A Study of the Key Factors on Production of Graphene Materials from Fe-Lignin Nanocomposites through a Molecular Cracking and Welding (MCW) Method
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Preparation of Iron (Fe)-Lignin Nanocomposites
2.3. Stabilization of Fe-Lignin Composites
2.4. Catalytic Graphitization of Fe-Lignin Composites to Graphene-Encapsulated Iron Nanoparticles (GEINs)
2.5. Production of Graphene Nanomaterials through the Molecular Cracking and Welding (MCW) Process
2.6. Characterization
3. Results and Discussion
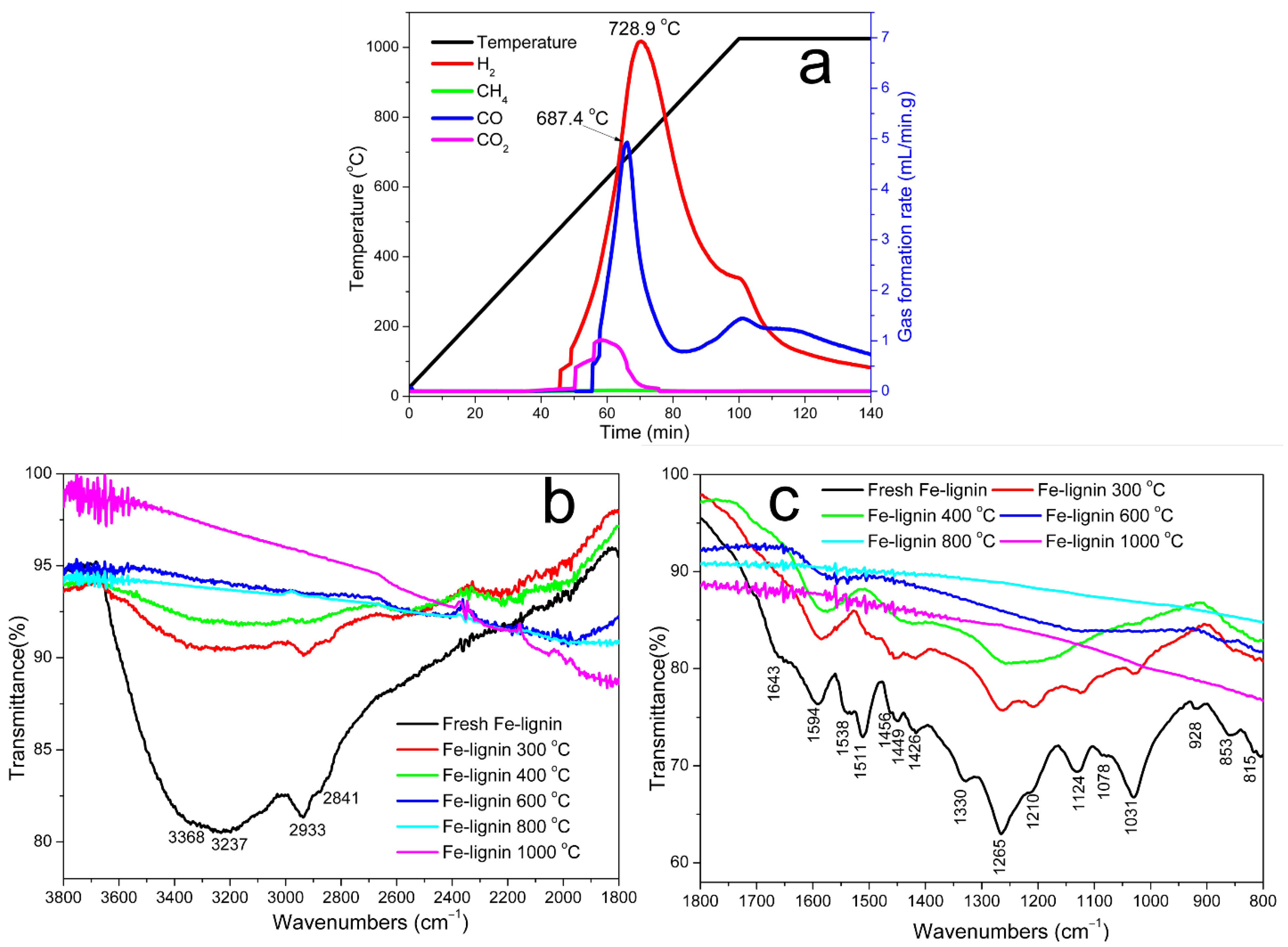
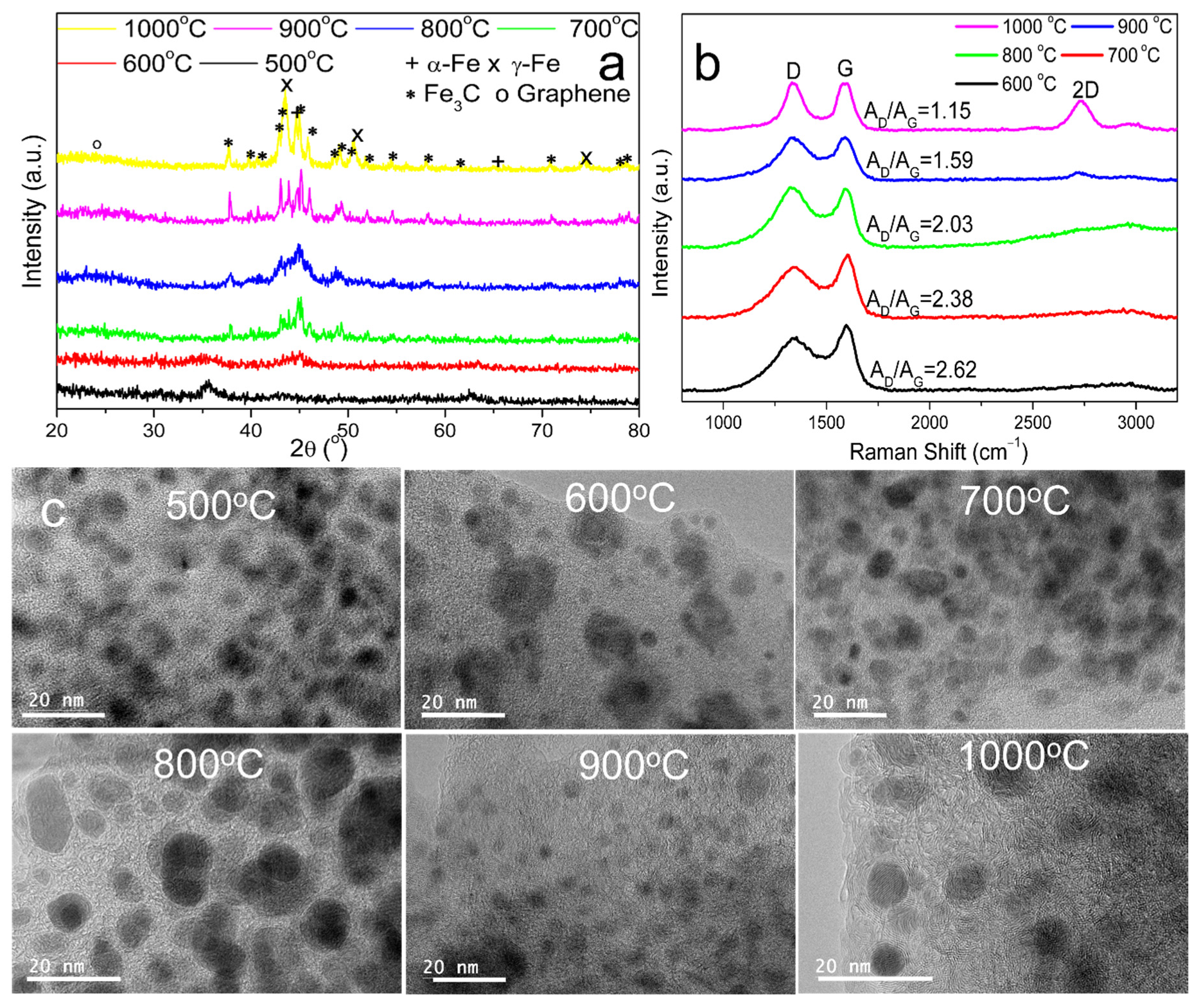
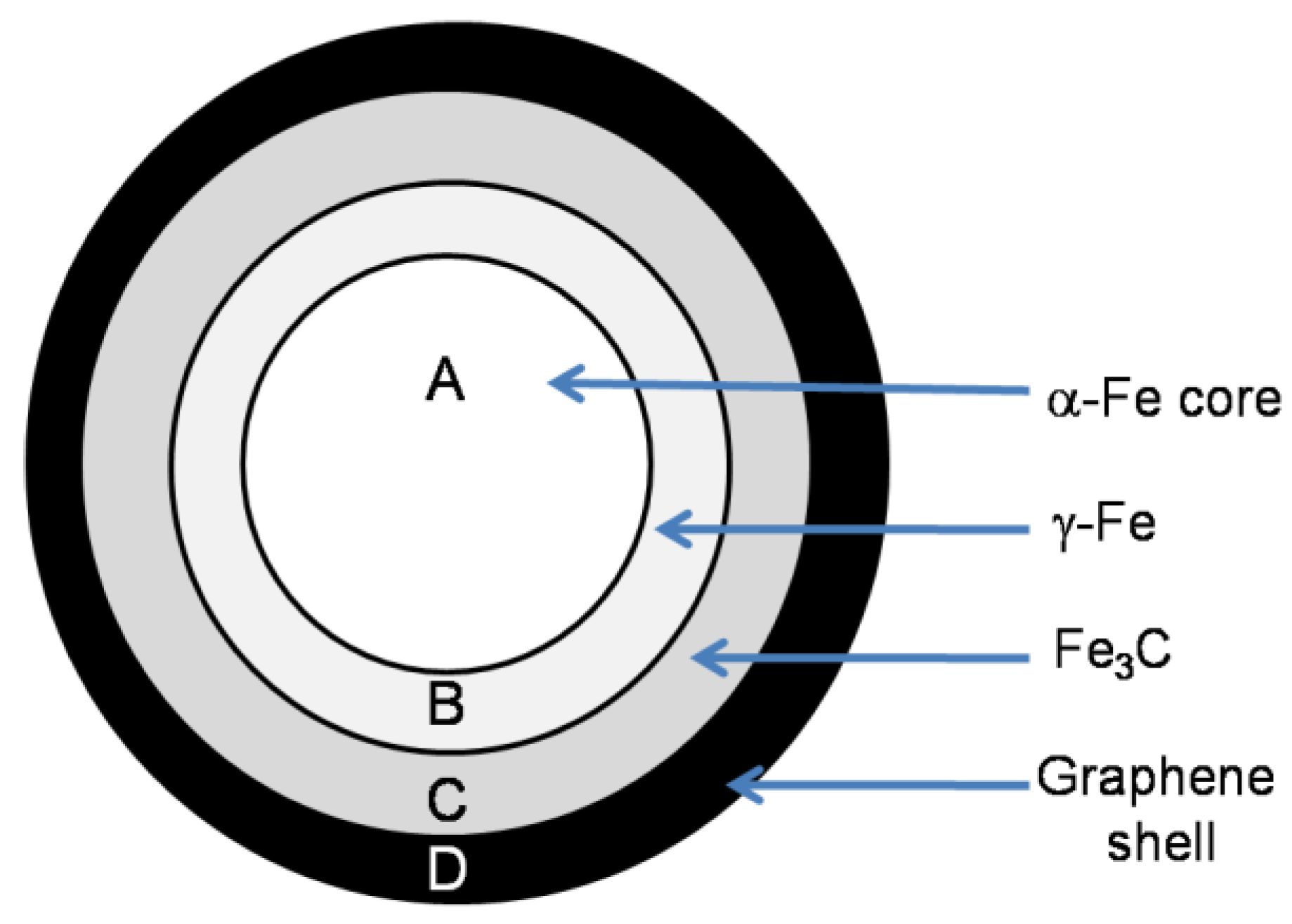
3.1. Formation of Graphene-Encapsulated Iron Nanoparticles (GEINs)
Effect of Temperature on Catalytic Graphitization Kraft Lignin to GEINs
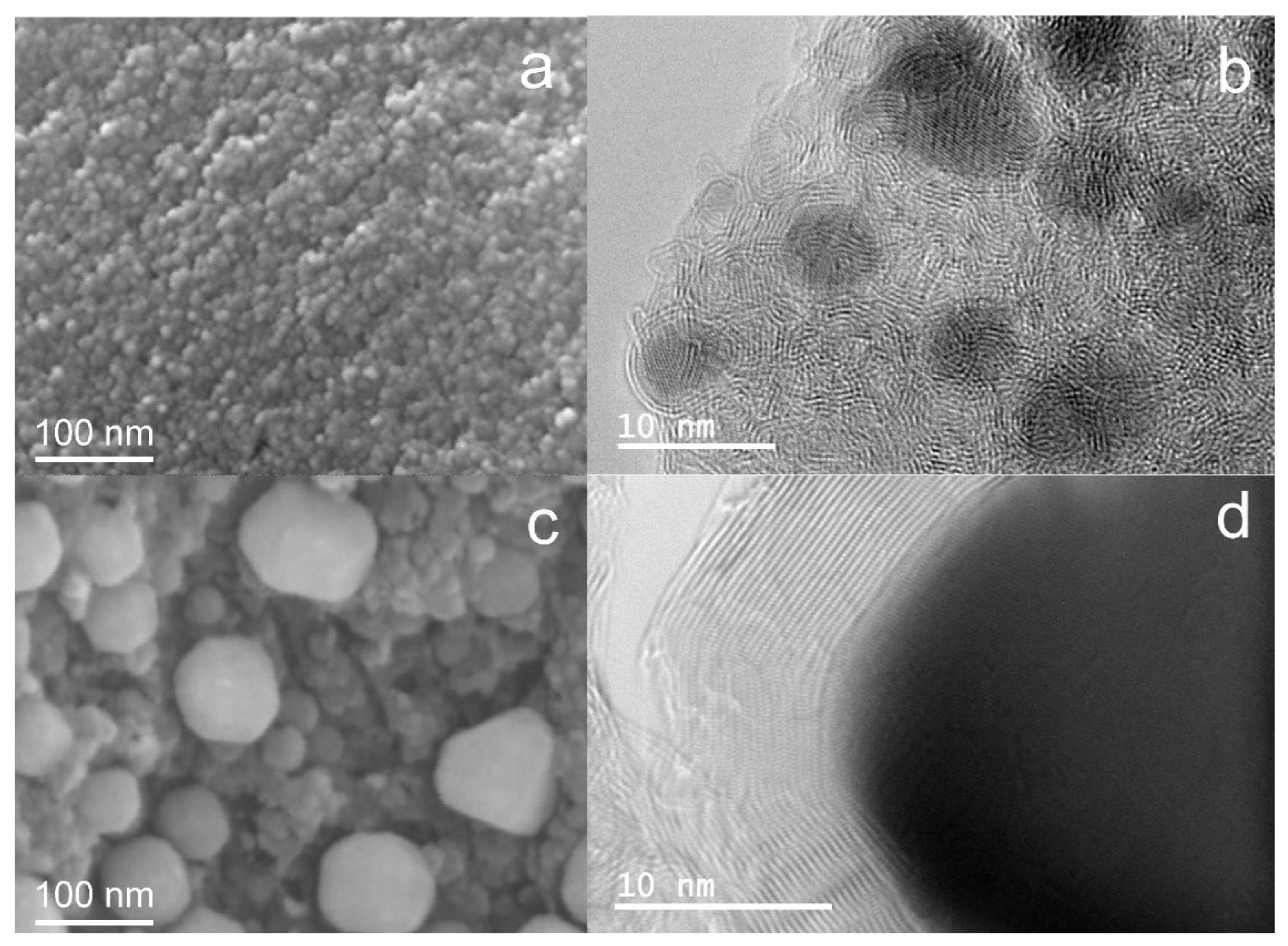
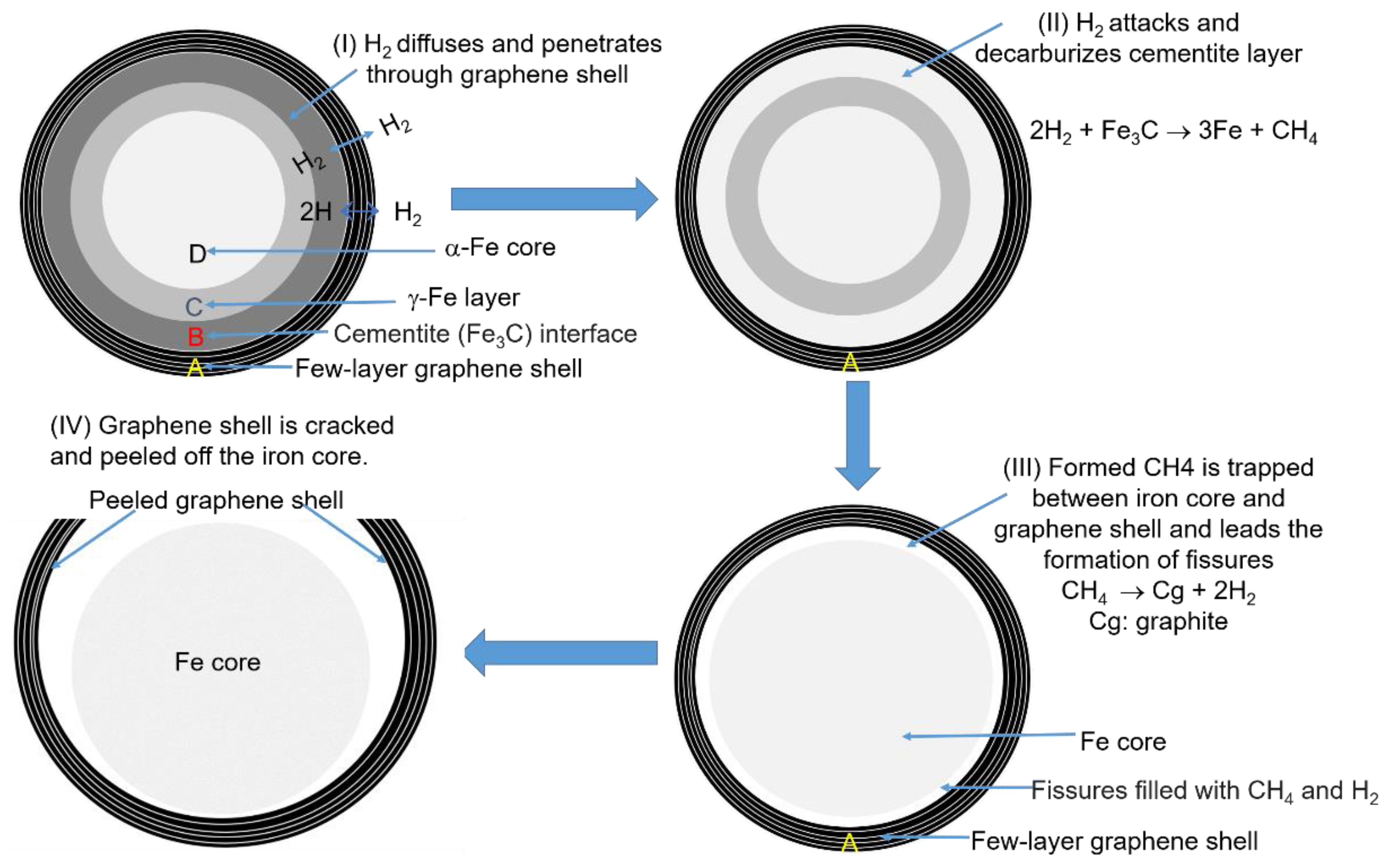
