Synthesis, Crystal Structures, and Spectroscopic Properties of Novel Gadolinium and Erbium Triphenylsiloxide Coordination Entities
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Syntheses
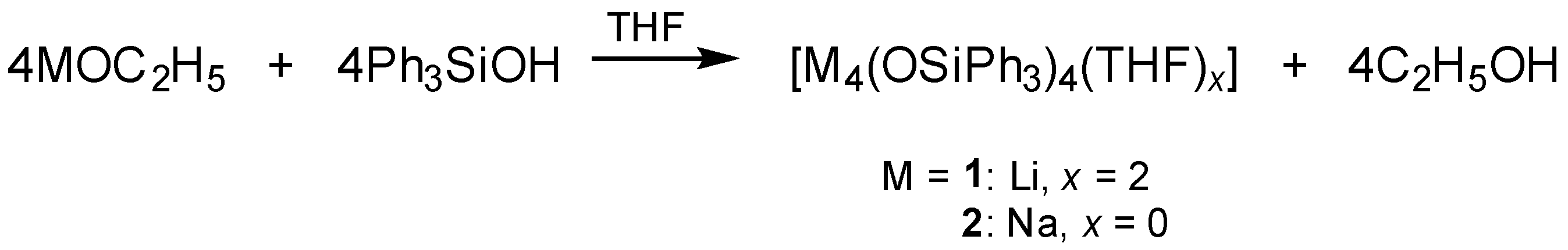
2.2.1. Synthesis of [Li4(OSiPh3)4(THF)2] (1)
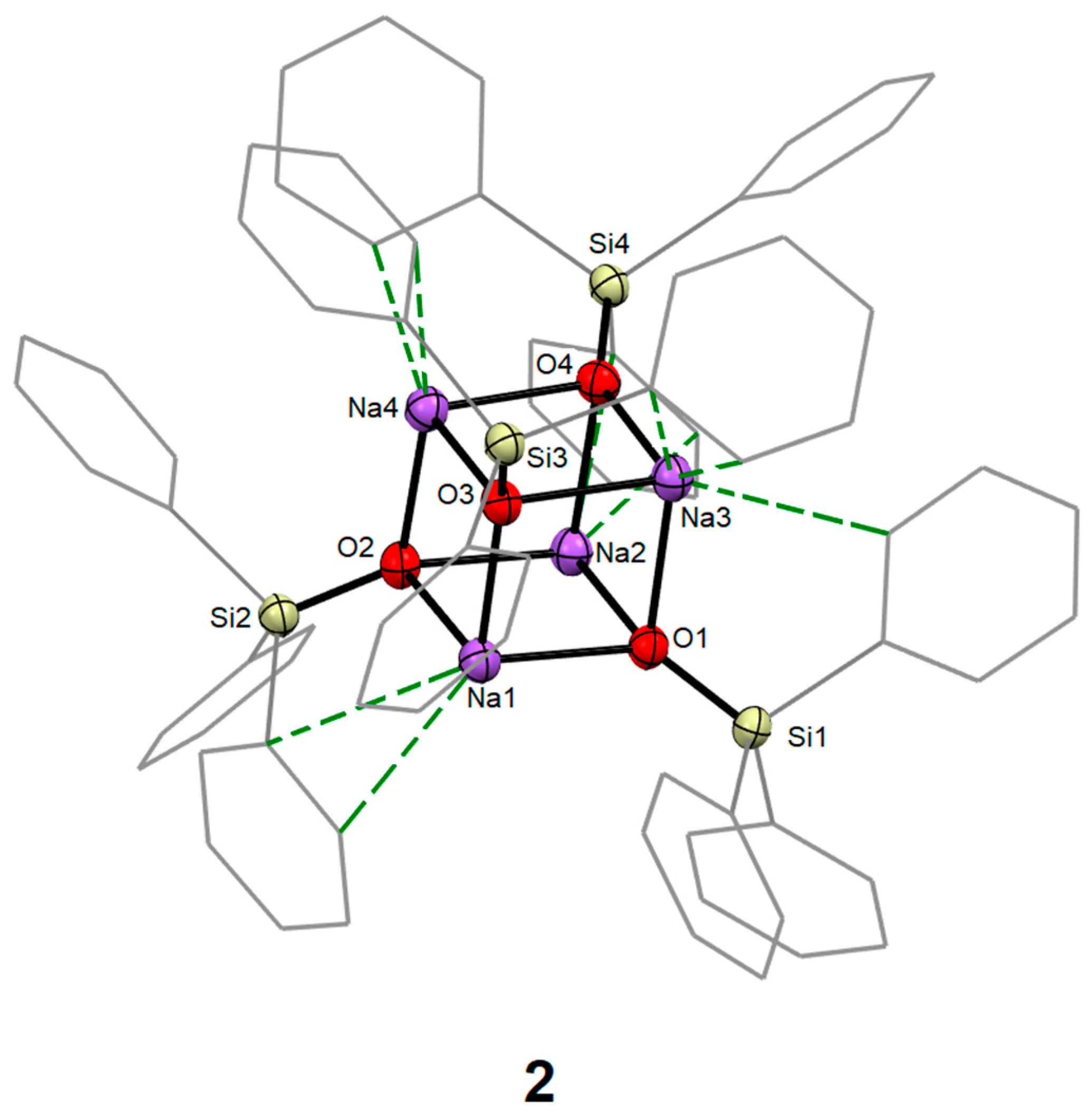
2.2.2. Synthesis of [Na4(OSiPh3)4] (2)
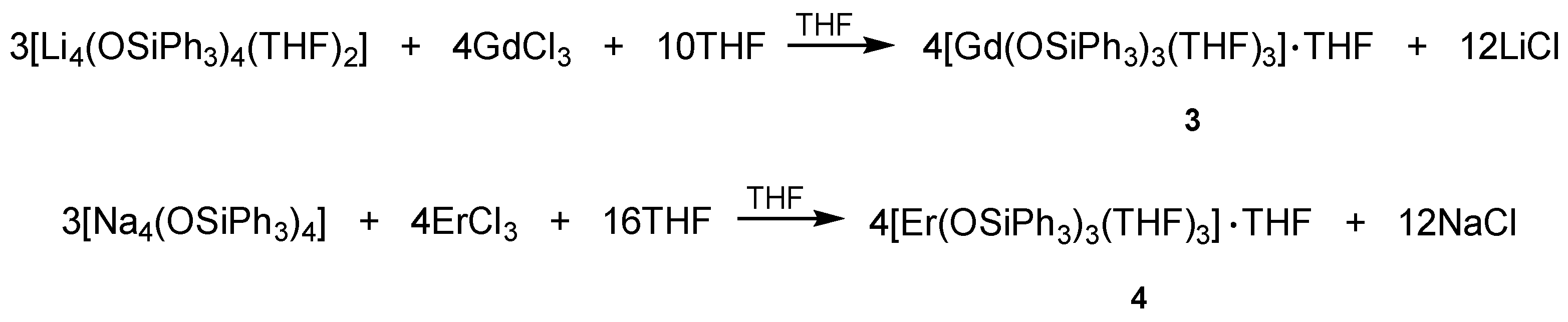
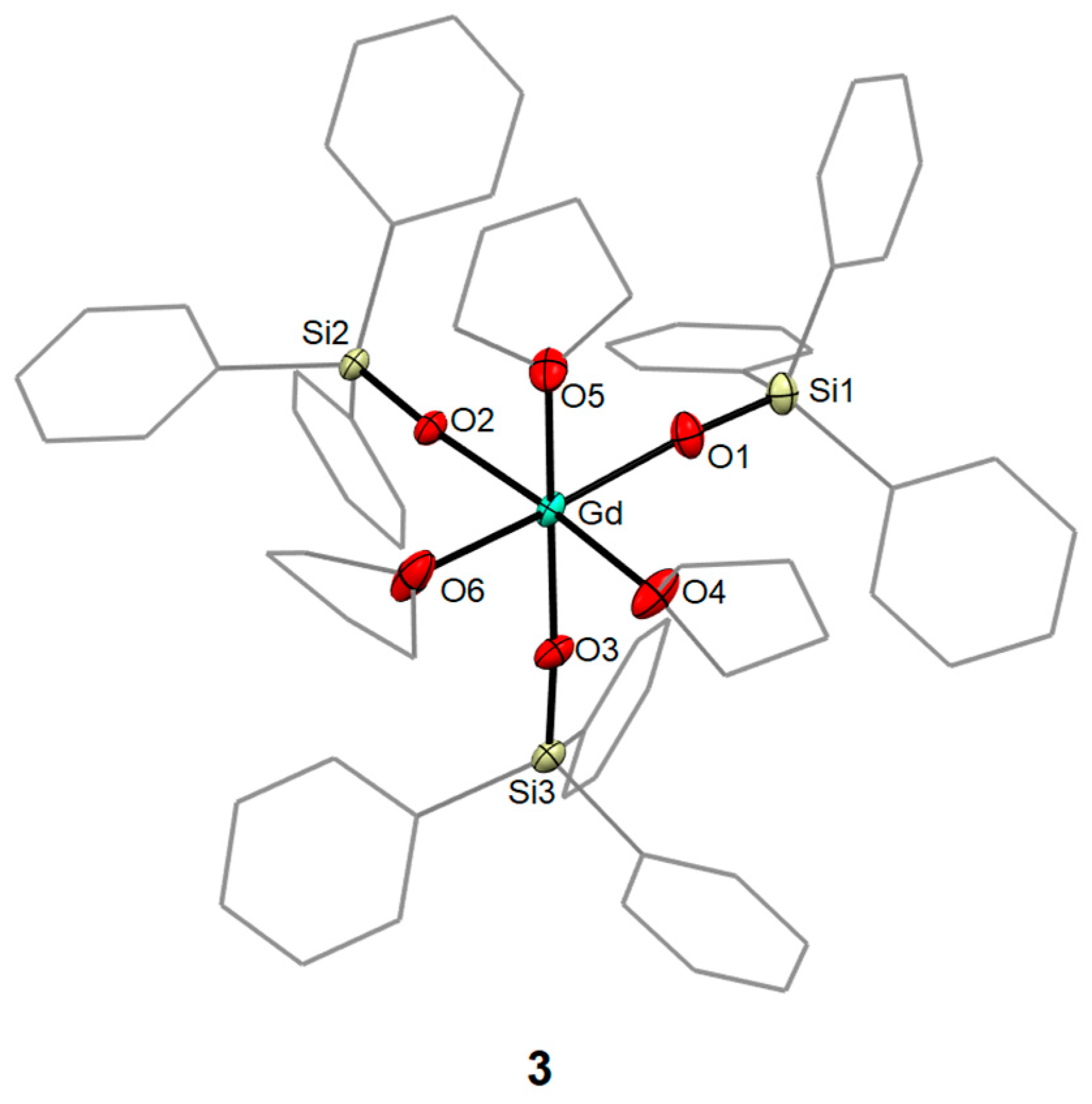
2.2.3. Synthesis of [Gd(OSiPh3)3(THF)3]·THF (3)
2.2.4. Synthesis of [Er(OSiPh3)3(THF)3]·THF (4)
3. Results and Discussion
3.1. Syntheses and Molecular Structures of 1–4
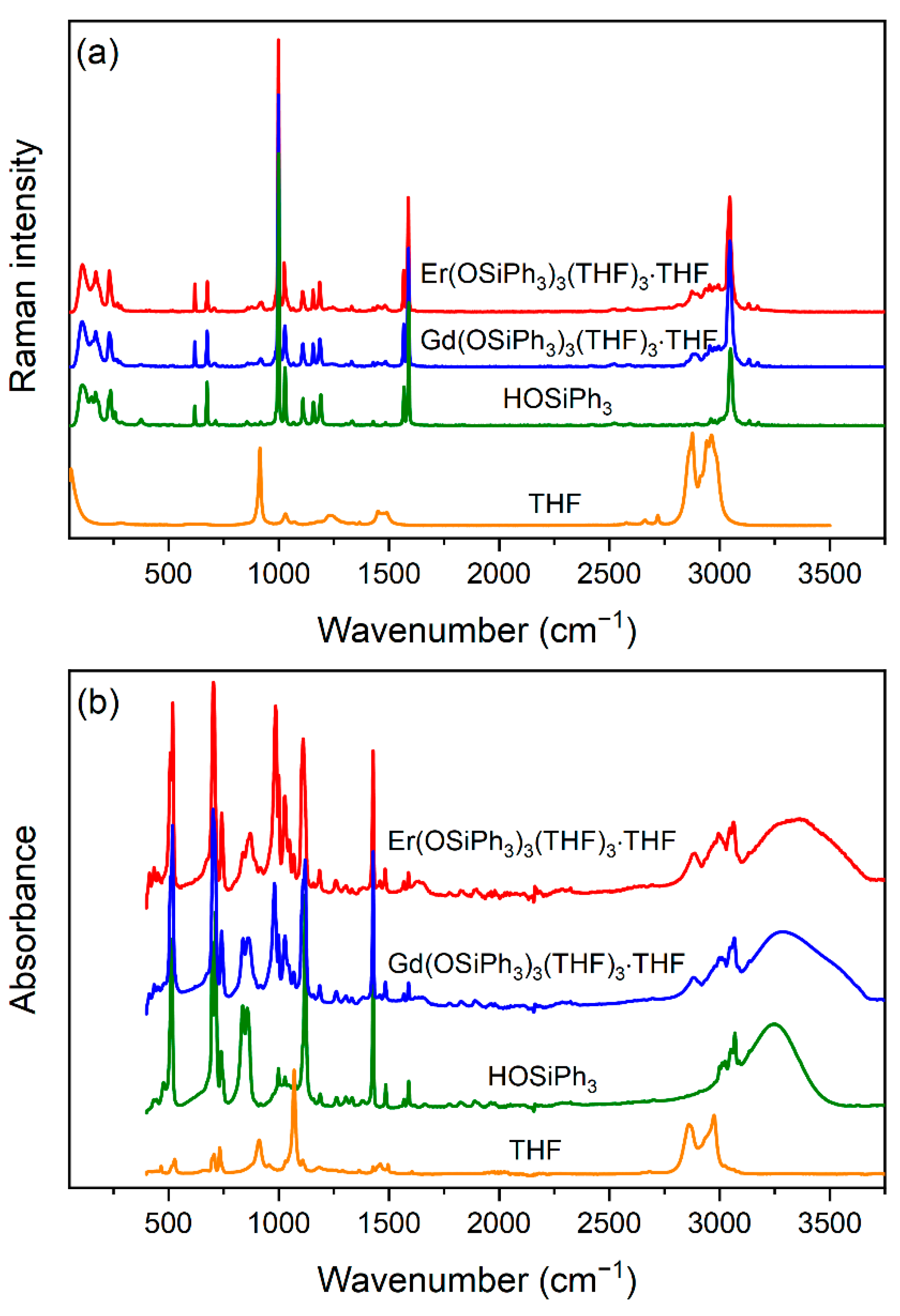
3.2. IR and Raman Spectroscopy of 3 and 4
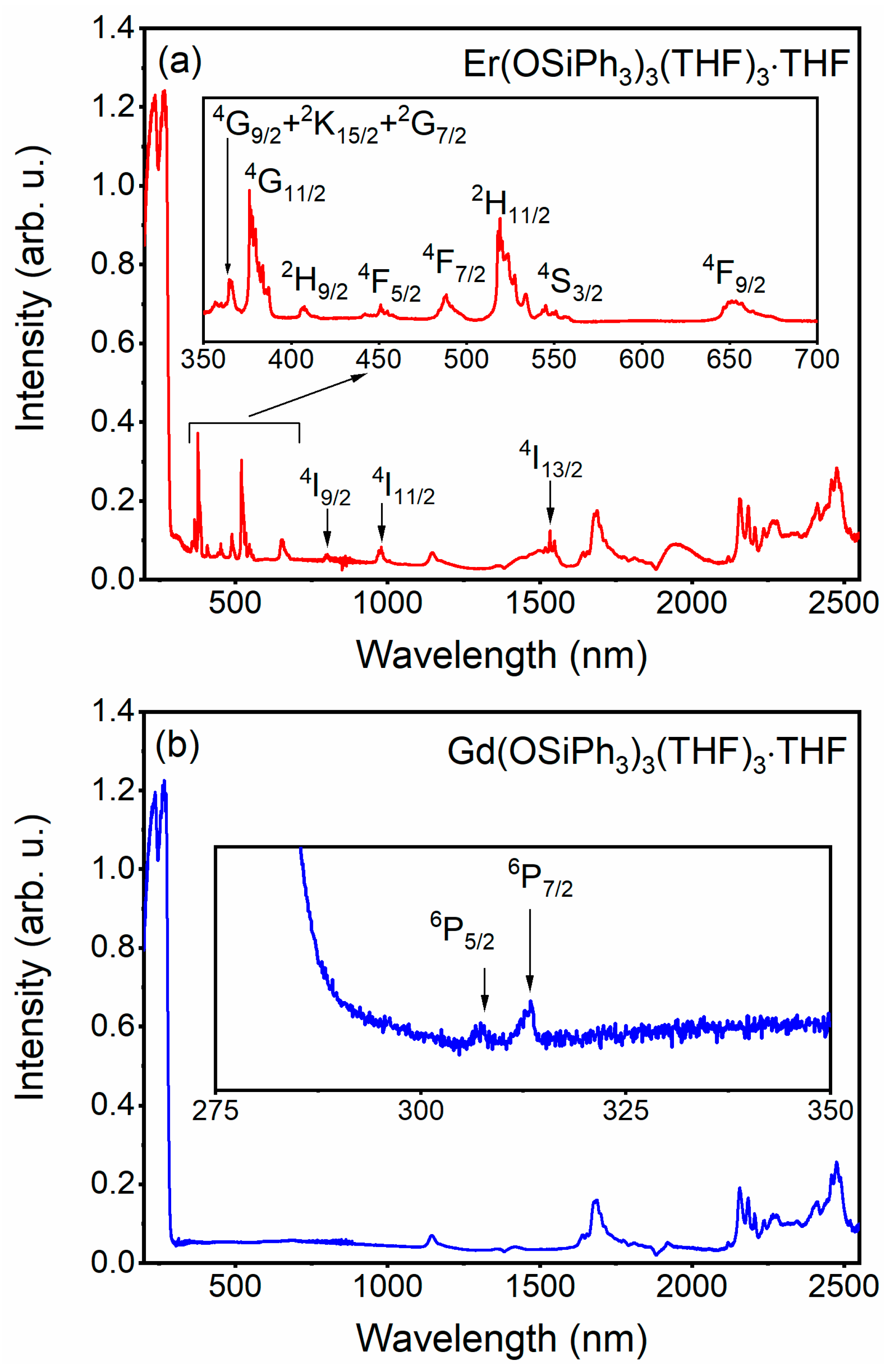
3.3. Absorption Spectra of 3 and 4
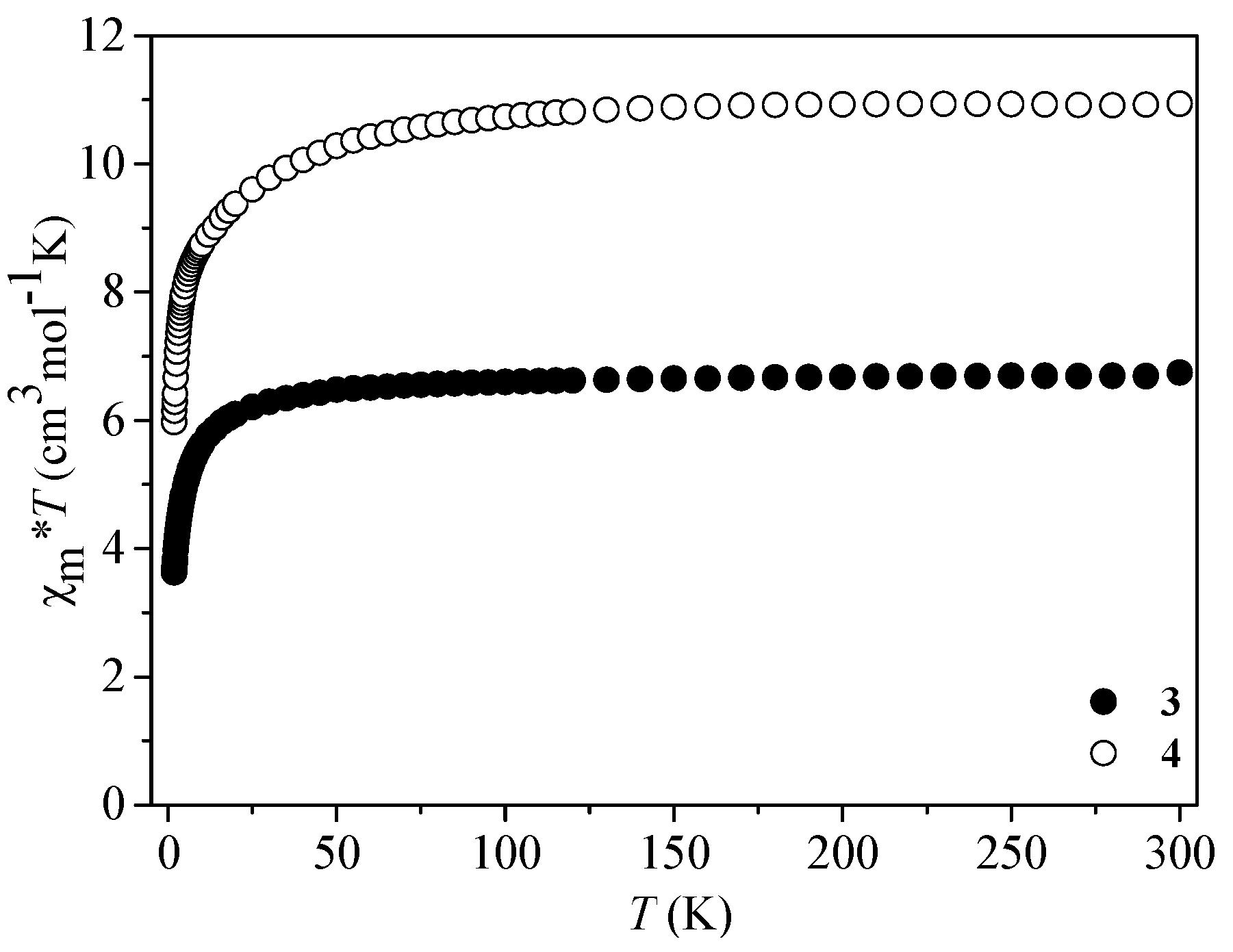
3.4. Magnetic Properties of 3 and 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Turova, N.Y.; Turevskaya, E.P.; Kessler, V.G.; Yanovskaya, M.I. The Chemistry of Metal. Alkoxides; Kluwer Academic Publishers: Boston, MA, USA, 2002. [Google Scholar]
- Bradley, D.C.; Mehrotra, R.C.; Rothwell, I.P.; Singh, A. Alkoxo and Aryloxo Derivatives of Metals; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Willauer, R.; Palumbo, C.T.; Fadaei-Tirani, F.; Zivkovic, I.; Douair, I.; Maron, L.; Mazzanti, M. Accessing the +IV Oxidation State in Molecular Complexes of Praseodymium. J. Am. Chem. Soc. 2020, 142, 5538–5542. [Google Scholar] [CrossRef] [PubMed]
- Quadri, C.C.; Larlempuia, R.; Hessevik, J.; Törnroos, K.W.; Le Roux, E. Structural Characterization of Tridentate N-Heterocyclic Carbene Titanium(IV) Benzyloxide, Silyloxide, Acetate, and Azide Complexes and Assessment of Their Efficacies for Catalyzing the Copolymerization of Cyclohexene Oxide with CO2. Organometallics 2017, 36, 4477–4489. [Google Scholar] [CrossRef]
