Comparative Chiral Separation of Thalidomide Class of Drugs Using Polysaccharide-Type Stationary Phases with Emphasis on Elution Order and Hysteresis in Polar Organic Mode
Abstract
:1. Introduction
2. Results
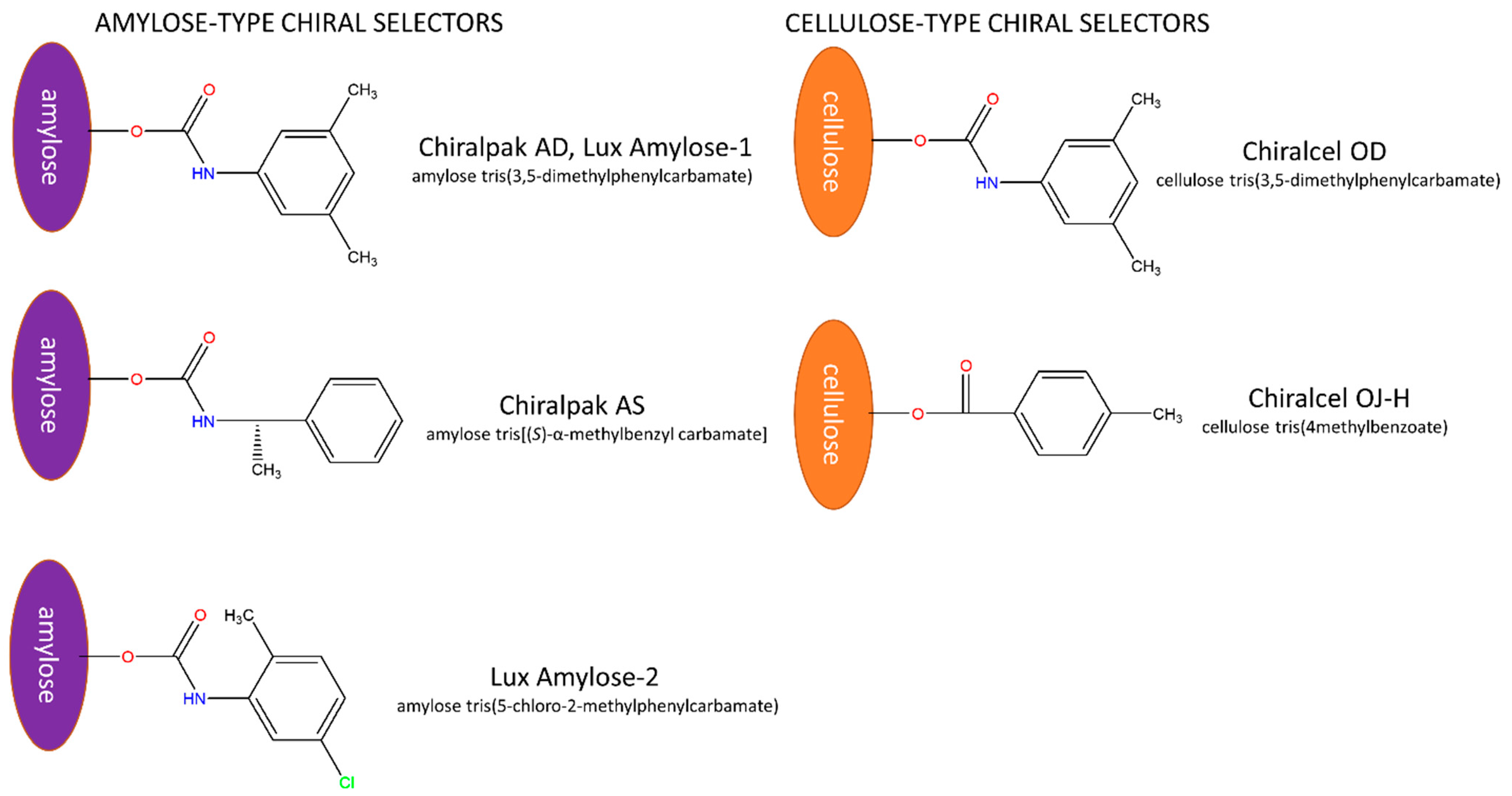
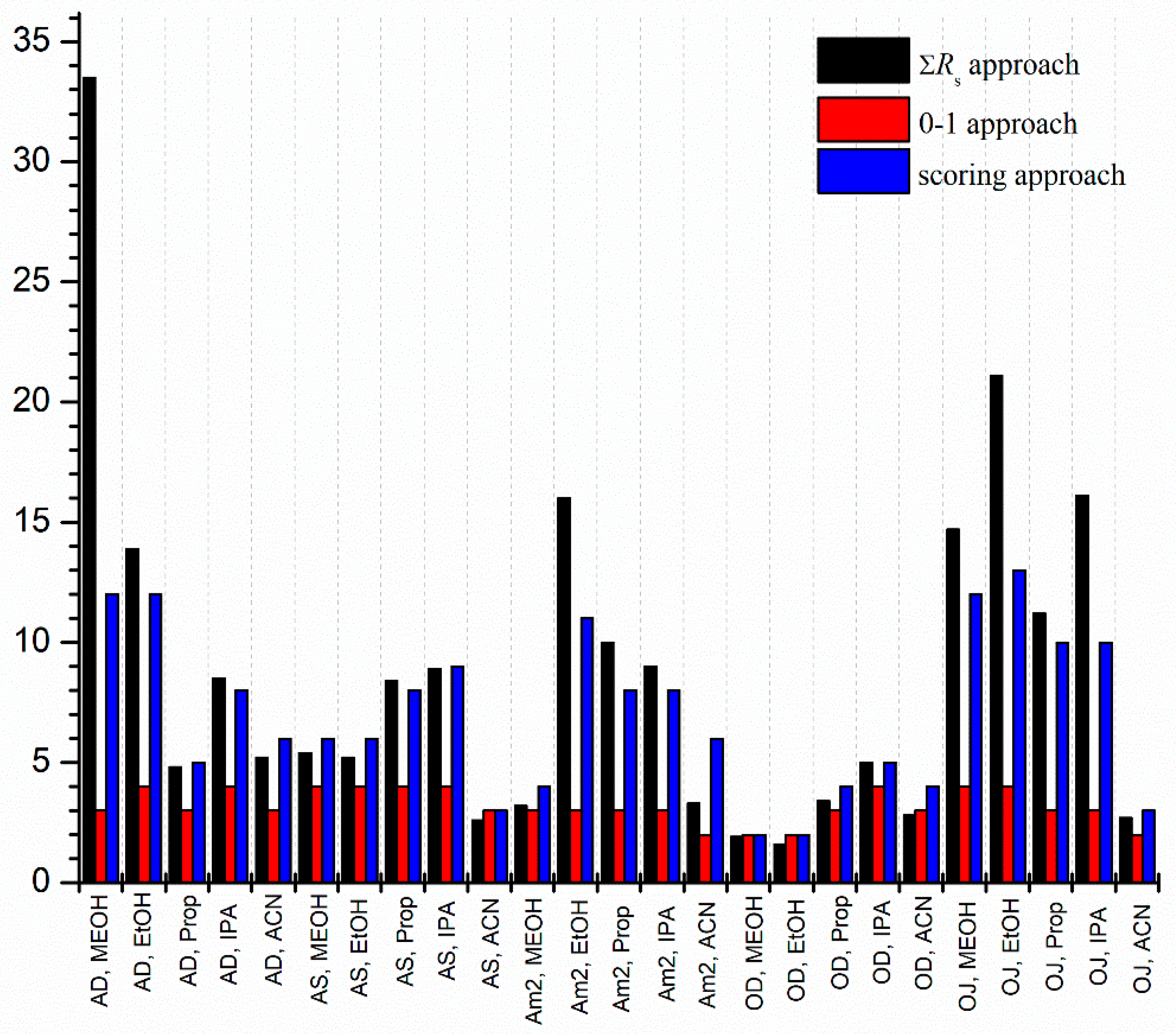
2.1. General Characterization of Enantioseparations
2.2. Enantiomer Elution Order
2.2.1. The Effect of the Type of Chiral Selector
2.2.2. Mobile Phase-Dependent Reversal of Elution Order
- Change from alcohol type modifier to ACN
- Change from MeOH or EtOH to PROP or IPA
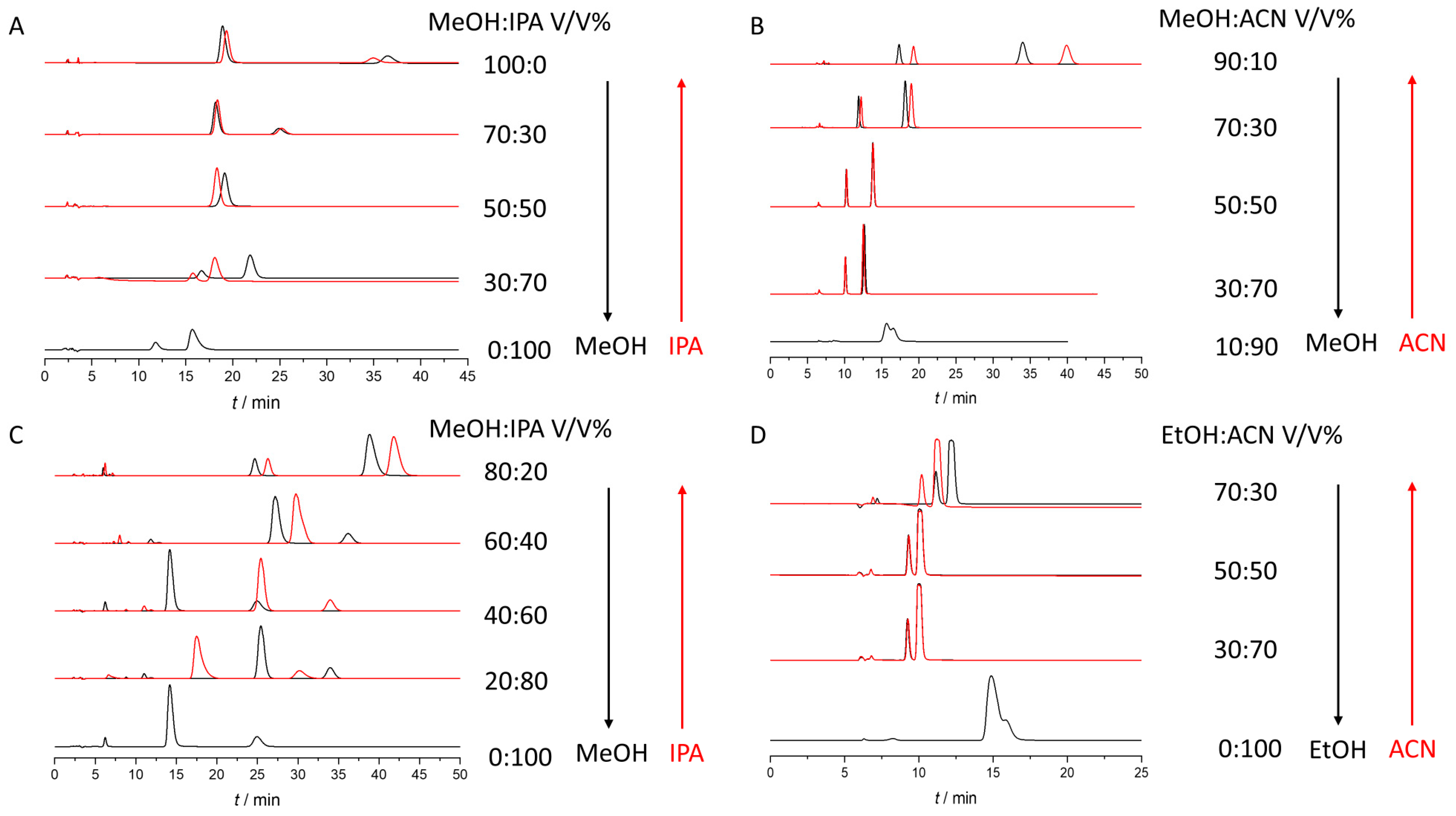
2.3. Enantioseparation of Mobile Phase Mixtures. Hysteresis of Retention and Selectivity
- Combination of MeOH/IPA applied on Chiralpak AD for THAL and POM
- Combination of MeOH/PROP applied on Chiralpak AD for THAL
- Combination of MeOH/ACN and EtOH/ACN applied on Chiralpak AD for THAL
- Combination of MeOH/IPA applied on Chiralcel OD for POM
- Combination of MeOH/IPA applied on Lux Amylose-2 for POM
3. Materials and Methods
3.1. Materials
3.2. LC-UV Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, J.; Okamoto, Y. Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers. Chem. Rev. 2015, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K. Chiral recognition in separation sciences. Part I: Polysaccharide and cyclodextrin selectors. TrAC Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Bajtai, A.; Ilisz, I.; Berkecz, R.; Fülöp, F.; Lindner, W.; Péter, A. Polysaccharide-based chiral stationary phases as efficient tools for diastereo- and enantioseparation of natural and synthetic Cinchona alkaloid analogs. J. Pharm. Biomed. Anal. 2021, 193, 113724. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B. Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. TrAC Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Dascalu, A.-E.; Ghinet, A.; Chankvetadze, B.; Lipka, E. Comparison of dimethylated and methylchlorinated amylose stationary phases, coated and covalently immobilized on silica, for the separation of some chiral compounds in supercritical fluid chromatography. J. Chromatogr. A 2020, 1621, 461053. [Google Scholar] [CrossRef]
