Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis
Abstract
:1. Introduction
2. Mechanistic Considerations of MCRs and Catalysis Roles
3. Catalyst-Free MCRs and Reasons to Use Catalysts
4. The Combined Role of Catalysis and Solvent Effects in MCRs
5. Catalysis in Stereoselective MCRs
6. Homogeneous and Heterogeneous Catalysis
7. Concluding Remarks
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weitz, E. New physical insights in experimental studies in catalysis. J. Phys. Chem. C 2017, 121, 23852. [Google Scholar] [CrossRef]
- Piumetti, M. A brief history of the science of catalysis-i: From the early concepts to single-site heterogeneous catalysts. Chim. Oggi. 2014, 32, 22–27. [Google Scholar]
- Lindstrom, B.; Pettersson, L.J. A brief history of catalysis. Cattech 2003, 7, 130–138. [Google Scholar] [CrossRef]
- Laidler, K.J. A glossary of terms used in chemical kinetics, including reaction dynamics. Pure Appl. Chem. 1996, 68, 149–192. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production? A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Shet, H.; Parmar, U.; Bhilare, S.; Kapdi, A.R. A comprehensive review of caged phosphines: Synthesis, catalytic applications, and future perspectives. Org. Chem. Front. 2021, 8, 1599–1656. [Google Scholar] [CrossRef]
- Ruslan, N.; Kan, S.Y.; Hamzah, A.S.; Chia, P.W. Natural food additives as green catalysts in organic synthesis: A review. Environ. Chem. Lett. 2021, 19, 3359–3380. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Demkiv, O.; Prokopiv, T.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Synthesis, catalytic properties and application in biosensorics of nanozymes and electronanocatalysts: A review. Sensors 2020, 20, 4509. [Google Scholar] [CrossRef]
- Murgolo, S.; De Ceglie, C.; Di Iaconi, C.; Mascolo, G. Novel tio2-based catalysts employed in photocatalysis and photoelectrocatalysis for effective degradation of pharmaceuticals (phacs) in water: A short review. Curr. Opin. Green Sustain. Chem. 2021, 30. [Google Scholar] [CrossRef]
- Mahajan, A.; Chundawat, T.S. Review on the role of the metal catalysts in the synthesis of pharmacologically important quinoline substrate. Mini-Rev. Org. Chem. 2019, 16, 631–652. [Google Scholar] [CrossRef]
- Siahrostami, S.; Villegas, S.J.; Mostaghimi, A.H.B.; Back, S.; Farimani, A.B.; Wang, H.T.; Persson, K.A.; Montoya, J. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide. ACS Catal. 2020, 10, 7495–7511. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by fischer tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Volkova, Y.; Baranin, S.; Zavarzin, I. A3 coupling reaction in the synthesis of heterocyclic compounds. Adv. Synth. Catal. 2021, 363, 40–61. [Google Scholar] [CrossRef]
- Biesen, L.; Mueller, T.J.J. Multicomponent and one-pot syntheses of quinoxalines. Adv. Synth. Catal. 2021, 363, 980–1006. [Google Scholar] [CrossRef]
- Luo, J.; Chen, G.S.; Chen, S.J.; Li, Z.D.; Liu, Y.L. Catalytic enantioselective isocyanide-based reactions: Beyond passerini and ugi multicomponent reactions. Chem.-Eur. J. 2021, 27, 6598–6619. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Manna, S.; Panda, S. Transition metal catalyzed asymmetric multicomponent reactions of unsaturated compounds using organoboron reagents. Chem. Commun. 2021, 57, 441–459. [Google Scholar]
- Singh, K.N. Metal-free multicomponent reactions: A benign access to monocyclic six-membered n-heterocycles. Org. Biomol. Chem. 2021, 19, 2622–2657. [Google Scholar]
- Liu, C.H.; Huang, W.B.; Zhang, J.H.; Rao, Z.H.; Gu, Y.L.; Jerome, F. Formaldehyde in multicomponent reactions. Green Chem. 2021, 23, 1447–1465. [Google Scholar] [CrossRef]
- Ma, X.; Zhi, S.; Zhang, W. Recent developments on five-component reactions. Molecules 2021, 26, 1986. [Google Scholar] [CrossRef]
- Imtiaz, S.; War, J.A.; Banoo, S.; Khan, S. A-aminoazoles/azines: Key reaction partners for multicomponent reactions. RSC Adv. 2021, 11, 11083–11165. [Google Scholar] [CrossRef]
- Ghosh, S.; Biswas, K. Metal-free multicomponent approach for the synthesis of propargylamine: A review. RSC Adv. 2021, 11, 2047–2065. [Google Scholar] [CrossRef]
- Fairoosa, J.; Neetha, M.; Anilkumar, G. Recent developments and perspectives in the copper-catalyzed multicomponent synthesis of heterocycles. RSC Adv. 2021, 11, 3452–3469. [Google Scholar] [CrossRef]
- Strecker, A. Ueber die künstliche bildung der milchsäure und einen neuen, dem glycocoll homologen körper. Ann. Chemie Pharm. 1850, 75, 27–45. [Google Scholar] [CrossRef] [Green Version]
- Masamba, W. Petasis vs. Strecker amino acid synthesis: Convergence, divergence and opportunities in organic synthesis. Molecules 2021, 26, 1707. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv. 2012, 2, 16–58. [Google Scholar] [CrossRef] [Green Version]
- Longo, L.S.; Siqueira, F.A.; Anjos, N.S.; Santos, G.F.D. Scandium(iii)-triflate-catalyzed multicomponent reactions for the synthesis of nitrogen heterocycles. Chemistryselect 2021, 6, 5097–5109. [Google Scholar] [CrossRef]
- Santra, S. Baker’s yeast catalyzed multicomponent reactions: A new hope? Chemistryselect 2019, 4, 12630–12637. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Eberlin, M.N.; Neto, B.A.D. How and why to investigate multicomponent reactions mechanisms? A critical review. Chem. Rec. 2021, 21. [Google Scholar] [CrossRef]
- Ugi, I.; Heck, S. The multicomponent reactions and their libraries for natural and preparative chemistry. Comb. Chem. High. Throughput Screen 2001, 4, 1–34. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; da Silva Junior, E.N.; Neto, B.A.D. What do we know about multicomponent reactions? Mechanisms and trends for the biginelli, hantzsch, mannich, passerini and ugi mcrs. RSC Adv. 2014, 4, 54282–54299. [Google Scholar] [CrossRef]
- Santos, V.G.; Godoi, M.N.; Regiani, T.; Gama, F.H.S.; Coelho, M.B.; de Souza, R.O.M.A.; Eberlin, M.N.; Garden, S.J. The multicomponent hantzsch reaction: Comprehensive mass spectrometry monitoring using charge-tagged reagents. Chem.-Eur. J. 2014, 20, 12808–12816. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; Bataglion, G.A.; Ramos, L.M.; de Oliveira, A.L.; de Oliveira, H.C.B.; Eberlin, M.N.; de Macedo, J.L.; da Silva, W.A.; Neto, B.A.D. Task-specific ionic liquid incorporating anionic heteropolyacid-catalyzed hantzsch and mannich multicomponent reactions. Ionic liquid effect probed by esi-ms(/ms). Tetrahedron 2014, 70, 3306–3313. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.M.; Wei, H.X.; Zhao, X.; Luo, J. Covalently anchored tertiary amine functionalized ionic liquid on silica coated nano-fe3o4 as a novel, efficient and magnetically recoverable catalyst for the unsymmetrical hantzsch reaction and knoevenagel condensation. RSC Adv. 2017, 7, 53861–53870. [Google Scholar] [CrossRef] [Green Version]
