. Chem. Soc. Rev. 2013, 42, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- McInnes, E.J.L.; Timco, G.A.; Whitehead, G.F.S.; Winpenny, R.E.P. Heterometallic rings: Their physics and use as supramolecular building blocks. Angew. Chem. Int. Ed. 2015, 54, 14244–14269. [Google Scholar] [CrossRef]
- Ferrando-Soria, J. Cr7Ni wheels: Supramolecular tectons for the physical implementation of quantum information processing. Magnetochemistry 2016, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-molecule magnets. MRS Bull. 2000, 25, 66–71. [Google Scholar] [CrossRef]
- Christou, G. Single-molecule magnets: A molecular approach to nanoscale magnetic materials. Polyhedron 2005, 24, 2065–2075. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Cornia, A. Single-molecule magnets based on iron(III) oxo clusters. Chem. Commun. 2010, 9, 725–732. [Google Scholar] [CrossRef]
- Beltran, L.M.C.; Long, J.R. Directed assembly of metal-cyanide cluster magnets. Acc. Chem. Res. 2005, 38, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, M.; Bleuzen, A.; Marvaud, V.; Vaissermann, J.; Seuleiman, M.; Desplanches, C.; Scuiller, A.; Train, C.; Garde, R.; Gelly, G.; et al. Molecules to build solids: High TC molecule-based magnets by design and recent revival of cyano complexes chemistry. Coord. Chem. Rev. 1999, 100–192, 1023–1047. [Google Scholar] [CrossRef]
- Lescouëzec, R.; Toma, L.M.; Vaissermann, J.; Verdaguer, M.; Delgado, F.S.; Ruiz-Pérez, C.; Lloret, F.; Julve, M. Design of single chain magnets through cyanide-bearing six-coordinate complexes. Coord. Chem. Rev. 2005, 495, 2691–2729. [Google Scholar] [CrossRef]
- Kaim, W.; Moscherosch, M. The coordination chemistry of TCNE, TCNQ and related polynitrile π acceptors. Coord. Chem. Rev. 1994, 129, 157–193. [Google Scholar] [CrossRef]
- Miller, J.S. Organic and molecule-based magnets. Mater. Today 2014, 17, 224–235. [Google Scholar] [CrossRef]
- Marinescu, G.; Andruh, M.; Lloret, F.; Julve, M. Bis(oxalato)chromium(III) complexes: Versatile tectons in designing heterometallic coordination compounds. Coord. Chem. Rev. 2011, 255, 161–185. [Google Scholar] [CrossRef]
- Gruselle, M.; Train, C.; Boubekeur, K.; Gredin, P.; Ovanesyan, N. Enantioselective self-assembly of chiral bimetallic oxalate-based networks. Coord. Chem. Rev. 2006, 250, 2491–2500. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Martí-Gastaldo, C.; Romero, F.M. Multifunctionality in hybrid materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Calatayud, M.L.; Yuste, C.; Castellano, M.; Ruiz-García, R.; Cano, J.; Faus, J.; Verdaguer, M.; Lloret, F. Dinuclear copper(II) complexes as testing ground for molecular magnetism theory. Polyhedron 2019, 348, 66–77. [Google Scholar] [CrossRef]