- Gumustas, M.; Ozkan, S.A.; Chankvetadze, B. Separation and elution order of the enantiomers of some β-agonists using polysaccharide-based chiral columns and normal phase eluents by high-performance liquid chromatography. J. Chromatogr. A 2016, 1467, 297–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Nguyen, D.; Franco, P. Reversed-phase screening strategies for liquid chromatography on polysaccharide-derived chiral stationary phases. J. Chromatogr. A 2010, 1217, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, N.; Maftouh, M.; Heyden, Y.V. Screening approach for chiral separation of pharmaceuticals: IV. Polar organic solvent chromatography. J. Chromatogr. A 2006, 1111, 48–61. [Google Scholar] [CrossRef]
- Chankvetadze, B. Recent developments on polysaccharide-based chiral stationary phases for liquid-phase separation of en-antiomers. J. Chromatogr. A 2012, 1269, 26–51. [Google Scholar] [CrossRef]
- Ates, H.; Younes, A.A.; Mangelings, D.; Heyden, Y.V. Enantioselectivity of polysaccharide-based chiral selectors in polar organic solvents chromatography: Implementation of chlorinated selectors in a separation strategy. J. Pharm. Biomed. Anal. 2013, 74, 1–13. [Google Scholar] [CrossRef]
- Ferencz, E.; Kovács, B.; Boda, F.; Foroughbakhshfasaei, M.; Kelemen, K.; Tóth, G.; Szabó, Z.-I. Simultaneous determination of chiral and achiral impurities of ivabradine on a cellulose tris(3-chloro-4-methylphenylcarbamate) chiral column using polar organic mode. J. Pharm. Biomed. Anal. 2020, 177, 112851. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.-I.; Foroughbakhshfasaei, M.; Gál, R.; Horváth, P.; Komjáti, B.; Noszál, B.; Tóth, G. Chiral separation of lenalidomide by liquid chromatography on polysaccharide-type stationary phases and by capillary electrophoresis using cyclodextrin selectors. J. Sep. Sci. 2018, 41, 1414–1423. [Google Scholar] [CrossRef]
