Pyrene-Based Fluorescent Porous Organic Polymers for Recognition and Detection of Pesticides
Abstract
:1. Introduction
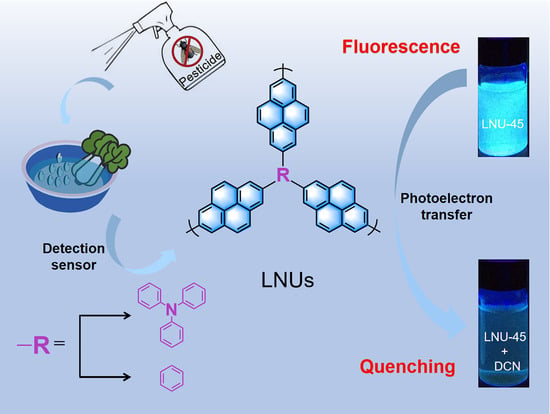
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of LNU-45 and LNU-47
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Di, L.; Xia, Z.Q.; Li, J. Selective sensing and visualization of pesticides by ABW-type metal-organic framework based luminescent sensors. RSC Adv. 2019, 9, 38469–38476. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.X.; Sui, Z.Y.; Chang, Z.S.; Wang, S.L.; Liang, Y.; Liu, X.; Feng, L.J.; Chen, Q.; Wang, N. A trifluoromethyl-grafted ultra-stable fluorescent covalent organic framework for adsorption and detection of pesticides. J. Mater. Chem. A 2020, 8, 25156–25164. [Google Scholar] [CrossRef]
- Wang, A.; Fang, Y. Applications of capillary electrophoresis with electrochemical detection in pharmaceutical and biomedical analyses. Electrophoresis 2000, 21, 1281–1290. [Google Scholar] [CrossRef]
- Samuel, B.; Elena, S. Chiral capillary electrophoresis. TrAC Trends Anal. Chem. 2020, 124, 115807–115825. [Google Scholar]
- Wen, Y.F.; Chen, S.; Yuan, Y.J.; Shao, Q.; He, X.J.; Qiao, H.Q. A quantitative HPLC method for simultaneous determination of prodrug of voriconazole and voriconazole in beagle plasma, and its application to a toxicokinetic study. Acta Chromatogr. 2022, 34, 162–169. [Google Scholar] [CrossRef]
- Li, J.; Yan, X. Comprehensive two-dimensional normal-phase liquid chromatography × reversed-phase liquid chromatography for analysis of toad skin. Anal. Chim. Acta 2017, 972, 114–120. [Google Scholar] [CrossRef]
- Köksoy, B.; Akyüz, D.; Şenocak, A.; Durmuş, M.; Demirbas, E. Sensitive, simple and fast voltammetric determination of pesticides in juice samples by novel BODIPY-phthalocyanine-SWCNT hybrid platform. Food Chem. Toxicol. 2021, 147, 111886–111897. [Google Scholar] [CrossRef]
- Tümay, S.O.; Şenocak, A.; Sarı, E.; Şanko, V.; Durmuş, M.; Demirbas, E. A new perspective for electrochemical determination of parathion and chlorantraniliprole pesticides via carbon nanotube-based thiophene-ferrocene appended hybrid nanosensor. Sens. Actuators B Chem. 2021, 345, 130344–130347. [Google Scholar] [CrossRef]
- Şenocak, A. Fast, simple and sensitive determination of coumaric acid in fruit juice samples by magnetite nanoparticleszeolitic imidazolate framework material. Electroanalysis 2020, 32, 2330–2339. [Google Scholar] [CrossRef]
- Polyakov, M.; Ivanova, V.; Klyamer, D.; Köksoy, B.; Şenocak, A.; Demirbaş, E.; Durmuş, M.; Basova, T. A hybrid nanomaterial based on singlewalled carbon nanotubes cross-linked via axially substituted silicon (iv) phthalocyanine for chemiresistive sensors. Molecules 2020, 25, 2073. [Google Scholar] [CrossRef]
- Şenocak, A.; Tümay, S.O.; Sarı, E.; Şanko, V.; Durmuş, M.; Demirbas, E. The simultaneously voltammetric determination of spinosad and chlorantraniliprole pesticides by carbazole-ferrocene functionalized carbon nanotube architecture. J. Electrochem. Soc. 2021, 168, 087513–087523. [Google Scholar] [CrossRef]
- Köksoy, B.; Akyüzb, D.; Şenocak, A.; Durmuş, M.; Demirbaş, E. Novel SWCNT-hybrid nanomaterial functionalized with subphthalocyanine substituted asymmetrical zinc (II) phthalocyanine conjugate: Design, synthesis, characterization and sensor properties for pesticides. Sens. Actuators B Chem. 2021, 329, 129198–129207. [Google Scholar] [CrossRef]