- Petrus, R.; Drąg-Jarząbek, A.; Utko, J.; Lis, T.; Sobota, P. Transformation of molecular compounds with Ba(Sr)/Al/Si and Ca(Sr, Ba)/Ti(Zr, Hf)/Si heteroelements as new efficient route to metal silicate materials. Dalton Trans. 2019, 48, 4283–4298. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.F.; Johannson, O.K.; Daudt, W.H.; Fleming, R.F.; Laudenslager, H.B.; Roche, M.P. Sodium and Potassium Salts of Triorganosilanols. J. Am. Chem. Soc. 1953, 75, 5615–5618. [Google Scholar] [CrossRef]
- McGeary, M.J.; Folting, K.; Streib, W.E.; Huffman, J.C.; Caulton, K.G. Oligomerization and Structural Variation of Alkali Metal Silyloxides. Polyhedron 1991, 10, 2699–2709. [Google Scholar] [CrossRef]
- Wagler, J.; Heimfarth, J. CSD Communication; Refcode COTFAT (Deposition, No. 720955); CCDC: Cambridge, UK, 2009. [Google Scholar]
- Clegg, W. CSD Communication; Refcode UQUGUK (Deposition, No. 1488886); CCDC: Cambridge, UK, 2016. [Google Scholar]
- Mommertz, A.; Dehnicke, K.; Magull, J. Die Kristallstruktur von [Na4(OSiPh3)4(H2O)3]. Z. Naturforsch. B 1996, 51, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- Wytrych, P.; Utko, J.; Lis, T.; John, Ł. Polyoxometalate-like structure of new potassium triphenylsiloxides: [K6(OSiPh3)6(C3H7OH)(H2O)]·2C6H5CH3 and [K6(OSiPh3)6(H2O)2]. Acta Cryst. 2021, C77, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Sobota, P.; Utko, J.; Ejfler, J.; Jerzykiewicz, L.B. Syntheses and molecular structures of [Mg4(THFFO)6(OSiPh3)2] and [Al3Mg(μ3-O)(THFFO)3(Me)6] relevant to Ziegler-Natta catalyst intermediates (THFFO = 2-tetrahydrofurfuroxide). Organometallics 2000, 19, 4929–4931. [Google Scholar] [CrossRef]