- Jibuti, G.; Mskhiladze, A.; Takaishvili, N.; Karchkhadze, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. HPLC separation of dihydropyridine derivatives enantiomers with emphasis on elution order using polysaccharide-based chiral columns. J. Sep. Sci. 2012, 35, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Mosiashvili, L.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. On the effect of basic and acidic additives on the separation of the enantiomers of some basic drugs with polysaccharide-based chiral selectors and polar organic mobile phases. J. Chromatogr. A 2013, 1317, 167–174. [Google Scholar] [CrossRef]
- Mskhiladze, A.; Karchkhadze, M.; Dadianidze, A.; Fanali, S.; Farkas, T.; Chankvetadze, B. Enantioseparation of Chiral Antimycotic Drugs by HPLC with Polysaccharide-Based Chiral Columns and Polar Organic Mobile Phases with Emphasis on Enantiomer Elution Order. Chromatographia 2013, 76, 1449–1458. [Google Scholar] [CrossRef]
- Gegenava, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. Enantioseparation of selected chiral sulfoxides in high-performance liquid chromatography with polysaccharide-based chiral selectors in polar organic mobile phases with emphasis on enantiomer elution order. J. Sep. Sci. 2014, 37, 1083–1088. [Google Scholar] [CrossRef]
- Matarashvili, I.; Shvangiradze, I.; Chankvetadze, L.; Sidamonidze, S.; Takaishvili, N.; Farkas, T.; Chankvetadze, B. High-performance liquid chromatographic separations of stereoisomers of chiral basic agrochemicals with polysaccharide-based chiral columns and polar organic mobile phases. J. Sep. Sci. 2015, 38, 4173–4179. [Google Scholar] [CrossRef]
- Matarashvili, I.; Chankvetadze, L.; Tsintsadze, T.; Farkas, T.; Chankvetadze, B. HPLC Separation of Enantiomers of Some Chiral Carboxylic Acid Derivatives Using Polysaccharide-Based Chiral Columns and Polar Organic Mobile Phases. Chromatographia 2015, 78, 473–479. [Google Scholar] [CrossRef]