- Karimi, B.; Mobaraki, A.; Mirzaei, H.M.; Zareyee, D.; Vali, H. Improving the selectivity toward three-component biginelli versus hantzsch reactions by controlling the catalyst hydrophobic/hydrophilic surface balance. ChemCatChem 2014, 6, 212–219. [Google Scholar] [CrossRef]
- Tamaddon, F.; Moradi, S. Controllable selectivity in biginelli and hantzsch reactions using nanozno as a structure base catalyst. J. Mol. Catal. A Chem. 2013, 370, 117–122. [Google Scholar] [CrossRef]
- Adibi, H.; Samimi, H.A.; Beygzadch, M. Iron(iii) trifluoro acetate and trifluoromethanesulfonate: Recyclable lewis acid catalysts for one-pot synthesis of 3,4-dihydropyrimidinones or their sulfur analogues and 1,4-dihydropyridines via solvent-free biginelli and hantzsch condensation protocols. Catal. Commun. 2007, 8, 2119–2124. [Google Scholar] [CrossRef]
- Ming, L.; Guo, W.S.; Wen, L.R.; Li, Y.F.; Yang, H.Z. One-pot synthesis of biginelli and hantzsch products catalyzed by non-toxic ionic liquid (bmimsac) and structural determination of two products. J. Mol. Catal. A Chem. 2006, 258, 133–138. [Google Scholar]
- Legeay, J.C.; Vanden Eynde, J.J.; Bazureau, J.P. Ionic liquid phase technology supported the three component synthesis of hantzsch 1,4-dihydropyridines and biginelli 3,4-dihydropyrimidin-2(1h)-ones under microwave dielectric heating. Tetrahedron 2005, 61, 12386–12397. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.; Reddy, C.S.; Yadav, J.S. A novel tmsi-mediated synthesis of hantzsch 1,4-dihydropyridines at ambient temperature. Tetrahedron Lett. 2003, 44, 4129–4131. [Google Scholar] [CrossRef]
- Undale, K.A.; Park, Y.; Park, K.; Dagade, D.H.; Pore, D.M. A revisit to the hantzsch reaction: Unexpected formation of tetrahydrobenzo b pyrans beyond polyhydroquinolines. Synlett 2011, 791–796. [Google Scholar] [CrossRef]
- Shen, L.; Cao, S.; Wu, J.J.; Zhang, J.; Li, H.; Liu, N.J.; Qian, X.H. A revisit to the hantzsch reaction: Unexpected products beyond 1,4-dihydropyridines. Green Chem. 2009, 11, 1414–1420. [Google Scholar] [CrossRef]
- Momeni, T.; Heravi, M.M.; Hosseinnejad, T.; Mirzaei, M.; Zadsirjan, V. H5bw12o40-catalyzed syntheses of 1,4-dihydropyridines and polyhydroquinolines via hantzsch reaction: Joint experimental and computational studies. J. Mol. Struct. 2020, 1199, 127011. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.J.; Tian, N.N.; Yan, H.; Wang, J.; Song, X.Q. Studies on chemoselective synthesis of 1,4-and 1,2-dihydropyridine derivatives by a hantzsch-like reaction: A combined experimental and dft study. Org. Biomol. Chem. 2021, 19, 3882–3892. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Minassi, A.; Appendino, G. Pietro biginelli: The man behind the reaction. Eur. J. Org. Chem. 2011, 5541–5550. [Google Scholar] [CrossRef]
- Freitas, E.F.; Souza, R.Y.; Passos, S.T.A.; Dias, J.A.; Dias, S.C.L.; Neto, B.A.D. Tuning the biginelli reaction mechanism by the ionic liquid effect: The combined role of supported heteropolyacid derivatives and acidic strength. RSC Adv. 2019, 9, 27125–27135. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.C.O.; Correa, J.R.; Rodrigues, M.O.; Alvim, H.G.O.; Guido, B.C.; Gatto, C.C.; Wanderley, K.A.; Fioramonte, M.; Gozzo, F.C.; de Souza, R.O.M.A.; et al. The biginelli reaction under batch and continuous flow conditions: Catalysis, mechanism and antitumoral activity. RSC Adv. 2015, 5, 48506–48515. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; Lima, T.B.; de Oliveira, A.L.; de Oliveira, H.C.B.; Silva, F.M.; Gozzo, F.C.; Souza, R.Y.; da Silva, W.A.; Neto, B.A.D. Facts, presumptions, and myths on the solvent-free and catalyst-free biginelli reaction. What is catalysis for? J. Org. Chem. 2014, 79, 3383–3397. [Google Scholar] [CrossRef]
- Ramos, L.M.; Guido, B.C.; Nobrega, C.C.; Corrêa, J.R.; Silva, R.G.; de Oliveira, H.C.B.; Gomes, A.F.; Gozzo, F.C.; Neto, B.A.D. The biginelli reaction with an imidazolium-tagged recyclable iron catalyst: Kinetics, mechanism, and antitumoral activity. Chem.-Eur. J. 2013, 19, 4156–4168. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; de Lima, T.B.; de Oliveira, H.C.B.; Gozzo, F.C.; de Macedo, J.L.; Abdelnur, P.V.; Silva, W.A.; Neto, B.A.D. Ionic liquid effect over the biginelli reaction under homogeneous and heterogeneous catalysis. ACS Catal. 2013, 3, 1420–1430. [Google Scholar] [CrossRef]
- De Souza, R.; da Penha, E.T.; Milagre, H.M.S.; Garden, S.J.; Esteves, P.M.; Eberlin, M.N.; Antunes, O.A.C. The three-component biginelli reaction: A combined experimental and theoretical mechanistic investigation. Chem.-Eur. J. 2009, 15, 9799–9804. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.G.S.; Silva, S.; Goncalves, R.H.; Leite, E.R.; Schwab, R.S.; Correa, A.G.; Paixao, M.W. Highly efficient and magnetically recoverable niobium nanocatalyst for the multicomponent biginelli reaction. ChemCatChem 2014, 6, 3455–3463. [Google Scholar] [CrossRef]
- Kappe, C.O. A reexamination of the mechanism of the biginelli dihydropyrimidine synthesis. Support for an n-acyliminium ion intermediate. J. Org. Chem. 1997, 62, 7201–7204. [Google Scholar] [CrossRef] [PubMed]
- Litvic, M.; Vecenaj, I.; Ladisic, Z.M.; Lovric, M.; Vinkovic, V.; Filipan-Litvic, M. First application of hexaaquaaluminium(iii) tetrafluoroborate as a mild, recyclable, non-hygroscopic acid catalyst in organic synthesis: A simple and efficient protocol for the multigram scale synthesis of 3,4-dihydropyrimidinones by biginelli reaction. Tetrahedron 2010, 66, 3463–3471. [Google Scholar] [CrossRef]
- Cepanec, I.; Litvic, M.; Filipan-Litvic, M.; Grungold, I. Antimony(iii) chloride-catalysed biginelli reaction: A versatile method for the synthesis of dihydropyrimidinones through a different reaction mechanism. Tetrahedron 2007, 63, 11822–11827. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J.; Sherwood, J. The combined role of catalysis and solvent effects on the biginelli reaction: Improving efficiency and sustainability. Chem.-Eur. J. 2013, 19, 5174–5182. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Man, Y.; Xu, Z.H.; Wu, T.T.; Xu, X.X.; Tang, B. A catalyst-free aqueous mediated multicomponent reaction of isocyanide: Expeditious synthesis of polyfunctionalized cyclo b fused mono-, di- and tricarbazoles. Org. Chem. Front. 2020, 7, 3720–3726. [Google Scholar] [CrossRef]
- Cao, W.X.; Dai, F.Y.; Hu, R.R.; Tang, B. Economic sulfur conversion to functional polythioamides through catalyst-free multicomponent polymerizations of sulfur, acids, and amines. J. Am. Chem. Soc. 2020, 142, 978–986. [Google Scholar] [CrossRef]