- Sobota, P.; Przybylak, S.; Ejfler, J.; Kobyłka, M.; Jerzykiewicz, L.B. Synthesis and structural characterization of magnesium and titanium siloxanes. Inorg. Chim. Acta 2002, 334, 159–164. [Google Scholar] [CrossRef]
- Willauer, A.R.; Polumbo, C.T.; Scopelliti, R.; Zivkovic, I.; Douair, I.; Moron, L.; Mazzanti, M. Stabilization of the Oxidation State +IV in Siloxide-Supported Terbium Compounds. Angew. Chem. Int. Ed. 2020, 59, 3549–3553. [Google Scholar] [CrossRef] [PubMed]
- Gradeff, P.S.; Yunlu, K.; Deming, T.J.; Olofson, J.M.; Doedens, R.J.; Evans, W.J. Synthesis of yttrium and lanthanide silyloxy complexes from anhydrous nitrate and oxoalkoxide precursors and the x-ray crystal structure of [Ce(OSiPh3)3(THF)3](THF). Inorg. Chem. 1990, 29, 420–424. [Google Scholar] [CrossRef]
- McGeary, M.J.; Coan, P.S.; Folting, K.; Streib, W.E.; Caulton, K.G. Yttrium and Lanthanum Silyloxide Complexes. Inorg. Chem. 1991, 30, 1723–1735. [Google Scholar] [CrossRef]
- Chakraborty, J.; Ray, A.; Pilet, G.; Chastanet, G.; Luneau, D.; Ziessel, R.F.; Charbonniere, L.J.; Carrella, L.; Rentschler, E.; Fallahe, M.S.E.; et al. Syntheses, characterisation, magnetism and photoluminescence of a homodinuclear Ln (III)-Schiff base family. Dalton Trans. 2009, 10263–10272. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Brooker, S. Review of purely 4f and mixed-metal nd-4f single-molecule magnets containing only one lanthanide Ion. Coord. Chem. Rev. 2014, 276, 1–13. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.Y.; Liu, C.M.; Li, Y.Z.; Zuo, J.L. A sandwich-type triple-decker lanthanide complex with mixed phthalocyanine and Schiff base ligands. Dalton Trans. 