- Şenocak, A.; Tümay, S.O.; Makhseed, S.; Demirbas, E.; Durmuş, M. A synergetic and sensitive physostigmine pesticide sensor using copper complex of 3D zinc (II) phthalocyanine-SWCNT hybrid material. Biosens. Bioelectron. 2021, 174, 112819–112826. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.L.; Chen, B.; Liu, X.G. A highly luminescent entangled metal-organic framework based on pyridine-substituted tetraphenylethene for efficient pesticide detection. Chem. Commun. 2017, 53, 9975–9978. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent organic polymers and frameworks for fluorescence-based sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Tümay, S.O.; Şanko, V.; Demirbas, E.; Şenocak, A. Fluorescence determination of trace level of cadmium with pyrene modified nanocrystalline cellulose in food and soil samples. Food Chem. Toxicol. 2020, 146, 111847–111857. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Wang, L.; Li, Y.; Feng, Y.Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Jarju, J.J.; Lavender, A.M.; Espiña, B.; Romero, V.; Salonen, L.M. Covalent organic framework composites: Synthesis and analytical applications. Molecules 2020, 25, 5404. [Google Scholar] [CrossRef]
- Diercks, C.S.; Kalmutzki, M.J.; Yaghi, O.M. Covalent organic frameworks-organic chemistry beyond the molecule. Molecules 2017, 22, 1575. [Google Scholar] [CrossRef] [Green Version]
- Geng, K.Y.; He, T.; Liu, R.Y.; Dalapati, S.; Tan, K.T.; Li, Z.P.; Tao, S.H.; Gong, Y.F.; Jiang, Q.H.; Jiang, D.L. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 16, 8814–8933. [Google Scholar] [CrossRef]
- Kotha, S.; Meshram, M.; Panguluri, N.R.; Shah, V.; Todeti, S.; Shirbhate, M.E. Synthetic approaches to star-shaped molecules with 1,3,5-trisubstituted aromatic cores. Chem. Asian J. 2019, 14, 1356–1403. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ren, H.; Sun, F.; Jing, X.; Cai, K.; Zhao, X.; Wang, Y.; Wei, Y.; Zhu, G. Sensitive detection of hazardous explosives via highly fluorescent crystalline porous aromatic frameworks. J. Mater. Chem. 2012, 22, 24558–24562. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.J.; Ren, H.; Ma, H.P.; Yuan, R.R.; Yuan, Y.; Zou, X.Q.; Sun, F.X.; Zhu, G.S. Construction and sorption properties of pyrene-based porous aromatic frameworks. Microporous Mesoporous Mater. 2013, 173, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Porcua, P.; Estrada-Montaño, A.S.; Vonlanthena, M.; Cuétara-Guadarrama, F.; González-Méndez, I.; Sorroza-Martínez, K.; Zaragoza-Galán, G.; Rivera, E. Azobenzene dyads containing fullerene, porphyrin and pyrene chromophores: Molecular design and optical properties. Dyes Pigm. 2022, 197, 109858–109879. [Google Scholar] [CrossRef]
- Hüsnüye, A.A.; Süreyya, O.T.; Ahmet, Ş.; Serkan, Y. Pyrene functionalized cyclotriphosphazene-based dyes: Synthesis, intramolecular excimer formation, and fluorescence receptor for the detection of nitro-aromatic compounds. Dyes Pigm. 2018, 153, 172–181. [Google Scholar]
- Alidagi, H.A.; Tümay, S.O.; Ahmet, Ş.; Çiftbudak, Ö.F.; Çoşut, B.; Yeşilot, S. Constitutional isomers of dendrimer-like pyrene substituted cyclotriphosphazenes: Synthesis, theoretical calculations, and use as fluorescence receptors for the detection of explosive nitroaromatics. New J. Chem. 2019, 43, 16738–16747. [Google Scholar] [CrossRef]
- Wu, H.T.; Xu, H.; Tao, F.R.; Su, X.; Yu, W.W.; Li, T.D.; Cui, Y.Z. Preparation of a hyperbranched porous polymer and its sensing performance for nitroaromatics. New J. Chem. 2018, 42, 12802–12810. [Google Scholar] [CrossRef]