- Liu, T.X.; Yue, S.S.; Wei, C.G.; Ma, N.N.; Zhang, P.L.; Liu, Q.F.; Zhang, G.S. Solvent-promoted catalyst-free regioselective n-incorporation multicomponent domino reaction: Rapid assembly of -functionalized 60 fullerene-fused dihydrocarbolines. Chem. Commun. 2018, 54, 13331–13334. [Google Scholar] [CrossRef]
- Ghosh, S.; Jana, C.K. Rapid access to cinnamamides and piper amides via three component coupling of arylaldehydes, amines, and meldrum’s acid. Green Chem. 2019, 21, 5803–5807. [Google Scholar] [CrossRef]
- Rocha, R.O.; Rodrigues, M.O.; Neto, B.A.D. Review on the ugi multicomponent reaction mechanism and the use of fluorescent derivatives as functional chromophores. ACS Omega 2020, 5, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, M.N.; Zhang, Z.H. Visible light-initiated catalyst-free one-pot, multicomponent construction of 5-substituted indole chromeno 2,3-b pyridines. Adv. Synth. Catal. 2019, 361, 5182–5190. [Google Scholar] [CrossRef]
- Kerru, N.; Maddila, S.; Jonnalagadda, S.B. A facile and catalyst-free microwave-promoted multicomponent reaction for the synthesis of functionalised 1,4-dihydropyridines with superb selectivity and yields. Front. Chem. 2021, 9, 638832. [Google Scholar] [CrossRef]
- Nazeef, M.; Saquib, M.; Tiwari, S.K.; Yadav, V.; Ansari, S.; Sagir, H.; Hussain, M.K.; Siddiqui, I.R. Catalyst free, multicomponent green approach to benzo a chromeno 2,3-c phenazines using glycerol as a recyclable and biodegradable promoting medium. Chemistryselect 2020, 5, 14447–14454. [Google Scholar] [CrossRef]
- Maury, S.K.; Kumari, S.; Kushwaha, A.K.; Kamal, A.; Singh, H.K.; Kumar, D.; Singh, S. Grinding induced catalyst free, multicomponent synthesis of indoloindole pyrimidine. Tetrahedron Lett. 2020, 61, 152383. [Google Scholar] [CrossRef]
- Franz, M.; Stalling, T.; Steinert, H.; Martens, J. First catalyst-free co2 trapping of n-acyliminium ions under ambient conditions: Sustainable multicomponent synthesis of thia- and oxazolidinyl carbamates. Org. Biomol. Chem. 2018, 16, 8292–8304. [Google Scholar] [CrossRef]
- Tian, T.; Hu, R.R.; Tang, B.Z. Room temperature one-step conversion from elemental sulfur to functional polythioureas through catalyst-free multicomponent polymerizations. J. Am. Chem. Soc. 2018, 140, 6156–6163. [Google Scholar] [CrossRef] [PubMed]
- Arfan, A.; Paquin, L.; Bazureau, J.P. Acidic task-specific ionic liquid as catalyst of microwave-assisted solvent-free biginelli reaction. Russ. J. Org. Chem. 2007, 43, 1058–1064. [Google Scholar] [CrossRef]
- Pramanik, M.; Bhaumik, A. Phosphonic acid functionalized ordered mesoporous material: A new and ecofriendly catalyst for one-pot multicomponent biginelli reaction under solvent-free conditions. ACS Appl. Mater. Interfaces 2014, 6, 933–941. [Google Scholar] [CrossRef]
- Rostamnia, S.; Morsali, A. Basic isoreticular nanoporous metal-organic framework for biginelli and hantzsch coupling: Irmof-3 as a green and recoverable heterogeneous catalyst in solvent-free conditions. RSC Adv. 2014, 4, 10514–10518. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Bronsted acidic ionic liquid based magnetic nanoparticles: A new promoter for the biginelli synthesis of 3,4-dihydropyrimidin-2(1h)-ones/thiones. New J. Chem. 2014, 38, 358–365. [Google Scholar] [CrossRef]
- Wang, A.Q.; Liu, X.; Su, Z.X.; Jing, H.W. New magnetic nanocomposites of zro2-al2o3-fe3o4 as green solid acid catalysts in organic reactions. Catal. Sci. Technol. 2014, 4, 71–80. [Google Scholar] [CrossRef]
- Liberto, N.A.; De Paiva Silva, S.; De Fátima, Â.; Fernandes, S.A. Β-cyclodextrin-assisted synthesis of biginelli adducts under solvent-free conditions. Tetrahedron 2013, 69, 8245–8249. [Google Scholar] [CrossRef]
- Joseph, J.K.; Jain, S.L.; Singhal, S.; Sain, B. Efficient synthesis of 3,4-dihydropyrimidinones in 1-n-butyl-3-methylimidazolium tetrachloroindate (bmi center dot incl(4)). Ind. Eng. Chem. Res. 2011, 50, 11463–11466. [Google Scholar] [CrossRef]
- Salama, S.K.; Mohamed, M.F.; Darweesh, A.F.; Elwahy, A.H.M.; Abdelhamid, I.A. Molecular docking simulation and anticancer assessment on human breast carcinoma cell line using novel bis(1,4-dihydropyrano[2,3-c] pyrazole-5-carbonitrile) and bis(1,4-dihydropyrazolo[4′,3′:5,6] pyrano[2,3-b] pyridine-6-carbonitrile) derivatives. Bioorg. Chem. 2017, 71, 19–29. [Google Scholar] [CrossRef]
- da Silva, D.L.; Fernandes, S.A.; Sabino, A.A.; de Fatima, A. P-sulfonic acid calixarenes as efficient and reusable organocatalysts for the synthesis of 3,4-dihydropyrimidin-2(1h)-ones/-thiones. Tetrahedron Lett. 2011, 52, 6328–6330. [Google Scholar] [CrossRef] [Green Version]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef]
- Studer, A.; Jeger, P.; Wipf, P.; Curran, D.P. Fluorous synthesis: Fluorous protocols for the ugi and biginelli multicomponent condensations. J. Org. Chem. 1997, 62, 2917–2924. [Google Scholar] [CrossRef]
- Keshavarz, M.; Dekamin, M.G.; Mamaghani, M.; Nikpassand, M. Tetramethylguanidine-functionalized melamine as a multifunctional organocatalyst for the expeditious synthesis of 1,2,4-triazoloquinazolinones. Sci. Rep. 2021, 11, 14457. [Google Scholar] [CrossRef]
- Jalili, Z.; Tayebee, R.; Zonoz, F.M. Eco-friendly synthesis of chromeno 4,3-b chromenes with a new photosensitized wo3/zno@nh2-ey nanocatalyst. RSC Adv. 2021, 11, 18026–18039. [Google Scholar] [CrossRef]
- Mardazad, N.; Khorshidi, A.; Shojaei, A.F. Efficient one-pot synthesis and dehydrogenation of tricyclic dihydropyrimidines catalyzed by oms-2-so3h, and application of the functional-chromophore products as colorimetric chemosensors. RSC Adv. 2021, 11, 12349–12360. [Google Scholar] [CrossRef]
- Yadav, M.B.; Lim, K.T.; Kim, J.S.; Jeong, Y.T. One-pot four-component synthesis of methyl 4-(4-chlorophenyl)-5,7-dioxo-1-phenyl-1,4,5,6,7,8-hexahydropyrazolo 4’, 3’: 5, 6 pyrano 2,3-d pyrimidine-3-carboxylate; a green approach. Tetrahedron Lett. 2021, 65, 1527542. [Google Scholar] [CrossRef]
- Lei, J.; Li, Y.; He, L.J.; Luo, Y.F.; Tang, D.Y.; Yan, W.; Lin, H.K.; Li, H.Y.; Chen, Z.Z.; Xu, Z.G. Expeditious access of chromone analogues via a michael addition-driven multicomponent reaction. Org. Chem. Front. 2020, 7, 987–992. [Google Scholar] [CrossRef]
- Saha, A.; Payra, S.; Banerjee, S. One-pot multicomponent synthesis of highly functionalized bio-active pyrano 2,3-c pyrazole and benzylpyrazolyl coumarin derivatives using zro2 nanoparticles as a reusable catalyst. Green Chem. 2015, 17, 2859–2866. [Google Scholar] [CrossRef]
- Tayade, Y.A.; Padvi, S.A.; Wagh, Y.B.; Dalai, D.S. Β-cyclodextrin as a supramolecular catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] in aqueous medium. Tetrahedron Lett. 2015, 56, 2441–2447. [Google Scholar] [CrossRef]