2013, 42, 11043–11046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Y.; Chen, Y.; Pregot, C.; Li, T.; Balasubramaniam, S.; Hobart, D.B.; Zhang, Y.; Wi, S.; Davis, R.M.; et al. Gd3N@C84(OH)x: A new egg-shaped metallofullerene magnetic resonance imaging contrast agent. J. Am. Chem. Soc. 2014, 136, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Deacon, G.B.; Gazukin, O.; Junk, P.C.; Kersting, B.; Langley, S.K.; Moubaraki, B.; Murray, K.S.; Schleife, F.; Shome, M.; et al. Structure, Magnetic Behavior, and Anisotropy of Homoleptic Trinuclear Lanthanoid 8-Quinolinolate Complexes. Inorg. Chem. 2014, 53, 2528–2534. [Google Scholar] [CrossRef]
- Upadhyay, A.; Komatireddy, N.; Ghirri, A.; Tuna, F.; Langley, S.K.; Srivastava, A.K.; Sanudo, E.C.; Moubaraki, B.; Murray, K.S.; McInnes, E.J.L.; et al. Synthesis and magnetothermal properties of a ferromagnetically coupledNiII–GdIII–NiII luster. Dalton Trans. 2014, 43, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Matalon, E.; Huber, T.; Hagelueken, G.; Graham, B.; Frydman, V.; Feintuch, A.; Otting, G.; Goldfarb, D. Gadolinium(III) spin labels for high-sensitivity distance measurements in transmembrane helices. Angew. Chem. Int. Ed. 2013, 52, 11831–11834. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Kückmann, T.I.; Bolte, M.; Wagner, M.; Lerner, H.W. Adducts of NaOSiMePh2 Oligomers with THF and Cyclopentadienyl Iron Dicarbonyl Dimer. Z. Anorg. Allg. Chem. 2007, 633, 290–297. [Google Scholar] [CrossRef]
- Lerner, H.W.; Scholz, S.; Bolte, S. The Sodium Siloxides (tBu3SiONa)4 and (tBu2PhSiONa)4: Synthesis and X-ray Crystal Structure Analysis. Organometallics 2002, 21, 3827–3830. [Google Scholar] [CrossRef]
- Boyle, T.J.; Ottley, L.A.M.; Brewer, L.N.; Sigman, J.; Clem, P.G.; Richardson, J.J. Structurally characterized erbium alkoxides for use as an amphoteric dopant in PErZT ceramic thin film and nanoparticles. Eur. J. Inorg. Chem. 2007, 24, 3805–3815. [Google Scholar] [CrossRef]