- Duggan, D.M.; Hendrickson, D.N. Magnetic exchange interactions in transition metal dimers. II. Copper and nickel di-μ-azido and μ-oxalato complexes. Inorg. Chem. 1973, 12, 2422–2431. [Google Scholar] [CrossRef]
- Hall, G.R.; Duggan, D.M.; Hendrickson, D.N. Magnetic exchange interactions in transition metal dimers. V. Copper(II)-diethylenetriamine complexes with oxalate, cyanate, thiocyanate, and azide inner- and outer-sphere bridging units. Electron paramagnetic resonance of alkali halide pelleted copper complexes. Inorg. Chem. 1975, 14, 1956–1964. [Google Scholar]
- Felthouse, T.R.; Laskowski, E.J.; Bieksza, D.S.; Hendrickson, D.N. Structural and magnetic properties of copper(II) dimers bridged by oxalate, azide, and cyanide ions; X-ray structures of [Cu2{EtN(CH2CH2NEt2)2}2(C2O4)][BPh4]2 and [Cu2{MeN(CH2CH2NMe2)2}2(N3)2][BPh4]2. The role of the transition-metal ion ground state in magnetic exchange interactions. J. Chem. Soc. Chem. Commun. 1976, 19, 777–778. [Google Scholar]
- Charlot, M.F.; Verdaguer, M.; Journaux, Y.; de Loth, P.; Daudey, J.P. Ab initio direct calculation of the singlet-triplet splitting in a μ-oxalato copper(II) binuclear complex. Inorg. Chem. 1984, 23, 3802–3808. [Google Scholar] [CrossRef]
- Julve, M.; Verdaguer, M.; Gleizes, A.; Philoche-Levisalles, M.; Kahn, O. Design of μ-oxalato copper(II) binuclear complexes exhibiting expected magnetic properties. Inorg. Chem. 1984, 23, 3808–3818. [Google Scholar] [CrossRef]
- Julve, M.; Faus, J.; Verdaguer, M.; Gleizes, A. Copper(II), a chemical Janus: Two different (oxalato)(bipyridyl)copper(II) complexes in one single crystal. Structure and magnetic properties. J. Am. Chem. Soc. 1984, 106, 8306–8308. [Google Scholar] [CrossRef]
- Alvarez, S.; Julve, M.; Verdaguer, M. Oxalato-bridged and related dinuclear copper(II) complexes: Theoretical analysis of their structures and magnetic coupling. Inorg. Chem. 1990, 29, 4500–4507. [Google Scholar] [CrossRef]
- Cano, J.; Alemany, P.; Alvarez, S.; Verdaguer, M.; Ruiz, E. Exchange coupling in oxalato-bridged copper(II) binuclear compounds: A density functional study. Chem. Eur. J. 1998, 4, 476–484. [Google Scholar] [CrossRef]
- Julve, M.; Gleizes, A.; Chamoreau, L.M.; Ruiz, E.; Verdaguer, M. Antiferromagnetic interactions in copper(II) μ-oxalato dinuclear complexes: The role of the counterion. Eur. J. Inorg. Chem. 2018, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, M.L.; Orts-Arroyo, M.; Julve, M.; Lloret, F.; Marino, N.; De Munno, G.; Ruiz-García, R.; Castro, I. Magneto-structural correlations in asymmetric oxalato-bridged dicopper(II) complexes with polymethyl-substituted pyrazole ligands. J. Coord. Chem. 2018, 71, 657–674. [Google Scholar] [CrossRef]