- Gallinella, B.; Ferretti, R.; Zanitti, L.; Sestili, I.; Mosca, A.; Cirilli, R. Comparison of reversed-phase enantioselective HPLC methods for determining the enantiomeric purity of (S)-omeprazole in the presence of its related substances. J. Pharm. Anal. 2016, 6, 132–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabó, Z.-I.; Foroughbakhshfasaei, M.; Noszál, B.; Tóth, G. Enantioseparation of racecadotril using polysaccharide-type chiral stationary phases in polar organic mode. Chirality 2018, 30, 95–105. [Google Scholar] [CrossRef]
- Horváth, S.; Németh, G. Hysteresis of retention and enantioselectivity on amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phases in mixtures of 2-propanol and methanol. J. Chromatogr. A 2018, 1568, 149–159. [Google Scholar] [CrossRef]
- Horváth, S.; Eke, Z.; Németh, G. Utilization of the hysteresis phenomenon for chiral high-performance liquid chromatographic method selection in polar organic mode. J. Chromatogr. A 2020, 1625, 461280. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.B.; Dredge, K.; Dalgleish, A.G. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat. Rev. Cancer 2004, 4, 314–322. [Google Scholar] [CrossRef]
- Man, H.-W.; Schafer, P.; Wong, L.M.; Patterson, R.T.; Corral, L.G.; Raymon, H.; Blease, K.; Leisten, J.; Shirley, M.A.; Tang, Y.; et al. Discovery of (S)-N-{2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl}acetamide (Apremilast), a Potent and Orally Active Phosphodiesterase 4 and Tumor Necrosis Factor-α Inhibitor. J. Med. Chem. 2009, 52, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Jacques, V.; Czarnik, A.W.; Judge, T.M.; Van der Ploeg, L.H.T.; DeWitt, S.H. Differentiation of antiinflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs. Proc. Natl. Acad. Sci. USA 2015, 112, E1471–E1479. [Google Scholar] [CrossRef] [Green Version]