- Khurana, J.M.; Chaudhary, A. Efficient and green synthesis of 4h-pyrans and 4h-pyrano[2,3-c] pyrazoles catalyzed by task-specific ionic liquid [bmim]oh under solvent-free conditions. Green Chem. Lett. Rev. 2012, 5, 633–638. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Mirhosseini-Eshkevari, B.; Abdollahi-Basir, M.H. Mil-53(fe) metal-organic frameworks (mofs) as an efficient and reusable catalyst for the one-pot four-component synthesis of pyrano[2,3-c]-pyrazoles. Appl. Organomet. Chem. 2019, 33, e4679. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Mishra, A.; Rai, P.; Pandey, Y.K.; Srivastava, M.; Yadav, S.; Singh, J.; Singh, J. A green and clean pathway: One pot, multicomponent, and visible light assisted synthesis of pyrano[2,3-c]pyrazoles under catalyst-free and solvent-free conditions. New J. Chem. 2017, 41, 11148–11154. [Google Scholar] [CrossRef]
- Maleki, B.; Ashrafi, S.S. Nano a-al2o3 supported ammonium dihydrogen phosphate (nh4h2po4/al2o3): Preparation, characterization and its application as a novel and heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. RSC Adv. 2014, 4, 42873–42891. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Khalil, K.D.; Adam, A.Y.; Elnagdi, M.H. Green one pot solvent-free synthesis of pyrano[2,3-c]-pyrazoles and pyrazolo[1,5-a]pyrimidines. Molecules 2010, 15, 6619–6629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, A.A.; Abd-Elmonem, M.; Hayallah, A.M.; Elsoud, F.A.A.; Sadek, K.U. Glycerol: A promising benign solvent for catalyst free one-pot multi-component synthesis of pyrano[2,3-c]pyrazoles and tetrahydro-benzo[b]pyrans at ambient temperature. Chemistryselect 2017, 2, 10689–10693. [Google Scholar] [CrossRef]
- Ali, E.; Naimi-Jamal, M.R.; Ghahramanzadeh, R. One-pot multicomponent synthesis of pyrano[2,3 c]pyrazole derivatives using cmcso3h as a green catalyst. Chemistryselect 2019, 4, 9033–9039. [Google Scholar] [CrossRef]
- Zakeri, M.; Nasef, M.M.; Kargaran, T.; Ahmad, A.; Abouzari-Lotf, E.; Asadi, J. Synthesis of pyrano[2,3-c] pyrazoles by ionic liquids under green and eco-safe conditions. Res. Chem. Interm. 2017, 43, 717–728. [Google Scholar] [CrossRef]
- Mecadon, H.; Rohman, M.R.; Rajbangshi, M.; Myrboh, B. Γ-alumina as a recyclable catalyst for the four-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles in aqueous medium. Tetrahedron Lett. 2011, 52, 2523–2525. [Google Scholar] [CrossRef]
- Karami, S.; Dekamin, M.G.; Valiey, E.; Shakib, P. Daba mnps: A new and efficient magnetic bifunctional nanocatalyst for the green synthesis of biologically active pyrano 2,3-c pyrazole and benzylpyrazolyl coumarin derivatives. New J. Chem. 2020, 44, 13952–13961. [Google Scholar] [CrossRef]
- Moustafa, M.S.; Al-Mousawi, S.M. Organic reactions under high pressure: Efficient multicomponent synthesis of novel tricyclic pyridazinonaphthyridine derivatives under high pressure. Curr. Org. Chem. 2018, 22, 268–275. [Google Scholar] [CrossRef]
- Moustafa, M.S.; Al-Mousawi, S.M.; Elnagdi, M.H. Use of a novel multicomponent reaction under high pressure for the efficient construction of a new pyridazino[5,4,3-de][1,6] naphthyridine tricyclic system. RSC Adv. 2016, 6, 90840–90845. [Google Scholar] [CrossRef]
- Kumamoto, K.; Iida, H.; Hamana, H.; Kotsuki, H.; Matsumoto, K. Are multicomponent strecker reactions of diketones with diamines under high pressure amenable to heterocyclic synthesis? Heterocycles 2005, 66, 675–681. [Google Scholar]
- Matsumoto, K.; Kim, J.C.; Iida, H.; Hamana, H.; Kumamoto, K.; Kotsuki, H.; Jenner, G. Multicomponent strecker reaction under high pressure. Helv. Chim. Acta 2005, 88, 1734–1753. [Google Scholar] [CrossRef]
- van Berkom, L.W.A.; Kuster, G.J.T.; Scheeren, H.W. High pressure: A promising tool for multicomponent reactions. Mol. Divers. 2003, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kaladevi, S.; Paul, N.; Muthusubramanian, S.; Sivakolunthu, S. Synthesis of unsymmetrical (1,3-diarylimidazolidin-4-yl) (aryl)methanone via mannich reaction. Tetrahedron Lett. 2013, 54, 3702–3705. [Google Scholar] [CrossRef]
- Reddy, M.V.; Jeong, Y.T. Incl3-catalyzed green synthesis of 1h-pyrazolo 1,2-b phthalazine-5,10-diones under solvent-free conditions. Tetrahedron Lett. 2013, 54, 3546–3549. [Google Scholar] [CrossRef]
- Udhaya Kumar, C.; Sethukumar, A.; Arul Prakasam, B. Synthesis and spectral studies of some 4h-pyran derivatives: Crystal and molecular structure of isobutyl 6-amino-5-cyano-2-methyl-4-phenyl-4h-pyran-3-carboxylate. J. Mol. Struct. 2012, 1036, 257–266. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M.K.; Kumar, M. Micelle promoted supramolecular carbohydrate scaffold-catalyzed multicomponent synthesis of 1,2-dihydro-1-aryl-3h-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthols derivatives in aqueous medium. RSC Adv. 2012, 2, 7371–7376. [Google Scholar] [CrossRef]
- Rahmatpour, A. Polystyrene-supported gacl3 as a highly efficient and reusable heterogeneous lewis acid catalyst for the three-component synthesis of benzoxanthene derivatives. Monatsh. Chem. 2013, 144, 1205–1212. [Google Scholar] [CrossRef]
- Gutierrez, R.U.; Correa, H.C.; Bautista, R.; Vargas, J.L.; Jerezano, A.V.; Delgado, F.; Tamariz, J. Regioselective synthesis of 1,2-dihydroquinolines by a solvent-free mgbr2-catalyzed multicomponent reaction. J. Org. Chem. 2013, 78, 9614–9626. [Google Scholar] [CrossRef]
- Fu, L.-P.; Shi, Q.-Q.; Shi, Y.; Jiang, B.; Tu, S.-J. Three-component domino reactions for regioselective formation of bis-indole derivatives. ACS Comb. Sci. 2013, 15, 135–140. [Google Scholar] [CrossRef]
- Safaei, S.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Diastereoselective synthesis of pyrazolines using a bifunctional bronsted acidic ionic liquid under solvent-free conditions. Adv. Synth. Catal. 2012, 354, 3095–3104. [Google Scholar] [CrossRef]
- Kumar, R.; Wadhwa, D.; Hussain, K.; Prakash, O. Modified one-pot multicomponent diastereoselective synthesis of trans-2,3-dihydrofuro[3,2-c]coumarins via in situ-generated α- tosyloxyketones. Synth. Commun. 2013, 43, 1802–1807. [Google Scholar] [CrossRef]
- Ganesan, S.S.; Ganesan, A. Zncl2 promoted efficient, one-pot synthesis of 3-arylmethyl and diarylmethyl indoles. Tetrahedron Lett. 2014, 55, 694–698. [Google Scholar]
- Pierce, C.J.; Larsen, C.H. Copper(ii) catalysis provides cyclohexanone-derived propargylamines free of solvent or excess starting materials: Sole by-product is water. Green Chem. 2012, 14, 2672–2676. [Google Scholar] [CrossRef]
- Quan, Z.J.; Hu, W.H.; Zhang, Z.; Da, Y.X.; Jia, X.D.; Wang, X.C. One-pot synthesis of allylamine derivatives by iodine- catalyzed three-component reaction of n-heterocycles, paraformaldehyde and styrenes. Adv. Synth. Catal. 2013, 355, 891–900. [Google Scholar] [CrossRef]