- Tang, C.; Xu, H.; Liu, F.; Liu, X.D.; Lai, W.Y.; Wang, X.L.; Huang, W. Alternating pyrene-fluorene linear copolymers: Influence of non-conjugated and conjugated pyrene on thermal and optoelectronic properties. Synth. Met. 2013, 174, 33–41. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Fenenko, L.; Yahiro, M.; Adachi, C. Alternating copolyfluorenevinyles with polynuclear aromatic moieties: Synthesis, photophysics, and electroluminescence. Polym. Chem. 2007, 45, 4661–4670. [Google Scholar] [CrossRef]
- Zhao, M.T.; Chen, J.Z.; Chen, B.; Zhang, X.; Shi, Z.Y.; Liu, Z.Q.; Ma, Q.L.; Peng, Y.W.; Tan, C.L.; Wu, X.J.; et al. Selective epitaxial growth of oriented hierarchical metal-organic framework heterostructures. J. Am. Chem. Soc. 2020, 19, 8953–8961. [Google Scholar] [CrossRef]
- Xia, L.X.; Zhang, H.C.; Feng, B.; Yang, D.Q.; Bu, N.S.; Zhao, Y.B.; Yan, Z.J.; Li, Z.N.; Yuan, Y.; Zhao, X.J. Facile strategy to prepare fluorescent porous aromatic frameworks for sensitive detection of nitroaromatic explosives. Chem. J. Chin. Univ. 2019, 40, 2456–2464. [Google Scholar]
- Zhang, X.H.; Wang, X.P.; Xiao, J.; Wang, S.Y.; Huang, D.K.; Ding, X.; Xiang, Y.G.; Chen, H. Synthesis of 1,4-diethynylbenzene-based conjugated polymer photocatalysts and their enhanced visible/near-infrared-light-driven hydrogen production activity. J. Catal. 2017, 350, 64–71. [Google Scholar] [CrossRef]
- Geng, T.M.; Zhang, C.; Hu, C.; Liu, M.; Fei, Y.T.; Xia, H.Y. Synthesis of 1,6-disubstituted pyrene-based conjugated microporous polymers for reversible adsorption and fluorescence sensing of iodine. New J. Chem. 2020, 44, 2312–2320. [Google Scholar] [CrossRef]
- Guo, L.; Cao, D.P. Color tunable porous organic polymer luminescent probes for selective sensing of metal ions and nitroaromatic explosives. J. Mater. Chem. C 2015, 3, 8490–8494. [Google Scholar] [CrossRef]
- Zhan, S.Z.; Li, M.; Zhou, X.P.; Wang, J.H.; Yang, J.R.; Li, D. When Cu4I4 cubane meets Cu3(pyrazolate)3 triangle: Dynamic interplay between two classical luminophores functioning in a reversibly thermochromic coordination polymer. Chem. Commun. 2011, 47, 12441–12443. [Google Scholar] [CrossRef]
- He, Q.J.; Shi, J.L.; Cui, X.Z.; Zhao, J.J.; Chen, Y.; Zhou, J. Rhodamine B-co-condensed spherical SBA-15 nanoparticles: Facile co-condensation synthesis and excellent fluorescence features. J. Mater. Chem. 2009, 19, 3395–3403. [Google Scholar] [CrossRef]
- Geng, T.M.; Li, D.K.; Zhu, Z.M. Fluorescent conjugated microporous polymer based on perylene tetraanhydride bisimide for sensing o-nitrophenol. Anal. Chim. Acta. 2018, 1011, 77–85. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z.G. Creation of carbazole-based fluorescent porous polymers for recognition and detection of various pesticides in water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef]
- Engelhard, M.H.; Baer, D.R.; Herrera-Gomez, A.; Sherwood, P.M.A. Introductory guide to backgrounds in XPS spectra and their impact on determining peak intensities. J. Vac. Sci. Technol. A 2020, 38, 063203–063227. [Google Scholar] [CrossRef]
- Tougaard, S. Practical guide to the use of backgrounds in quantitative XPS. J. Vac. Sci. Technol. A 2021, 39, 011201–011222. [Google Scholar] [CrossRef]
- Shard, A.G.; Spencer, S.J. A simple approach to measuring thick organic films using the XPS inelastic background. Surf. Interface Anal. 2017, 49, 1256–1270. [Google Scholar] [CrossRef]
- Müller, A.; Sparnacci, K.; Unger, W.E.S.; Tougaard, S. Determining nonuniformities of core-shell nanoparticle coatings by analysis of the inelastic background of X-ray photoelectron spectroscopy survey spectra. Surf. Interface Anal. 2020, 52, 770–777. [Google Scholar] [CrossRef]