- Bruker. SAINT (Version 7.68A); Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker. SADABS (Version 2004/4); Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- CrystalMaker; CrystalMaker Software: Bicester, UK, 2015.
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; pp. 227 and 244. [Google Scholar]
- Rosenthal, M.R. The Myth of the Non-Coordinating Anion. J. Chem. Educ. 1973, 50, 331–335. [Google Scholar] [CrossRef]
- Buvailo, A.I.; Tomyn, S.V.; Haukka, M.; Pavlenko, V.A.; Fritsky, I.O. Aquabis(3,5-dimethyl-1H-pyrazole-κN)-(oxalato-κ2O,O’)copper(II). Acta Cryst. 2008, E64, m37–m38. [Google Scholar] [CrossRef] [Green Version]
- Switlicka-Olszewska, A.; Machura, B.; Mrozinski, J.; Kalinska, B.; Kruszynski, R.; Penkala, M. Effect of N-donor ancillary ligands on structural and magnetic properties of oxalate copper(II) complexes. New J. Chem. 2014, 38, 1611–1626. [Google Scholar] [CrossRef] [Green Version]
- Plasseraud, L.; Maid, H.; Hampel, F.; Saalfrank, R.W. A meso-helical coordination polymer from achiral dinuclear [Cu2(H3CCN)2(m-pydz)2][PF6]2 and 1,3-bis(diphenylphosphanyl)propane—Synthesis and crystal structure of {[Cu(m-pydz)2][PF6]} (pydz = pyridazine). Chem. Eur. J. 2001, 7, 4007–4011. [Google Scholar] [CrossRef]
- Pardo, E.; Bernot, K.; Lloret, F.; Julve, M.; Ruiz-García, R.; Pasán, J.; Ruiz-Pérez, C.; Cangussu, D.; Costa, V.; Lescouëzec, R.; et al. Solid-state anion-guest encapsulation by metallosupramoleculat capsules made from two tetranuclear copper(II) complexes. Eur. J. Inorg. Chem. 2007, 4569–4573. [Google Scholar] [CrossRef]
- Pardo, E.; Cangussu, D.; Lescouëzec, R.; Journaux, Y.; Pasán, J.; Delgado, F.S.; Ruiz-Pérez, C.; Ruiz-García, R.; Cano, J.; Julve, M.; et al. Molecular-programmed self-assembly of Homo- and Heterometallic tetranuclear coordination compounds: Synthesis, crystal structures and Magnetic properties of rack-type CuII2MII2 complexes (M = Cu and Ni) with tetranucleating phenylenedioxamato bridging ligands. Inorg. Chem. 2009, 48, 4661–4673. [Google Scholar]
- Bonner, J.C.; Fisher, M.E. Linear magnetic chains with anisotropic coupling. Phys. Rev. A 1964, 135, 640–658. [Google Scholar] [CrossRef] [Green Version]
- Bleaney, B.; Bowers, K.D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond. A 1952, 214, 451–465. [Google Scholar]
- Cano, J. VPMAG; University of Valencia: Valencia, Spain, 2001. [Google Scholar]
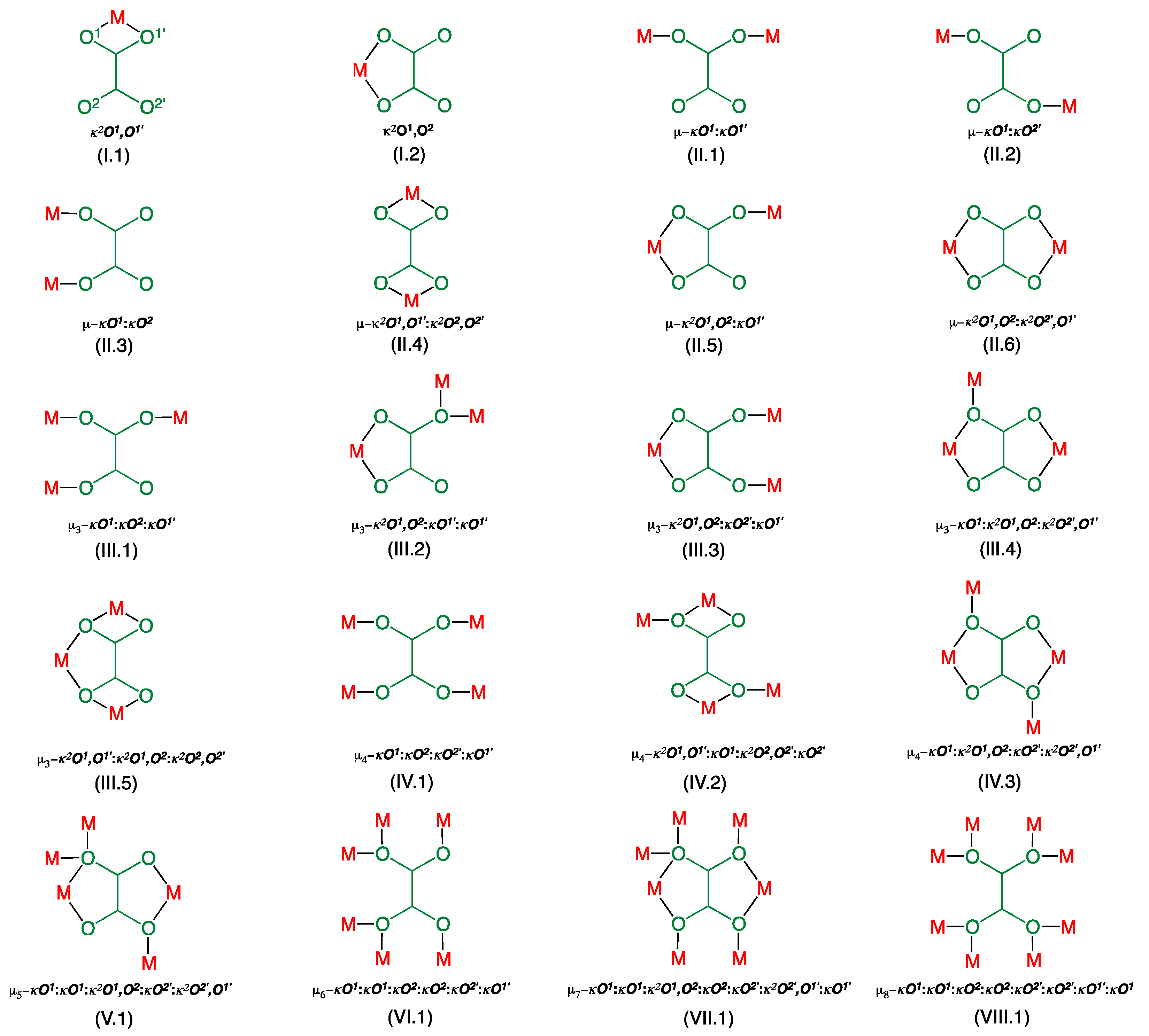





| Compound | 1 | 2 |
|---|---|---|
| CCDC number | 2076622 | 2076621 |
| Formula | C26H36Cl2Cu2N12O12 | C102H176Cl6Cu6N32O44 |
| Fw | 906.65 | 3148.68 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P21/c |