- Maiti, B.; Chanda, K.; Selvaraju, M.; Tseng, C.-C.; Sun, C.-M. Multicomponent solvent-free synthesis of benzimidazolyl imidazo 1,2-a -pyridine under microwave irradiation. ACS Comb. Sci. 2013, 15, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kasapidou, P.M.; Zarganes-Tzitzikas, T.; Tsoleridis, C.A.; Stephanidou-Stephanatou, J.; Neochoritis, C.G. Manipulating a multicomponent reaction: A straightforward approach to chromenopyrazole hybrid scaffolds. Synthesis 2017, 49, 3619–3632. [Google Scholar]
- Aliaga, M.J.; Ramon, D.J.; Yus, M. Impregnated copper on magnetite: An efficient and green catalyst for the multicomponent preparation of propargylamines under solvent free conditions. Org. Biomol. Chem. 2010, 8, 43–46. [Google Scholar] [CrossRef]
- Schneider, A.E.; Manolikakes, G. Bi(otf)3-catalyzed multicomponent alpha-amidoalkylation reactions. J. Org. Chem. 2015, 80, 6193–6212. [Google Scholar] [CrossRef]
- Safaei, S.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Khavasi, H.R. One-pot three-component synthesis of pyrano 3,2-b pyrazolo 4,3-e pyridin-8(1h)-ones. ACS Comb. Sci. 2013, 15, 141–146. [Google Scholar] [CrossRef]
- Hajjami, M.; Ghorbani, F.; Bakhti, F. Mcm-41-n-propylsulfamic acid: An efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphtols. Appl. Catal. A Gen. 2014, 470, 303–310. [Google Scholar] [CrossRef]
- Appun, J.; Stolz, F.; Naumov, S.; Abel, B.; Schneider, C. Modular synthesis of dipyrroloquinolines: A combined synthetic and mechanistic study. J. Org. Chem. 2018, 83, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, C.K.; Nipate, A.S.; Chate, A.V.; Dofe, V.S.; Sangshetti, J.N.; Khedkar, V.M.; Gill, C.H. Rapid construction of substituted dihydrothiophene ureidoformamides at room temperature using diisopropyl ethyl ammonium acetate: A green perspective. ACS Omega 2020, 5, 29055–29067. [Google Scholar] [CrossRef] [PubMed]
- Rostamnia, S.; Xin, H.C.; Liu, X.; Lamei, K. Simultaneously application of sba-15 sulfonic acid nanoreactor and ultrasonic irradiation as a very useful novel combined catalytic system: An ultra-fast, selective, reusable and waste-free green approach. J. Mol. Catal. A Chem. 2013, 374, 85–93. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Jonnalagadda, S.B. Eco-efficient ultrasonic responsive synthesis of pyrimidines/pyridines. Ultrason. Sonochem. 2014, 21, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Padala, A.K.; Dar, B.A.; Yadav, R.R.; Singh, B.; Vishwakarma, R.A. Montmorillonite clay cu(ii) catalyzed domino one-pot multicomponent synthesis of 3,5-disubstituted isoxazoles. Tetrahedron Lett. 2013, 54, 3558–3561. [Google Scholar] [CrossRef]
- Shaabani, A.; Soleimani, E.; Maleki, A. Ionic liquid promoted one-pot synthesis of 3-aminoimidazo[1,2-α]pyridines. Tetrahedron Lett. 2006, 47, 3031–3034. [Google Scholar] [CrossRef]
- Pawlowski, R.; Zaorska, E.; Staszko, S.; Szadkowska, A. Copper(i)-catalyzed multicomponent reactions in sustainable media. Appl. Organomet. Chem. 2018, 32, e4256. [Google Scholar] [CrossRef]
- Zhu, A.L.; Liu, R.X.; Du, C.Y.; Li, L.J. Betainium-based ionic liquids catalyzed multicomponent hantzsch reactions for the efficient synthesis of acridinediones. RSC Adv. 2017, 7, 6679–6684. [Google Scholar] [CrossRef] [Green Version]
- Kazemnejadi, M.; Nasseri, M.A.; Sheikh, S.; Rezazadeh, Z.; Gol, S.A.A. Fe3o4@sap/cu(ii): An efficient magnetically recoverable green nanocatalyst for the preparation of acridine and quinazoline derivatives in aqueous media at room temperature. RSC Adv. 2021, 11, 15989–16003. [Google Scholar] [CrossRef]
- Palermo, V.; Sathicq, A.; Liberto, N.; Fernandes, S.; Langer, P.; Jios, J.; Romanelli, G. Calix[n]arenes: Active organocatalysts for the synthesis of densely functionalized piperidines by one-pot multicomponent procedure. Tetrahedron Lett. 2016, 57, 2049–2054. [Google Scholar] [CrossRef]
- Brahmachari, G.; Das, S. Bismuth nitrate-catalyzed multicomponent reaction for efficient and one-pot synthesis of densely functionalized piperidine scaffolds at room temperature. Tetrahedron Lett. 2012, 53, 1479–1484. [Google Scholar] [CrossRef]
- Kilbas, B.; Ergen, S.; Cakici, D. Highly efficient and reusable pd/alo(oh) catalyzed synthesis of acridinedione derivatives. Curr. Organocatalysis 2019, 6, 257–265. [Google Scholar] [CrossRef]
- Thorat, K.G.; Tayade, R.P.; Sekar, N. Acridine-1, 8-diones—a new class of thermally stable nlophores: Photophysical, (hyper)polarizability and td-dft studies. Opt. Mater. 2016, 62, 306–319. [Google Scholar] [CrossRef]
- Muscia, G.C.; Buldain, G.Y.; Asis, S.E. Only acridine derivative from hantzsch-type one-pot three-component reactions. Monatsh. Chem. 2009, 140, 1529–1532. [Google Scholar] [CrossRef]
- Rajput, J.K. Autocombustion-promoted synthesis of lanthanum iron oxide: Application as heterogeneous catalyst for synthesis of piperidines, substituted amines and light-assisted degradations. Chemistryselect 2020, 5, 10863–10881. [Google Scholar]
- Ghalandarzehi, Y.; Shahraki, M.; Habibi-Khorassani, S.M. Synthesis and kinetics of highly substituted piperidines in the presence of tartaric acid as a catalyst. Comb. Chem. High. Throughput Screen 2018, 21, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.R.; Bahekar, S.P.; Sarode, P.B.; Zade, S.S.; Chandak, H.S. L-proline nitrate: A recyclable and green catalyst for the synthesis of highly functionalized piperidines. RSC Adv. 2015, 5, 47053–47059. [Google Scholar] [CrossRef]
- Varghese, J.J.; Mushrif, S.H. Origins of complex solvent effects on chemical reactivity and computational tools to investigate them: A review. React. Chem. Eng. 2019, 4, 165–206. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Winterton, N. Green chemistry: Deliverance or distraction? Clean Techn. Environ. Policy 2016, 18, 991–1001. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Gupta, M.K.; Kumar, M. L-proline catalysed multicomponent synthesis of 3-amino alkylated indoles via a mannich-type reaction under solvent-free conditions. Green Chem. 2012, 14, 290–295. [Google Scholar] [CrossRef]
- Li, M.; Cao, H.; Wang, Y.; Lv, X.L.; Wen, L.R. One-pot multicomponent cascade reaction of n,s-ketene acetal: Solvent-free synthesis of imidazo[1,2-a]thiochromeno[3,2-e]pyridines. Org. Lett. 2012, 14, 3470–3473. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, X.L.; Wen, L.R.; Hu, Z.Q. Direct solvent-free regioselective construction of pyrrolo[1,2-a][1,10]phenanthrolines based on isocyanide-based multicomponent reactions. Org. Lett. 2013, 15, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Hashemi, E.; Beheshtiha, Y.S.; Kamjou, K.; Toolabi, M.; Hosseintash, N. Solvent-free multicomponent reactions using the novel n-sulfonic acid modified poly(styrene-maleic anhydride) as a solid acid catalyst. J. Mol. Catal. A Chem. 2014, 392, 173–180. [Google Scholar] [CrossRef]