- Oku, M.; Shishido, T.; Matsuta, H.; Wagatsuma, K. Comparison of the background corrected valence band XPS spectra of Fe and Co aluminides and silicides with their electronic structures. J. Electron. Spectros. Relat. Phenomena 2006, 153, 75–80. [Google Scholar] [CrossRef]
- Turner, N.H. Estimates of peak areas and relative atomic amounts from wide-scan XPS spectra. Surf. interface Anal. 1992, 18, 47–51. [Google Scholar] [CrossRef]
- Dwyer, V.M. Background intensity determination in AES/XPS. Surf. Sci. 1988, 193, 549–568. [Google Scholar] [CrossRef]
- Krishnan, S.; Suneesh, C.V. Fluorene—Triazine conjugated porous organic polymer framework for superamplified sensing of nitroaromatic explosives. J. Photochem. 2019, 371, 414–422. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Q.; Xin, C.; Liu, Q.Y.; Deng, X.; Liu, T.T.; Xu, X.H.; Zhang, X.M. Covalent organic framework nanofiber with bidentate ligand as enhanced fluorescent sensor for Cu2+. Microporous Mesoporous Mater. 2020, 299, 110122–110130. [Google Scholar] [CrossRef]
- Geethanjalia, H.S.; Nagaraja, D.; Melavanki, R.M. Exploring the mechanism of fluorescence quenching in two biologically active boronic acid derivatives using Stern-Volmer kinetics. J. Mol. Liq. 2015, 209, 669–675. [Google Scholar] [CrossRef]
- Ma, L.N.; Zhang, B.; Wang, Z.H.; Hou, L.; Zhu, Z.H.; Wang, Y.Y. Efficient gas and VOC separation and pesticide detection in a highly stable interpenetrated Indium-Organic framework. Inorg. Chem. 2021, 60, 10698–10706. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Wang, X.W.; Gu, L.; Yu, Y.H.; Gao, J.S. Three pairs of luminescent coordination polymers based on CoII and CdII clusters for the detection of antibiotics, pesticides and chiral nitro aromatic compounds. RSC Adv. 2020, 10, 9475–9476. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Zhou, T.Y.; Wang, Y.P.; Gong, L.S.; Liu, J.B. Transformation from gold nanoclusters to plasmonic nanoparticles: A general strategy towards selective detection of organophosphorothioate pesticides. Biosens. Bioelectron. 2018, 99, 274–280. [Google Scholar] [CrossRef] [PubMed]






| Sample | SBET (m2 g−1) | Smicro (m2 g−1) | Vmicro (cm3 g−1) | Vtotal (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|---|---|
| LNU-45 | 322.401 | 121.989 | 0.065 | 0.131 | 1.165–1.809 |
| LNU-47 | 181.924 | 31.808 | 0.017 | 0.049 | 1.810 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Liu, J.; Miao, C.; Su, P.; Zheng, G.; Cui, B.; Geng, T.; Fan, J.; Yu, Z.; Bu, N.; et al. Pyrene-Based Fluorescent Porous Organic Polymers for Recognition and Detection of Pesticides. Molecules 2022, 27, 126. https://doi.org/10.3390/molecules27010126
Yan Z, Liu J, Miao C, Su P, Zheng G, Cui B, Geng T, Fan J, Yu Z, Bu N, et al. Pyrene-Based Fluorescent Porous Organic Polymers for Recognition and Detection of Pesticides. Molecules. 2022; 27(1):126. https://doi.org/10.3390/molecules27010126
Chicago/Turabian StyleYan, Zhuojun, Jinni Liu, Congke Miao, Pinjie Su, Guiyue Zheng, Bo Cui, Tongfei Geng, Jiating Fan, Zhiyi Yu, Naishun Bu, and et al. 2022. "Pyrene-Based Fluorescent Porous Organic Polymers for Recognition and Detection of Pesticides" Molecules 27, no. 1: 126. https://doi.org/10.3390/molecules27010126
APA StyleYan, Z., Liu, J., Miao, C., Su, P., Zheng, G., Cui, B., Geng, T., Fan, J., Yu, Z., Bu, N., Yuan, Y., & Xia, L. (2022). Pyrene-Based Fluorescent Porous Organic Polymers for Recognition and Detection of Pesticides. Molecules, 27(1), 126. https://doi.org/10.3390/molecules27010126