- Szabó, Z.-I.; Mohammadhassan, F.; Szőcs, L.; Nagy, J.; Komjáti, B.; Noszál, B.; Tóth, G. Stereoselective interactions and liquid chromatographic enantioseparation of thalidomide on cyclodextrin-bonded stationary phases. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 227–236. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Szőcs, L.; Muntean, D.L.; NoszáL, B.; Tóth, G. Chiral Separation of uncharged pomalidomide enantiomers using carboxymethyl-cyclodetrin: A validated capillary electrophoretic method. Chirality 2016, 28, 199–203. [Google Scholar] [CrossRef]
- Szabó, Z.-I.; Szőcs, L.; Horváth, P.; Komjáti, B.; Nagy, J.; Jánoska, A.; Muntean, D.-L.; Noszál, B.; Tóth, G. Liquid chromatography with mass spectrometry enantioseparation of pomalidomide on cyclodextrin-bonded chiral stationary phases and the elucidation of the chiral recognition mechanisms by NMR spectroscopy and molecular modeling. J. Sep. Sci. 2016, 39, 2941–2949. [Google Scholar] [CrossRef] [PubMed]
- Foroughbakhshfasaei, M.; Szabó, Z.-I.; Tóth, G. Validated LC Method for Determination of Enantiomeric Purity of Apremilast Using Polysaccharide-Type Stationary Phases in Polar Organic Mode. Chromatographia 2018, 81, 1613–1621. [Google Scholar] [CrossRef]
- Wenslow, R.M.; Wang, T. Solid-State NMR Characterization of Amylose Tris(3,5-dimethylphenylcarbamate) Chiral Stationary-Phase Structure as a Function of Mobile-Phase Composition. Anal. Chem. 2001, 73, 4190–4195. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M. Reversal of elution order during the chiral separation in high performance liquid chromatography. J. Pharm. Biomed. Anal. 2001, 27, 401–407. [Google Scholar] [CrossRef]
- Chankvetadze, L.; Ghibradze, N.; Karchkhadze, M.; Peng, L.; Farkas, T. Enantiomer elution order reversal of fluorenylmethoxycarbonyl-isoleucine in high-performance liquid chromatography by changing the mobile phase temperature and composition. J. Chromatogr. A 2011, 1218, 6554–6560. [Google Scholar] [CrossRef] [PubMed]