- Katkar, S.V.; Jayaram, R.V. Cu-ni bimetallic reusable catalyst for synthesis of propargylamines via multicomponent coupling reaction under solvent-free conditions. RSC Adv. 2014, 4, 47958–47964. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S. Recent developments in solvent-free multicomponent reactions: A perfect synergy for eco-compatible organic synthesis. RSC Adv. 2012, 2, 4547–4592. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships.23. A comprehensive collection of the solvatochromic parameters, π*, 〈 and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. Solvatochromic comparison method.6. Π* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. Solvatochromic comparison method.2. A-scale of solvent hydrogen-bond donor (hbd) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. Solvatochromic comparison method.1. B-scale of solvent hydrogen-bond acceptor (hba) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Marcus, Y. The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 1993, 22, 409–416. [Google Scholar] [CrossRef]
- Noppawan, P.; Sangon, S.; Supanchaiyamat, N.; Hunt, A.J. Vegetable oil as a highly effective 100% bio-based alternative solvent for the one-pot multicomponent biginelli reaction. Green Chem. 2021, 23, 5766–5774. [Google Scholar] [CrossRef]
- Sherwood, J.; Granelli, J.; McElroy, C.R.; Clark, J.H. A method of calculating the kamlet-abboud-taft solvatochromic parameters using cosmo-rs. Molecules 2019, 24, 2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramozzi, R.; Morokuma, K. Revisiting the passerini reaction mechanism: Existence of the nitrilium, organocatalysis of its formation, and solvent effect. J. Org. Chem. 2015, 80, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Komagawa, S.; Uchiyama, M.; Morokuma, K. Finding reaction pathways for multicomponent reactions: The passerini reaction is a four-component reaction. Angew. Chem. Int. Ed. 2011, 50, 644–649. [Google Scholar] [CrossRef]
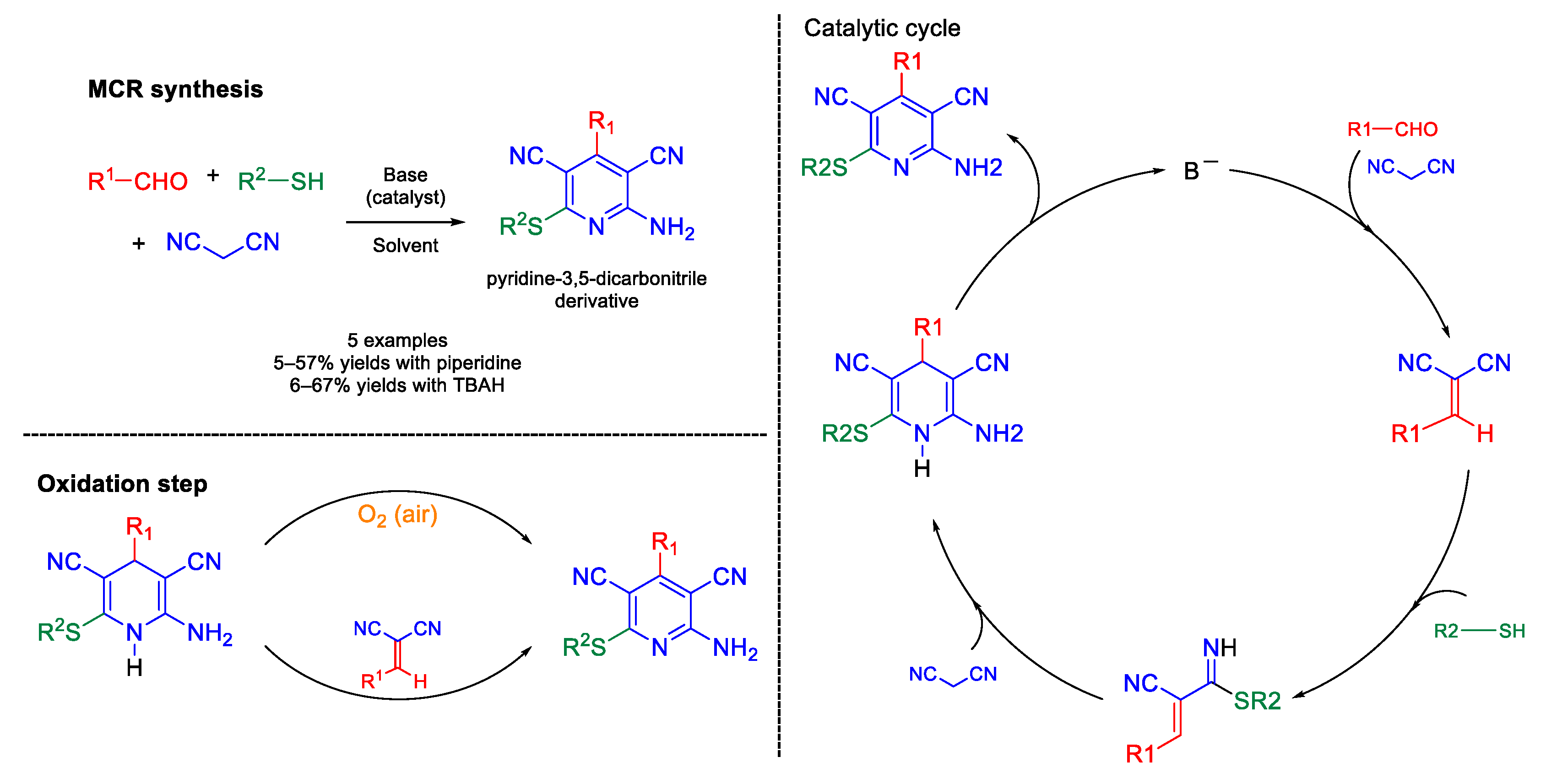
- Guo, K.; Thompson, M.J.; Chen, B.N. Exploring catalyst and solvent effects in the multicomponent synthesis of pyridine-3,5-dicarbonitriles. J. Org. Chem. 2009, 74, 6999–7006. [Google Scholar] [CrossRef] [PubMed]
- Price, G.A.; Brisdon, A.K.; Flower, K.R.; Pritchard, R.G.; Quayle, P. Solvent effects in gold-catalysed a(3)-coupling reactions. Tetrahedron Lett. 2014, 55, 151–154. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Ghorbannezhad, F.; Sajadi, S.M. A review on recent advances in the application of nanocatalysts in a(3) coupling reactions. Chem. Rec. 2018, 18, 1409–1473. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the a3-coupling. Chem. Soc. Rev. 2012, 41, 3790–3807. [Google Scholar] [CrossRef]
- Rokade, B.V.; Barker, J.; Guiry, P.J. Development of and recent advances in asymmetric a3 coupling. Chem. Soc. Rev. 2019, 48, 4766–4790. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Kafle, B.; Aher, N.G.; Khadka, D.; Park, H.; Cho, H. Isoxazol-5(4h) one derivatives as ptp1b inhibitors showing an anti-obesity effect. Chem.-Asian J. 2011, 6, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yang, J.; Lee, S.; Park, C.; Kang, D.; Akter, J.; Ullah, S.; Kim, Y.J.; Chun, P.; Moon, H.R. The tyrosinase inhibitory effects of isoxazolone derivatives with a (z)-beta-phenyl-alpha, beta-unsaturated carbonyl scaffold. Bioorg. Med. Chem. 2018, 26, 3882–3889. [Google Scholar] [CrossRef]
- Oraby, A.K.; Abdellatif, K.R.A.; Abdelgawad, M.A.; Attia, K.M.; Dawe, L.N.; Georghiou, P.E. 2,4-disubstituted phenylhydrazonopyrazolone and isoxazolone derivatives as antibacterial agents: Synthesis, preliminary biological evaluation and docking studies. Chemistryselect 2018, 3, 3295–3301. [Google Scholar] [CrossRef]
- Mazimba, O.; Wale, K.; Loeto, D.; Kwape, T. Antioxidant and antimicrobial studies on fused-ring pyrazolones and isoxazolones. Bioorg. Med. Chem. 2014, 22, 6564–6569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-M.; Xu, P.; Wang, S.-Y.; Ji, S.-J. Palladium catalyzed insertion reaction of isocyanides with 3-arylisoxazol-5(4h)-ones: Synthesis of 4-aminomethylidene isoxazolone derivates. J. Org. Chem. 2019, 84, 11007–11013. [Google Scholar] [CrossRef]
- Ishioka, T.; Kubo, A.; Koiso, Y.; Nagasawa, K.; Itai, A.; Hashimoto, Y. Novel non-steroidal/non-anilide type androgen antagonists with an isoxazolone moiety. Bioorg. Med. Chem. 2002, 10, 1555–1566. [Google Scholar] [CrossRef]
- Ferreira, J.G.L.; Ramos, L.M.; de Oliveira, A.L.; Orth, E.S.; Neto, B.A.D. An ionically tagged water-soluble artificial enzyme promotes the dephosphorylation reaction with nitroimidazole: Enhanced ionic liquid effect and mechanism. J. Org. Chem. 2015, 80, 5979–5983. [Google Scholar] [CrossRef]
- Oliveira, G.H.C.; Ramos, L.M.; de Paiva, R.K.C.; Passos, S.T.A.; Simoes, M.M.; Machado, F.; Correa, J.R.; Neto, B.A.D. Synthetic enzyme-catalyzed multicomponent reaction for isoxazol-5(4h)-one syntheses, their properties and biological application; why should one study mechanisms? Org. Biomol. Chem. 2021, 19, 1514–1531. [Google Scholar] [CrossRef]
- Fozooni, S.; Hosseinzadeh, N.G.; Hamidian, H.; Akhgar, M.R. Nano fe2o3, clinoptilolite and h3pw12o40 as efficient catalysts for solvent-free synthesis of 5(4h)-isoxazolone under microwave irradiation conditions. J. Braz. Chem. Soc. 2013, 24, 1649–1655. [Google Scholar]
- Konkala, V.S.; Dubey, P.K. One-pot synthesis of 3-phenyl-4-pyrazolylmethylene-isoxazol-(5h)-ones catalyzed by sodium benzoate in aqueous media under the influence of ultrasound waves: A green chemistry approach. J. Heterocycl. Chem. 2017, 54, 2483–2492. [Google Scholar] [CrossRef]
- Shanshak, M.; Budagumpi, S.; Malecki, J.G.; Keri, R.S. Green synthesis of 3,4-disubstituted isoxazol-5(4h)-ones using zno@fe3o4 core-shell nanocatalyst in water. Appl. Organomet. Chem. 2020, 34, e5544. [Google Scholar] [CrossRef]
- Kasar, S.B.; Thopate, S.R. Ultrasonically assisted efficient and green protocol for the synthesis of 4h-isoxazol-5-ones using itaconic acid as a homogeneous and reusable organocatalyst. Curr. Organocatalysis 2019, 6, 231–237. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, K.D.; Patel, H.D. Fruit juice of citrus limon as a biodegradable and reusable catalyst for facile, eco-friendly and green synthesis of 3,4-disubstituted isoxazol-5(4h)-ones and dihydropyrano 2,3-c -pyrazole derivatives. Res. Chem. Interm. 2016, 42, 7559–7579. [Google Scholar] [CrossRef]