| a/Ǻ | 12.3020(3) | 15.3910(14) |
| b/Ǻ | 23.0520(6) | 30.496(3) |
| c/Ǻ | 13.6840(2) | 17.1782(17) |
| α/° | 90 | 90 |
| β/° | 95.1130(13) | 113.241(4) |
| γ/° | 90 | 90 |
| V/Ǻ3 | 3865.15(15) | 7408.6(12) |
| Z | 4 | 2 |
| Dc/g cm−3 | 1.558 | 1.411 |
| T/K | 293(2) | 293(2) |
| μ/mm−1 | 1.310 | 1.039 |
| F(000) | 1856 | 3280 |
| Refl. Collected | 8741 | 116,008 |
| Refl. indep. [Rint] | 4430 [0.0215] | 15,028 [0.0789] |
| Refl. obs. [I > 2σ(I)] | 10,171 | 10,171 |
| Goodness-of-fit on F2 | 1.059 | 1.031 |
| R1a [I > 2σ(I)] (all) | 0.0360 (0.0523) | 0.0489 (0.0827) |
| wR2b [I > 2σ(I)] (all) | 0.0978 (0.1031) | 0.1246 (0.1432) |
| Δρmax, min/e Å−3 | 0.485 and −0.433 | 0.653 and −0.566 |
| Compound a | Coordination Mode | Rb/Å | R’c/Å | ∆Rd/Å | ϕe/° | ψf/° | αg/° | β/°h | ri/Å | Jj/cm−1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | µ3-κ2O1,O2:κO2′:κO1′ | 1.959 | 2.377 | 0.418 | 6.09 | 85.7 | 147.7 | 21.9 | 5.949 | −0.42 | This work |
| 2 | µ3-κ2O1,O2:κO2′:κO1 µ-κ2O1,O2:κ2O2′,O1′ | 1.948 1.994 1.994 2.016 | 2.257 2.341 | 0.309 0.347 | 13.9 5.4 | 77.5 87.8 0 | 126.5 138.1 | 19.0 17.7 | 5.418 5.703 5.224 | n.a. +0.28 −348 | This work |
| 4 | µ-κ2O1,O2:κO1 | 1.950 | 2.416 | 0.466 | 5.8 | 81.5 | 177.0 | 20.2 | 6.300 | −0.74 | [59] |
| 5a | µ-κ2O1,O2:κ2O2′,O1′ | 1.976 | 2.122 | 0.146 | 114.9 | 2.0 | −129 | [50] | |||
| 5b | µ-κ2O1,O2:κ2O2′,O1′ | 1.973 | 2.110 | 0.137 | 114.4 | 2.5 | −161 | [50] | |||
| 6 | µ4-κO1:κ2O1,O2:κO2′:κ2O2′,O1′ | 1.959 | 2.340 | 0.381 | 86.0 | 0 | 126.4 | 19.3 | 4.237 5.553 | 0 0 | [59] |
| µ-κ2O1,O2:κ2O2′,O1′ | 1.984 1.985 | 2.8 | 0 | 5.176 | −312 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, I.; Calatayud, M.L.; Orts-Arroyo, M.; Moliner, N.; Marino, N.; Lloret, F.; Ruiz-García, R.; Munno, G.D.; Julve, M. Ferro- and Antiferromagnetic Interactions in Oxalato-Centered Inverse Hexanuclear and Chain Copper(II) Complexes with Pyrazole Derivatives. Molecules 2021, 26, 2792. https://doi.org/10.3390/molecules26092792
Castro I, Calatayud ML, Orts-Arroyo M, Moliner N, Marino N, Lloret F, Ruiz-García R, Munno GD, Julve M. Ferro- and Antiferromagnetic Interactions in Oxalato-Centered Inverse Hexanuclear and Chain Copper(II) Complexes with Pyrazole Derivatives. Molecules. 2021; 26(9):2792. https://doi.org/10.3390/molecules26092792
Chicago/Turabian StyleCastro, Isabel, M. Luisa Calatayud, Marta Orts-Arroyo, Nicolás Moliner, Nadia Marino, Francesc Lloret, Rafael Ruiz-García, Giovanni De Munno, and Miguel Julve. 2021. "Ferro- and Antiferromagnetic Interactions in Oxalato-Centered Inverse Hexanuclear and Chain Copper(II) Complexes with Pyrazole Derivatives" Molecules 26, no. 9: 2792. https://doi.org/10.3390/molecules26092792