- Xie, Z.; Chui, K.; Yang, Q.; Mak, T.C.W.; Sun, J. Synthesis, Molecular Structure, and Reactivity of Organolanthanide Fluoride Complexes, [{(Me3Si)2C5H3}2Ln(μ-F)]2 (Ln = La, Nd, Sm, Gd) and [(C5H5)2Ln(μ-F)(THF)]2 (Ln = Y, Yb). Organometallics 1998, 17, 3937–3944. [Google Scholar] [CrossRef]
- Boyle, T.J.; Bunge, S.D.; Clem, P.G.; Richardson, J.; Dawley, J.T.; Ottley, L.A.M.; Rodriguez, M.A.; Tuttle, B.A.; Avilucea, G.R.; Tissot, R.G. Synthesis and Characterization of a Family of Structurally Characterized Dysprosium Alkoxides for Improved Fatigue-Resistance Characteristics of PDyZT Thin Films. Inorg. Chem. 2005, 44, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, D.J.; Howard, K.E.; Payne, D.A.; Wilson, S.R. Crystal structure of lithium niobium ethoxide (LiNb(OCH2CH3)6: A precursor for lithium niobate ceramics. Inorg. Chem. 1990, 29, 1458–1459. [Google Scholar] [CrossRef]
- Shurvell, H.F.; Southby, M.C. Infrared and Raman spectra of tetrahydrofuran hydroperoxide. Vib. Spectrosc. 1997, 15, 137–146. [Google Scholar] [CrossRef]
- Billes, F.; Böhling, H.; Ackermann, M.; Kudra, M. A vibrational spectroscopic study on furan and its hydrated derivatives. J. Mol. Struct. 2004, 672, 1–16. [Google Scholar] [CrossRef]
- Ishida, H.; Koenig, J.L. Vibrational Assignments of Organosilanetriols. I. Vinylsilanetriol and Vinylsilanetriol-d3 in Aqueous Solutions. Appl. Spect. 1978, 32, 462–469. [Google Scholar] [CrossRef]
- Carteret, C. Vibrational properties of silanol group: From alkylsilanol to small silica cluster: Effects of silicon substituents. Spectrochim. Acta A 2006, 64, 670–680. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Gong, X.; Luo, Z.; Huang, Y. Spectroscopic properties and laser performance of Er3+ and Yb3+ co-doped GdAl3(BO3)4 crystal. IEEE J. Quantum Electron. 2007, 43, 950–956. [Google Scholar] [CrossRef]