- Kiyani, H.; Ghorbani, F. Potassium phthalimide as efficient basic organocatalyst for the synthesis of 3,4-disubstituted isoxazol-5(4h)-ones in aqueous medium. J. Saudi Chem. Soc. 2017, 21, S112–S119. [Google Scholar] [CrossRef] [Green Version]
- Kiyani, H.; Ghorbani, F. Efficient tandem synthesis of a variety of pyran-annulated heterocycles, 3,4-disubstituted isoxazol-5(4h)-ones, and alpha,beta-unsaturated nitriles catalyzed by potassium hydrogen phthalate in water. Res. Chem. Interm. 2015, 41, 7847–7882. [Google Scholar] [CrossRef]
- Kalinski, C.; Lemoine, H.; Schmidt, J.; Burdack, C.; Kolb, J.; Umkehrer, M.; Ross, G. Multicomponent reactions as a powerful tool for generic drug synthesis. Synthesis 2008, 4007–4011. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, P.; Li, S.-Y.; Sun, H.; Xiang, S.-H.; Wang, J.; Houk, K.N.; Tan, B. Asymmetric phosphoric acid-catalyzed four-component ugi reaction. Science 2018, 361, eaas8707. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.P.; Bouma, M.J.; Alcaraz, L.; Stocks, M.; Furber, M.; Masson, G.; Zhu, J.P. Organocatalytic enantioselective one-pot four-component ugi-type multicomponent reaction for the synthesis of epoxy-tetrahydropyrrolo 3,4-b pyridin-5-ones. Chem.-Eur. J. 2012, 18, 12624–12627. [Google Scholar] [CrossRef] [PubMed]
- Ramaraju, P.; Mir, N.A.; Singh, D.; Kumar, I. Enantioselective synthesis of 1,2,5,6-tetrahydropyridines (thps) via proline-catalyzed direct mannich-cyclization/domino oxidation-reduction sequence: Application for medicinally important n-heterocycles. RSC Adv. 2016, 6, 60422–60432. [Google Scholar] [CrossRef]
- Frey, R.; Galbraith, S.G.; Guelfi, S.; Lamberth, C.; Zeller, M. First examples of a highly stereoselective passerini reaction: A new access to enantiopure mandelamides. Synlett 2003, 1536–1538. [Google Scholar] [CrossRef]
- Denmark, S.E.; Fan, Y. The first catalytic, asymmetric alpha-additions of isocyanides. Lewis-base-catalyzed, enantioselective passerini-type reactions. J. Am. Chem. Soc. 2003, 125, 7825–7827. [Google Scholar] [CrossRef] [PubMed]
- Barbero, M.; Cadamuro, S.; Dughera, S. A bronsted acid catalysed enantioselective biginelli reaction. Green Chem. 2017, 19, 1529–1535. [Google Scholar] [CrossRef]
- Chen, X.H.; Xu, X.Y.; Liu, H.; Cun, L.F.; Gong, L.Z. Highly enantioselective organocatalytic biginelli reaction. J. Am. Chem. Soc. 2006, 128, 14802–14803. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Yang, F.Y.; Zhu, C.J. Highly enantioseletive biginelli reaction using a new chiral ytterbium catalyst: Asymmetric synthesis of dihydropyrimidines. J. Am. Chem. Soc. 2005, 127, 16386–16387. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.S.G.; Vidal, H.D.A.; Correa, A.G. Recent advances in catalytic enantioselective multicomponent reactions. Org. Biomol. Chem. 2020, 18, 7751–7773. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Jiang, R.R.; Wang, Y.M.; Zhou, Z.H. Enantioselective construction of spiro thiazol-4-one derivatives with multiple stereocenters via an organocatalyzed multicomponent cascade reaction. Adv. Synth. Catal. 2018, 360, 1961–1966. [Google Scholar] [CrossRef]
- Ramos, L.M.; Rodrigues, M.O.; Neto, B.A.D. Mechanistic knowledge and noncovalent interactions as the key features for enantioselective catalysed multicomponent reactions: A critical review. Org. Biomol. Chem. 2019, 17, 7260–7269. [Google Scholar] [CrossRef]
- Horino, Y.; Ishibashi, M.; Sakamoto, J.; Murakami, M.; Korenaga, T. Palladium-catalyzed diastereoselective synthesis of (z)-conjugated enynyl homoallylic alcohols. Adv. Synth. Catal. 2021, 363, 3592–3599. [Google Scholar] [CrossRef]
- He, D.D.; Zhuang, Z.Y.; Wang, X.; Li, J.W.; Li, J.X.; Wu, W.Q.; Zhao, Z.J.; Jiang, H.F.; Tang, B.Z. Assembly of 1h-isoindole derivatives by selective carbon-nitrogen triple bond activation: Access to aggregation-induced emission fluorophores for lipid droplet imaging. Chem. Sci. 2019, 10, 7076–7081. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.D.; Cao, C.P.; Lin, W.; Hu, M.H.; Huang, Z.B.; Shi, D.Q. Selective synthesis of polyfunctionalized pyrido[2,3-b]indoles by multicomponent domino reactions. J. Org. Chem. 2014, 79, 7935–7944. [Google Scholar] [CrossRef]
- Lebrene, A.; Martzel, T.; Gouriou, L.; Sanselme, M.; Levacher, V.; Oudeyer, S.; Afonso, C.; Loutelier-Bourhis, C.; Briere, J.F. The catalytic regio- and stereoselective synthesis of 1,6-diazabicyclo[4.3.0]nonane-2,7-diones. J. Org. Chem. 2021, 86, 8600–8609. [Google Scholar] [CrossRef] [PubMed]
- Alvim, H.G.O.; Pinheiro, D.L.J.; Carvalho-Silva, V.H.; Fioramonte, M.; Gozzo, F.C.; da Silva, W.A.; Amarante, G.W.; Neto, B.A.D. Combined role of the asymmetric counteranion-directed catalysis (acdc) and ionic liquid effect for the enantioselective biginelli multicomponent reaction. J. Org. Chem. 2018, 83, 12143–12153. [Google Scholar] [CrossRef]
- Wan, J.P.; Lin, Y.F.; Liu, Y.Y. Catalytic asymmetric biginelli reaction for the enantioselective synthesis of 3,4-dihydropyrimidinones (dhpms). Curr. Org. Chem. 2014, 18, 687–699. [Google Scholar] [CrossRef]
- Pham, H.T.; Chataigner, I.; Renaud, J.L. New approaches to nitrogen containing heterocycles: Enantioselective organocatalyzed synthesis of dihydropyridines (dhp’s), quinolizidine derivatives and dihydropyrimidines (dhpm’s). Curr. Org. Chem. 2012, 16, 1754–1775. [Google Scholar] [CrossRef]
- Xin, J.G.; Chang, L.; Hou, Z.R.; Shang, D.J.; Liu, X.H.; Feng, X.M. An enantioselective biginelli reaction catalyzed by a simple chiral secondary amine and achiral bronsted acid by a dual-activation route. Chem.-Eur. J. 2008, 14, 3177–3181. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, L.G.; Dias, I.M.; de Souza, G.B.M.; Dancini-Pontes, I.; Fernandes, N.R.C.; de Souza, P.S.; de Oliveira, G.R.; Alonso, C.G. Niobium oxides as heterogeneous catalysts for biginelli multicomponent reaction. J. Org. Chem. 2020, 85, 11170–11180. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, K.E.; Odom, A.L. A silica-supported titanium catalyst for heterogeneous hydroamination and multicomponent coupling reactions. Dalton Trans. 2019, 48, 11352–11360. [Google Scholar] [CrossRef]
- Diaz-Marta, A.S.; Tubio, C.R.; Carbajales, C.; Fernandez, C.; Escalante, L.; Sotelo, E.; Guitian, F.; Barrio, V.L.; Gil, A.; Coelho, A. Three-dimensional printing in catalysis: Combining 3d heterogeneous copper and palladium catalysts for multicatalytic multicomponent reactions. ACS Catal. 2018, 8, 392–404. [Google Scholar] [CrossRef]