- Tok, K.C.; Gumustas, M.; Jibuti, G.; Suzen, H.S.; Ozkan, S.A.; Chankvetadze, B. The Effect of Enantiomer Elution Order on the Determination of Minor Enantiomeric Impurity in Ketoprofen and Enantiomeric Purity Evaluation of Commercially Available Dexketoprofen Formulations. Molecules 2020, 25, 5865. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.I.; Suhail, M.; Asnin, L.; Aboul-Enein, H.Y. Effect of Various Parameters and Mechanism of Reversal Order of Elution in Chiral HPLC. Curr. Anal. Chem. 2020, 16, 59–78. [Google Scholar] [CrossRef]
- Matarashvili, I.; Kobidze, G.; Chelidze, A.; Dolidze, G.; Beridze, N.; Jibuti, G.; Farkas, T.; Chankvetadze, B. The effect of temperature on the separation of enantiomers with coated and covalently immobilized polysaccharide-based chiral stationary phases. J. Chromatogr. A 2019, 1599, 172–179. [Google Scholar] [CrossRef]
- Kasat, R.B.; Wang, N.-H.L.; Franses, E.I. Effects of Backbone and Side Chain on the Molecular Environments of Chiral Cavities in Polysaccharide-Based Biopolymers. Biomacromolecules 2007, 8, 1676–1685. [Google Scholar] [CrossRef]
- Pierini, M.; Carradori, S.; Menta, S.; Secci, D.; Cirilli, R. 3-(Phenyl-4-oxy)-5-phenyl-4,5-dihydro-(1 H )-pyrazole: A fascinating molecular framework to study the enantioseparation ability of the amylose (3,5-dimethylphenylcarbamate) chiral stationary phase. Part II. Solvophobic effects in enantiorecognition process. J. Chromatogr. A 2017, 1499, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Köteles, I.; Foroughbakhshfasaei, M.; Dobó, M.; Ádám, M.; Boldizsár, I.; Szabó, Z.-I.; Tóth, G. Determination of the Enantiomeric Purity of Solriamfetol by High-Performance Liquid Chromatography in Polar Organic Mode Using Polysaccharide-Type Chiral Stationary Phases. Chromatographia 2020, 83, 909–913. [Google Scholar] [CrossRef]
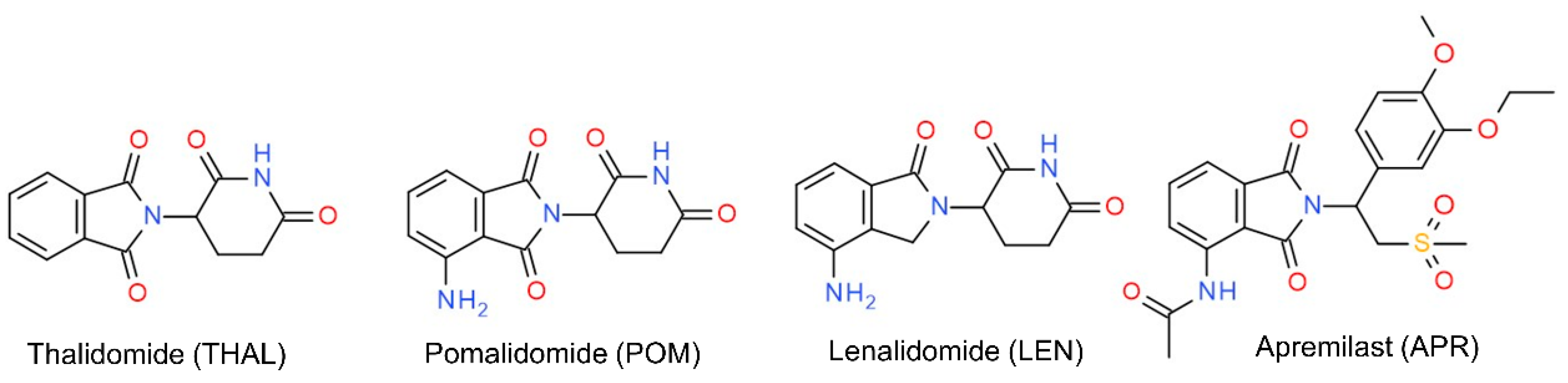


| Thalidomide | Pomalidomide | Lenalidomide | Apremilast | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Column | Mobile Phase | t1 | t2 | Rs | EEO | t1 | t2 | Rs | EEO | t1 | t2 | Rs | EEO | t1 | t2 | Rs | EEO |