- Lira, A.C.; Flores, M.; Arroyo, R.; Caldiño, U. Optical spectroscopy of Er3+ ions in poly(acrylicacid). Opt. Mater. 2006, 28, 1171–1177. [Google Scholar] [CrossRef]
- Martincik, J.; Ishizu, S.; Fukuda, K.; Suyama, T.; Cechak, T.; Beitlerova, A.; Yoshikawa, A.; Nikl, M. Concentration dependence study of VUV-UV-visible luminescence of Nd 3+ and Gd3+ in LuLiF4. Opt. Mater. 2012, 34, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef]
- Biswas, B.; Raghavaiah, P.; Aliaga-Alcalde, N.; Chen, J.D.; Ghosh, R. Syntheses, crystal structures and properties of a new family of isostructural and isomorphous compounds of type [M(L)(NCS)3] [M = La, Gd, Tb and Dy; L = a neutral hexadentate Schiff base]. Polyhedron 2010, 29, 2716–2721. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Martínez-Pérez, M.J.; Cardona-Serra, S.; Schlegel, C.; Moro, F.; Alonso, P.J.; Prima-García, H.; Clemente-Juan, J.M.; Evangelisti, M.; Gaita-Ariño, A.; Sesé, J.; et al. Gd-based single-ion magnets with tunable magnetic anisotropy: Molecular design of spin qubits. Phys. Rev. Lett. 2012, 108, 247213–247217. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Das, C.; Shanmugam, M.; Langley, S.K.; Murray, K.S.; Shanmugam, M. Electronic and Magnetic Properties of a Gadolinium(III) Schiff Base Complex. Eur. J. Inorg. Chem. 2014, 26, 4320–4325. [Google Scholar] [CrossRef]
- Gao, T.; Yan, P.F.; Li, G.M.; Zhang, J.W.; Sun, W.B.; Suda, M.; Einaga, Y. Correlations between structure and magnetism of three N,N′-ethylene-bis(3-methoxysalicylideneimine) gadolinium complexes. Solid State Sci. 2010, 12, 597–604. [Google Scholar] [CrossRef]


| 1. | Li1···O1 | 2.005(2) | Si1···C1A | 1.8798(14) |
| Li1···O2 | 2.003(2) | Si1···C1B | 1.8945(14) | |
| Li1···O2i | 1.996(2) | Si1···C1C | 1.8913(14) | |
| Li1···O3 | 1.942(2) | Si2···O2 | 1.6056(9) | |