- D’Oca, C.R.M.; Naciuk, F.F.; Silva, J.C.; Guedes, E.P.; Moro, C.C.; D’Oca, M.G.M.; Santos, L.S.; Natchigall, F.M.; Russowsky, D. New multicomponent reaction for the direct synthesis of beta-aryl-gamma-nitroesters promoted by hydrotalcite-derived mixed oxides as heterogeneous catalyst. J. Braz. Chem. Soc. 2017, 28, 285–298. [Google Scholar]
- Song, W.T.; Tao, S.Y.; Yu, Y.X.; Du, X.L.; Wang, S. Preparing magnetic multicomponent catalysts via a bio-inspired assembly for heterogeneous reactions. RSC Adv. 2016, 6, 69909–69918. [Google Scholar] [CrossRef]
- Amoozadeh, A.; Rahmani, S.; Bitaraf, M.; Abadi, F.B.; Tabrizian, E. Nano-zirconia as an excellent nano support for immobilization of sulfonic acid: A new, efficient and highly recyclable heterogeneous solid acid nanocatalyst for multicomponent reactions. New J. Chem. 2016, 40, 770–780. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, T.; Wang, L.; Ren, Z.; Zhang, W.N.; Huo, F.W. Programmable logic in metal-organic frameworks for catalysis. Adv. Mater. 2021, 33, 2007442. [Google Scholar] [CrossRef]
- Yadav, S.; Dixit, R.; Sharma, S.; Dutta, S.; Solanki, K.; Sharma, R.K. Magnetic metal-organic framework composites: Structurally advanced catalytic materials for organic transformations. Mater. Adv. 2021, 2, 2153–2187. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Mirhosseini-Eshkevari, B.; Tavakoli, M.; Zamani, F. Metal-organic frameworks: Advanced tools for multicomponent reactions. Green Chem. 2020, 22, 7265–7300. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Hu, K.Q.; Zhou, Y.Q.; Xiong, Q.; Cao, W.D.; Feng, X.M. Enantioselective isocyanide-based multicomponent reaction with alkylidene malonates and phenols. Org. Lett. 2021, 23, 5261–5265. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Li, G.L.; Dong, S.X.; Liu, X.H.; Feng, X.M. Enantioselective synthesis of hydrothiazole derivatives via an isocyanide-based multicomponent reaction. Org. Lett. 2019, 21, 8771–8775. [Google Scholar] [CrossRef]
- Wei, Q.H.; Ma, X.C.; Chen, J.H.; Niu, L.; Yang, X.; Xia, F.; Liu, S.Y. A triple-functionalised metal centre-catalyzed enantioselective multicomponent reaction. Org. Chem. Front. 2018, 5, 2799–2804. [Google Scholar] [CrossRef]
- Khopade, T.M.; Mete, T.B.; Arora, J.S.; Bhat, R.G. An adverse effect of higher catalyst loading and longer reaction time on enantioselectivity in an organocatalytic multicomponent reaction. Chem.-Eur. J. 2018, 24, 6036–6040. [Google Scholar] [CrossRef]
- Li, M.F.; Guo, X.; Zheng, Q.; Hu, W.H.; Liu, S.Y. Enantioselective multicomponent reaction for rapid construction of 1,2,5-triol derivatives with vicinal chiral centers. J. Org. Chem. 2017, 82, 5212–5221. [Google Scholar] [CrossRef]
- Barbato, K.S.; Luan, Y.; Ramella, D.; Panek, J.S.; Schaus, S.E. Enantioselective multicomponent condensation reactions of phenols, aldehydes, and boronates catalyzed by chiral biphenols. Org. Lett. 2015, 17, 5812–5815. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, M.A.; Tanaka, N.; Yokosaka, T.; Uraguchi, D.; Johnson, J.S.; Ooi, T. Enantioselective reductive multicomponent coupling reactions between isatins and aldehydes. Chem. Sci. 2015, 6, 6086–6090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calleja, J.; Gonzalez-Perez, A.B.; de Lera, A.R.; Alvarez, R.; Fananas, F.J.; Rodriguez, F. Enantioselective synthesis of hexahydrofuro 3,2-c quinolines through a multicatalytic and multicomponent process. A new “aromatic sandwich” model for binol-phosphoric acid catalyzed reactions. Chem. Sci. 2014, 5, 996–1007. [Google Scholar] [CrossRef]
- Cala, L.; Mendoza, A.; Fananas, F.J.; Rodriguez, F. A catalytic multicomponent coupling reaction for the enantioselective synthesis of spiroacetals. Chem. Commun. 2013, 49, 2715–2717. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.F.; Wang, Y.; Xu, P.F. Organocatalytic enantioselective multicomponent cascade reaction: Facile access to tetrahydropyridines with c3 all-carbon quaternary stereocenters. Tetrahedron 2011, 67, 3273–3277. [Google Scholar] [CrossRef]
- Qian, Y.; Jing, C.C.; Shi, T.D.; Ji, J.J.; Tang, M.; Zhou, J.; Zhai, C.W.; Hu, W.H. Dual catalysis in highly enantioselective multicomponent reaction with water: An efficient approach to chiral beta-amino-alpha-hydroxy acid derivatives. ChemCatChem 2011, 3, 653–656. [Google Scholar] [CrossRef]
- Guan, X.Y.; Yang, L.P.; Hu, W.H. Cooperative catalysis in multicomponent reactions: Highly enantioselective synthesis of gamma-hydroxyketones with a quaternary carbon stereocenter. Angew. Chem. Int. Ed. 2010, 49, 2190–2192. [Google Scholar] [CrossRef]
- Dagousset, G.; Drouet, F.; Masson, G.; Zhu, J.P. Chiral Bronsted acid-catalyzed enantioselective multicomponent Mannich reaction: Synthesis of anti-1,3-diamines using enecarbamates as nucleophiles. Org. Lett. 2009, 11, 5546–5549. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, J.C.; Foubelo, F.; Yus, M. Tandem enantioselective conjugate addition-Mannich reactions: Efficient multicomponent assembly of dialkylzincs, cyclic enones and chiral N-sulfinimines. Tetrahedron Lett. 2008, 49, 2343–2347. [Google Scholar] [CrossRef]
- Oisaki, K.; Zhao, D.B.; Kanai, M.; Shibasaki, M. Catalytic enantioselective alkylative aldol reaction: Efficient multicomponent assembly of dialkylzincs, allenic esters, and ketones toward highly functionalized delta-lactones with tetrasubstituted chiral centers. J. Am. Chem. Soc. 2007, 129, 7439–7443. [Google Scholar] [CrossRef]
- Marigo, M.; Schulte, T.; Franzen, J.; Jorgensen, K.A. Asymmetric multicomponent domino reactions and highly enantioselective conjugated addition of thiols to alpha,beta-unsaturated aldehydes. J. Am. Chem. Soc. 2005, 127, 15710–15711. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-C.; Wang, P.-S.; Tao, Z.-L.; Han, Z.-Y.; Gong, L.-Z. An enantioselective multicomponent carbonyl allylation of aldehydes with dienes and alkynyl bromides enabled by chiral palladium phosphate. Adv. Synth. Catal. 2017, 359, 2383–2389. [Google Scholar] [CrossRef]
- Antenucci, A.; Marra, F.; Dughera, S. Silica gel-immobilised chiral 1,2-benzenedisulfonimide: A bronsted acid heterogeneous catalyst for enantioselective multicomponent passerini reaction. RSC Adv. 2021, 11, 26083–26092. [Google Scholar] [CrossRef]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, B.A.D.; Rocha, R.O.; Rodrigues, M.O. Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis. Molecules 2022, 27, 132. https://doi.org/10.3390/molecules27010132
Neto BAD, Rocha RO, Rodrigues MO. Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis. Molecules. 2022; 27(1):132. https://doi.org/10.3390/molecules27010132
Chicago/Turabian StyleNeto, Brenno A. D., Rafael O. Rocha, and Marcelo O. Rodrigues. 2022. "Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis" Molecules 27, no. 1: 132. https://doi.org/10.3390/molecules27010132