| Chiralpak AD | MeOH | 29.8 | 76.4 | 14.4 | S < R | 19.8 | 37.2 | 12.8 | S < R | 4.9 | 5.5 | 6.3 | S < R | 26.1 | - | - | - |
| EtOH | 49.8 | 64.2 | 4.4 | S < R | 28.2 | 39.0 | 5.1 | S < R | 22.8 | 26.9 | 3.2 | S < R | 22.7 | 25.0 | 1.2 | R < S | |
| PROP | 39.8 | 41.9 | 1.1 | R < S | 25.2 | 27.2 | 1.2 | R < S | 19.2 | 23.2 | 2.5 | S < R | 15.9 | - | - | - | |
| IPA | 14.7 | 25.5 | 4.8 | R < S | 14.4 | 16.3 | 1.9 | R < S | 10.9 | 12.8 | 1.4 | S < R | 16.2 | 17.1 | 0.8 | R < S | |
| ACN | 14.5 | 16.1 | 1.7 | R < S | 25.2 | - | - | - | 11 | 12.2 | 1.7 | S < R | 6.4 | 8.0 | 1.8 | R < S | |
| Chiralpak AS | MeOH | 8.9 | 9.2 | 0.8 | R < S | 9.0 | 9.3 | 0.9 | R < S | 7.4 | 7.6 | 0.3 | R < S | 15.9 | 23.2 | 3.4 | R < S |
| EtOH | 10.9 | 12.3 | 1.4 | R < S | 11.6 | 12.2 | 0.9 | R < S | 8.2 | 8.6 | 0.3 | R < S | 29.5 | 35.0 | 2.6 | R < S | |
| PROP | 17.7 | 19.4 | 1.2 | R < S | 17.0 | 19.3 | 1.5 | R < S | 10.7 | 11.7 | 1.1 | R < S | 28.1 | 42.1 | 4.6 | R < S | |
| IPA | 21.1 | 23.7 | 1.5 | R < S | 19.7 | 23.4 | 2.1 | R < S | 15.3 | 17.7 | 1.1 | R < S | 20.1 | 38.3 | 4.2 | R < S | |
| ACN | 6.4 | 6.7 | 0.6 | R < S | 6.9 | 7.3 | 0.8 | R < S | 5.5 | 5.8 | 1.2 | R < S | 6.6 | - | - | - | |
| Lux Amylose-2 | MeOH | 8.1 | 8.3 | 0.6 | R < S | 7.3 | 7.8 | 1.1 | R < S | 5.1 | 5.6 | 1.5 | R < S | 11.6 | - | - | - |
| EtOH | 16.2 | 27.4 | 8.2 | R < S | 10.7 | 14.5 | 4.5 | R < S | 6.9 | 8.7 | 3.3 | R < S | 13.9 | - | - | - | |
| PROP | 18.9 | 25.8 | 5.3 | R < S | 12.8 | 16.5 | 3.4 | R < S | 8.4 | 9.6 | 1.3 | R < S | 21.2 | - | - | - | |
| IPA | 19.6 | 28.7 | 4.5 | R < S | 16.8 | 24.6 | 3.1 | R < S | 9.1 | 10.4 | 1.4 | R < S | 28.7 | - | - | - | |
| ACN | 5.3 | 5.6 | 1.6 | R < S | 4.3 | 5.2 | 1.7 | R < S | 5.3 | - | - | - | 4.28 | - | - | - | |
| Chiralcel OD | MeOH | 11.4 | 11.8 | 0.8 | R < S | 9.7 | 12.3 | 3.4 | R < S | 9.3 | - | - | - | 17.3 | - | - | - |
| EtOH | 18.9 | 19.6 | 0.8 | R < S | 10.7 | 11.6 | 0.8 | R < S | 10.4 | - | - | - | 24.9 | - | - | - | |