| Li2···O1 | 1.900(2) | Si2···C1D | 1.8944(13) | |
| Li2···O1i | 1.928(2) | Si2···C1E | 1.8797(14) | |
| Li2···O2 | 1.890(2) | Si2···C1F | 1.8912(13) | |
| Si1···O1 | 1.6055(9) | O3···Li1···O2 | 115.08(11) | |
| O2···Li1···O1 | 95.59(10) | O3···Li1···O2i | 125.10(12) | |
| O2···Li1···O1i | 94.59(10) | O2···Li2···O1 | 103.12(11) | |
| O2···Li1···O2i | 96.68(10) | O2···Li2···O1i | 100.73(11) | |
| O3···Li1···O1 | 123.17(12) | O1···Li2···O1i | 99.60(11) | |
| 2 | Na1···O1 | 2.239(3) | Si1···C1B | 1.908(3) |
| Na1···O2 | 2.346(3) | Si1···C1C | 1.895(3) | |
| Na1···O3 | 2.235(3) | Si2···O2 | 1.589(2) | |
| Na2···O1 | 2.231(3) | Si2···C1D | 1.895(4) | |
| Na2···O2 | 2.209(3) | Si2···C1E | 1.881(3) | |
| Na2···O4 | 2.331(3) | Si2···C1F | 1.904(3) | |
| Na3···O1 | 2.307(3) | Si3···O3 | 1.589(2) | |
| Na3···O3 | 2.311(3) | Si3···C1G | 1.897(4) | |
| Na3···O4 | 2.198(3) | Si3···C1H | 1.907(3) | |
| Na4···O2 | 2.248(3) | Si3···C1I | 1.886(3) | |
| Na4···O3 | 2.262(3) | Si4···O4 | 1.587(2) | |
| Na4···O4 | 2.281(3) | Si4···C1J | 1.894(3) | |
| Si1···O1 | 1.588(3) | Si4···C1K | 1.896(4) | |
| Si1···C1A | 1.895(3) | Si4···C1L | 1.882(3) | |
| O1···Na1···O2 | 90.33(9) | O1···Na3···O3 | 89.24(9) | |
| O1···Na1···O3 | 92.93(9) | O1···Na3···O4 | 93.45(9) | |
| O2···Na1···O3 | 91.99(9) | O3···Na3···O4 | 92.58(9) | |
| O1···Na2···O2 | 94.19(9) | O2···Na4···O3 | 93.87(9) | |
| O1···Na2···O4 | 91.94(9) | O2···Na4···O4 | 91.59(9) | |
| O2···Na2···O4 | 91.25(9) | O3···Na4···O4 | 91.71(9) |
| 3 | 4 | ||
|---|---|---|---|
| Gd···O1 | 2.170(3) | Er···O1 | 2.119(8) |
| Gd···O2 | 2.157(3) | Er···O2 | 2.118(9) |
| Gd···O3 | 2.146(3) | Er···O3 | 2.108(8) |
| Gd···O4 | 2.461(3) | Er···O4 | 2.428(9) |
| Gd···O5 | 2.497(3) | Er···O5 | 2.407(9) |
| Gd···O6 | 2.478(3) | Er···O6 | 2.375(9) |