| PROP | 14.2 | - | - | - | 16.8 | 18.2 | 1.0 | R < S | 16.2 | 17.2 | 0.6 | R < S | 24.1 | 28.0 | 1.8 | S < R | |
| IPA | 17.3 | 18.7 | 1.2 | R < S | 17.4 | 24.7 | 2.3 | R < S | 18.1 | 22.1 | 0.9 | R < S | 52.6 | 61.4 | 1.2 | S < R | |
| ACN | 8.2 | 8.6 | 0.7 | R < S | 8.1 | 8.7 | 1.6 | R < S | 9.2 | 10.1 | 0.5 | S < R | 7.4 | - | - | - | |
| Chiralcel OJ-H | MeOH | 11.1 | 14.3 | 7.2 | R < S | 13.1 | 14.5 | 2.9 | R < S | 7.6 | 8.0 | 3.1 | R < S | 41.2 | 46.0 | 1.5 | S < R |
| EtOH | 16.0 | 26.6 | 10.7 | R < S | 17.2 | 22.7 | 5.5 | R < S | 10.3 | 13.2 | 4.3 | R < S | 55.0 | 59.3 | 0.6 | S < R | |
| PROP | 14.7 | 19.8 | 4.7 | R < S | 25.9 | 32.5 | 4.3 | R < S | 10.3 | 15.2 | 2.2 | R < S | 6.8 | - | - | - | |
| IPA | 20.7 | 36.7 | 9.7 | R < S | 20.2 | 26.5 | 3.9 | R < S | 11.6 | 16.0 | 2.5 | R < S | 51.5 | - | - | - | |
| ACN | 7.4 | 7.6 | 0.5 | R < S | 7.0 | 7.7 | 2.2 | R < S | 7.3 | - | - | - | 59.2 | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroughbakhshfasaei, M.; Dobó, M.; Boda, F.; Szabó, Z.-I.; Tóth, G. Comparative Chiral Separation of Thalidomide Class of Drugs Using Polysaccharide-Type Stationary Phases with Emphasis on Elution Order and Hysteresis in Polar Organic Mode. Molecules 2022, 27, 111. https://doi.org/10.3390/molecules27010111
Foroughbakhshfasaei M, Dobó M, Boda F, Szabó Z-I, Tóth G. Comparative Chiral Separation of Thalidomide Class of Drugs Using Polysaccharide-Type Stationary Phases with Emphasis on Elution Order and Hysteresis in Polar Organic Mode. Molecules. 2022; 27(1):111. https://doi.org/10.3390/molecules27010111
Chicago/Turabian StyleForoughbakhshfasaei, Mohammadhassan, Máté Dobó, Francisc Boda, Zoltán-István Szabó, and Gergő Tóth. 2022. "Comparative Chiral Separation of Thalidomide Class of Drugs Using Polysaccharide-Type Stationary Phases with Emphasis on Elution Order and Hysteresis in Polar Organic Mode" Molecules 27, no. 1: 111. https://doi.org/10.3390/molecules27010111