| O1···Gd···O4 | 90.24(12) | O1···Er···O4 | 88.9(3) |
| O1···Gd···O5 | 86.47(12) | O1···Er···O5 | 86.8(4) |
| O1···Gd···O6 | 164.51(13) | O1···Er···O6 | 164.5(4) |
| O2···Gd···O1 | 101.37(12) | O2···Er···O1 | 102.2(4) |
| O2···Gd···O4 | 163.71(10) | O2···Er···O4 | 85.8(3) |
| O2···Gd···O5 | 88.23(11) | O2···Er···O5 | 162.3(4) |
| O2···Gd···O6 | 86.42(12) | O2···Er···O6 | 88.6(4) |
| O3···Gd···O1 | 100.83(12) | O3···Er···O1 | 100.6(3) |
| O3···Gd···O2 | 101.42(11) | O3···Er···O2 | 101.3(4) |
| O3···Gd···O4 | 87.37(11) | O3···Er···O4 | 166.6(3) |
| O3···Gd···O5 | 166.36(11) | O3···Er···O5 | 91.9(4) |
| O3···Gd···O6 | 90.56(13) | O3···Er···O6 | 88.0(3) |
| O4···Gd···O5 | 81.05(11) | O5···Er···O4 | 79.1(4) |
| O4···Gd···O6 | 79.74(12) | O6···Er···O4 | 80.7(3) |
| O6···Gd···O5 | 80.35(13) | O6···Er···O5 | 80.0(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wytrych, P.; Utko, J.; Kłak, J.; Ptak, M.; Stefanski, M.; Lis, T.; Ejfler, J.; John, Ł. Synthesis, Crystal Structures, and Spectroscopic Properties of Novel Gadolinium and Erbium Triphenylsiloxide Coordination Entities. Molecules 2022, 27, 147. https://doi.org/10.3390/molecules27010147
Wytrych P, Utko J, Kłak J, Ptak M, Stefanski M, Lis T, Ejfler J, John Ł. Synthesis, Crystal Structures, and Spectroscopic Properties of Novel Gadolinium and Erbium Triphenylsiloxide Coordination Entities. Molecules. 2022; 27(1):147. https://doi.org/10.3390/molecules27010147
Chicago/Turabian StyleWytrych, Patrycja, Józef Utko, Julia Kłak, Maciej Ptak, Mariusz Stefanski, Tadeusz Lis, Jolanta Ejfler, and Łukasz John. 2022. "Synthesis, Crystal Structures, and Spectroscopic Properties of Novel Gadolinium and Erbium Triphenylsiloxide Coordination Entities" Molecules 27, no. 1: 147. https://doi.org/10.3390/molecules27010147