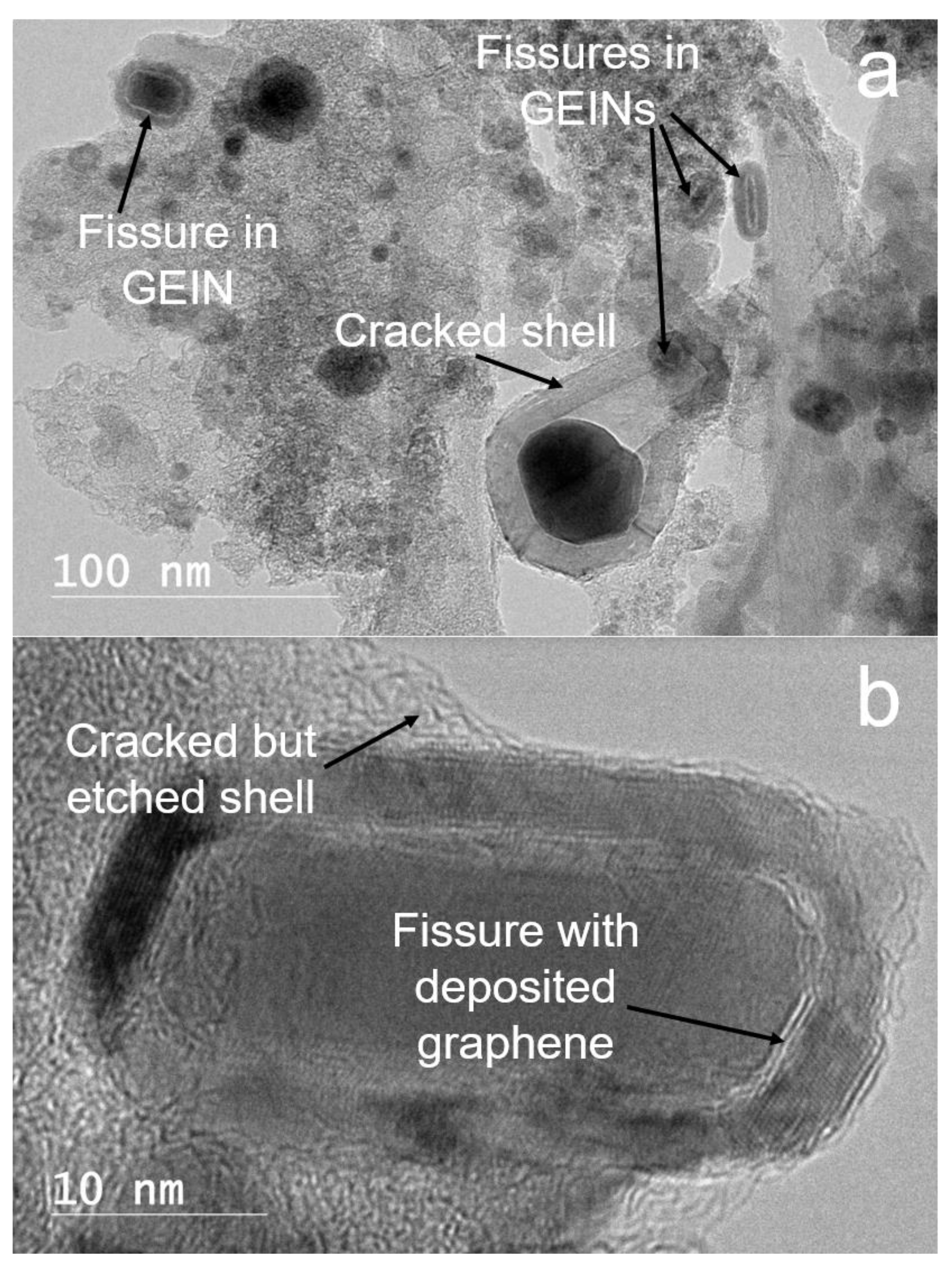
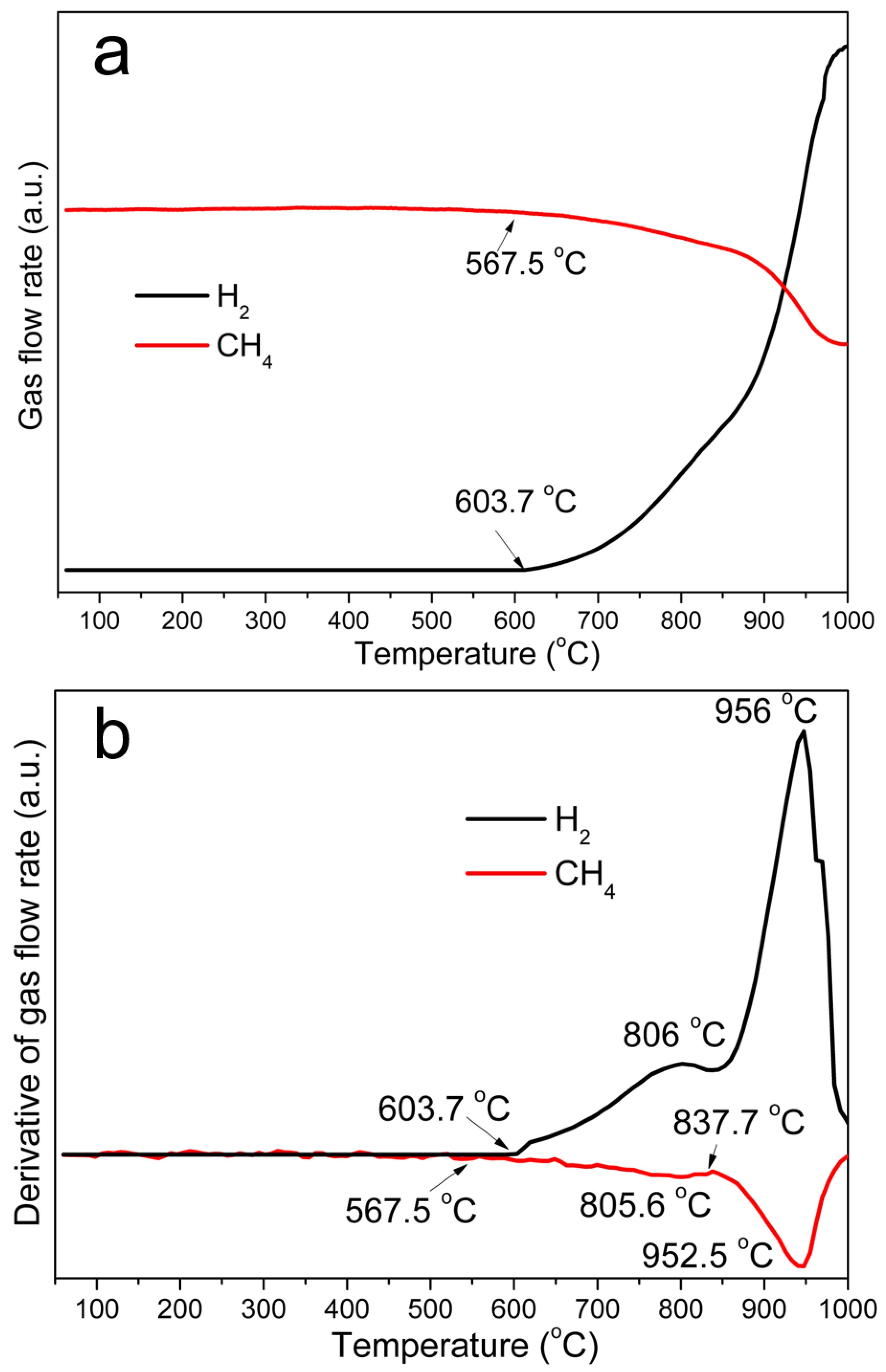
3.2. Breaking of the GEINs by Cracking Reagents and Peeling the Graphene Shells off the Iron Cores
3.3. Effect of Process Variables on Graphene Formation through MCW Process
3.3.1. Effect of Heating Temperature on Formation of Graphene Materials through MCW Process
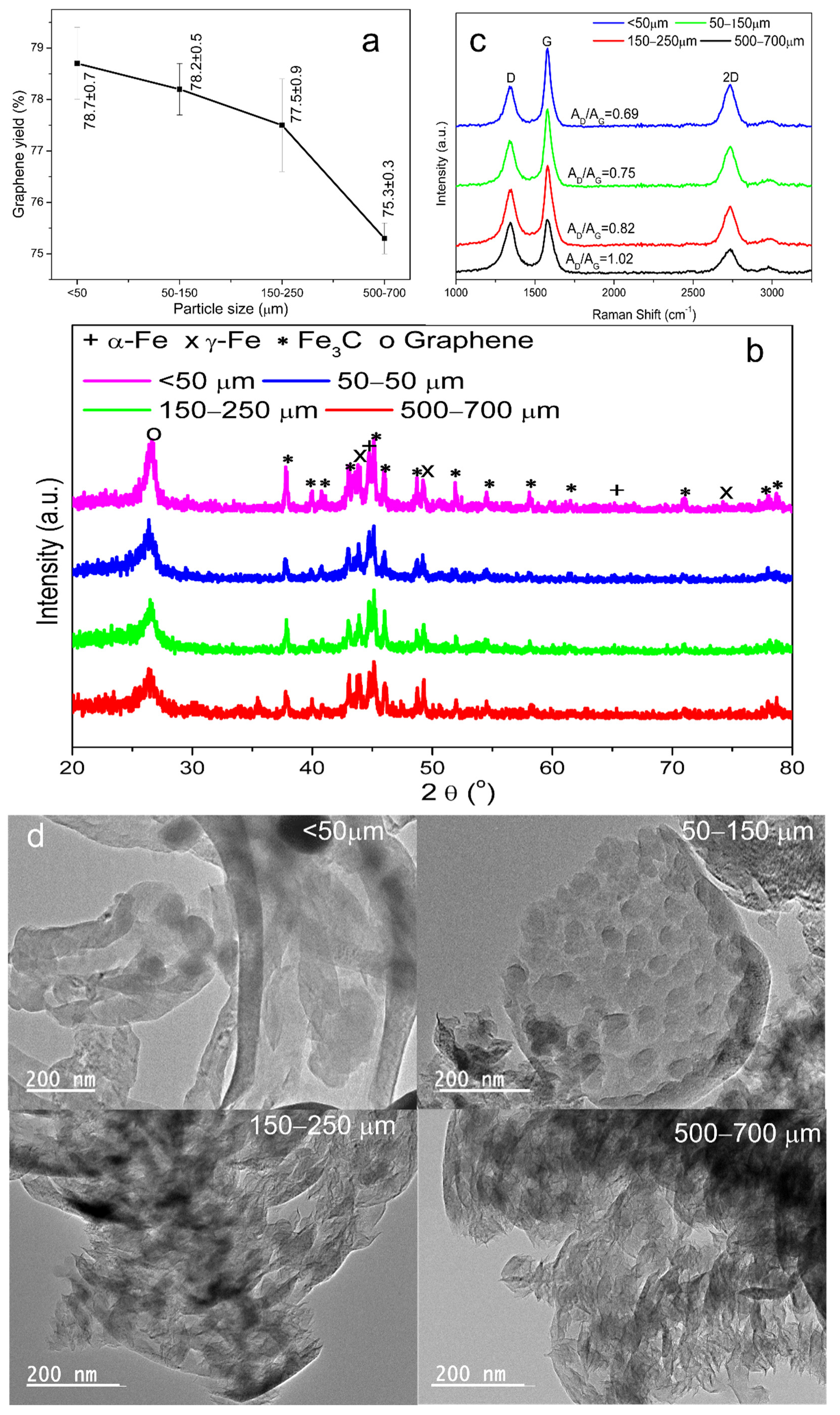
3.3.2. Effects of GEIN Grain Particle Sizes
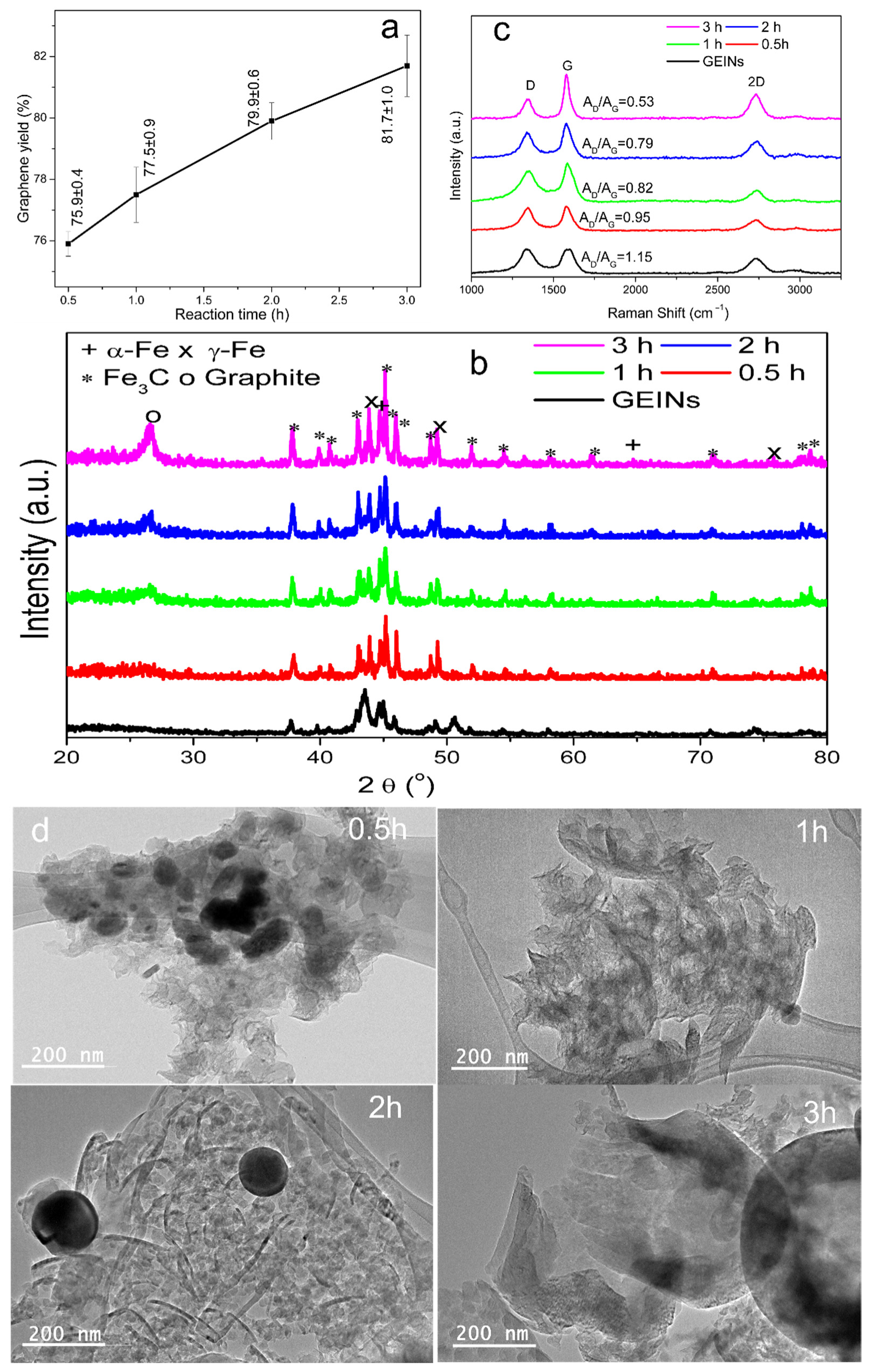
3.3.3. Effects of MCW Reaction Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefin. 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Pérez, A.D.; Fiskari, J.; Schuur, B. Delignification of Low-Energy Mechanical Pulp (Asplund Fibers) in a Deep Eutectic Solvent System of Choline Chloride and Lactic Acid. Front. Chem. 2021, 9, 688291. [Google Scholar] [CrossRef]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Mendonça, R.T. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Thermal Decomposition of Kraft Lignin under Gas Atmospheres of Argon, Hydrogen, and Carbon Dioxide. Polymers 2018, 10, 729. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Suhas, P.J.M.; Carrott, M.M.L.R. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Poursorkhabi, V.; Abdelwahab, M.A.; Misra, M.; Khalil, H.; Gharabaghi, B.; Mohanty, A.K. Processing, Carbonization, and Characterization of Lignin Based Electrospun Carbon Fibers: A Review. Front. Energy Res. 2020, 8, 208. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Innocent, M.T.; Wang, Q.; Xiang, H.; Tang, J.; Zhu, M. Lignin-based carbon fibers: Formation, modification and potential applications. Green Energy Environ. 2021, in press. [Google Scholar] [CrossRef]
- Ponomarev, N.; Sillanpaa, M. Combined chemical-templated activation of hydrolytic lignin for producing porous carbon. Ind. Crop. Prod. 2019, 135, 30–38. [Google Scholar] [CrossRef]
- Rosas, J.M.; Berenguer, R.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of different carbon materials by thermochemical conversion of lignin. Front. Mater. 2014, 1, 29. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Li, J.; Zhang, X.; Hassan, E.B.; Wang, C.; Zhang, J.; Cai, Z. Catalytic graphitization of kraft lignin to graphene-based structures with four different transitional metals. J. Nanopart. Res. 2018, 20, 223. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, X.; Li, J.; Hassan, E.B.; Wang, C.; Zhang, J.; Cai, Z. Catalytic conversion of Kraft lignin to bio-multilayer graphene materials under different atmospheres. J. Mater. Sci. 2018, 53, 8020–8029. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. Mass production of graphene materials from solid carbon sources using a molecular cracking and welding method. J. Mater. Chem. A 2019, 7, 13978–13985. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Arango, R.; Li, J.; Cai, Z. Fabrication and characterization of carbon foams using 100% Kraft lignin. Mater. Des. 2021, 201, 109460–109470. [Google Scholar] [CrossRef]
- Cai, Z.; Yan, Q.; Zhang, J.; Li, J.; Marcoccia, B.S.; Freiberg, J.D. Method for Synthesizing Graphene from Encapsulated Particles. U.S. Patent 10,669,155, 2 June 2020. [Google Scholar]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Lin, L.; Kasper, F.K.; Qin, Y.-X.; Mikos, A.G.; Sitharaman, B. Two-Dimensional Nanostructure-Reinforced Biodegradable Polymeric Nanocomposites for Bone Tissue Engineering. Biomacromolecules 2013, 14, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Akinwande, D.; Tao, L.; Yu, Q.; Lou, X.; Peng, P.; Kuzum, D. Large-Area Graphene Electrodes: Using CVD to facilitate applications in commercial touchscreens, flexible nanoelectronics, and neural interfaces. IEEE Nanotechnol. Mag. 2015, 9, 6–14. [Google Scholar] [CrossRef]
- Fei, H.; Ye, R.; Ye, G.; Gong, Y.; Peng, Z.; Fan, X.; Samuel, E.L.G.; Ajayan, P.M.; Tour, J.M. Boron- and Nitrogen-Doped Graphene Quantum Dots/Graphene Hybrid Nanoplatelets as Efficient Electrocatalysts for Oxygen Reduction. ACS Nano 2014, 8, 10837–10843. [Google Scholar] [CrossRef]
- Zhong, M.; Xu, D.; Yu, X.; Huang, K.; Liu, X.; Qu, Y.; Xu, Y.; Yang, D. Interface coupling in graphene/fluorographene heterostructure for high-performance graphene/silicon solar cells. Nano Energy 2016, 28, 12–18. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Güneş, F.; Ta, H.Q.; Lee, S.M.; Chae, S.J.; Sheem, K.Y.; Cojocaru, C.S.; Xie, S.S.; Lee, Y.H. Diffusion Mechanism of Lithium Ion through Basal Plane of Layered Graphene. J. Am. Chem. Soc. 2012, 134, 8646–8654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, S.; Garay, J.; Liu, G.; Balandin, A.A.; Abbaschian, R. Growth of large-area graphene films from metal-carbon melts. J. Appl. Phys. 2010, 108, 94321. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Han, T.Y.-J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Majumder, M.; Alemany, L.B.; Narayanan, T.N.; Ibarra, M.A.; Pradhan, B.K.; Ajayan, P.M. Engineered Graphite Oxide Materials for Application in Water Purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef]
- Yan, Q.; Cai, Z. Issues in Preparation of Metal-Lignin Nanocomposites by Coprecipitation Method. J. Inorg. Organomet. Polym. Mater. 2020, 31, 978–996. [Google Scholar] [CrossRef]
- Yan, Q.; Boardman, C.R.; Cai, Z. Thermal stability of metal-lignin composites prepared by coprecipitation method. Thermochim. Acta 2020, 690, 178659. [Google Scholar] [CrossRef]
- Kicinski, W.; Dyjak, S. Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon 2020, 168, 748–845. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Tite, T.; Loir, A.-S.; Maddi, C.; Donnet, C.; Garrelie, F. Review of Graphene Growth from a Solid Carbon Source by Pulsed Laser Deposition (PLD). Front. Chem. 2018, 6, 572. [Google Scholar] [CrossRef] [Green Version]
- Oya, A.; Otani, S. Catalytic graphitization of carbons by various metals. Carbon 1979, 17, 131–137. [Google Scholar] [CrossRef]
- Lin, Q.; Feng, Z.; Liu, Z.; Guo, Q.; Hu, Z.; He, L.; Ye, H. Atomic scale investigations of catalyst and catalytic graphitization in a silicon and titanium doped graphite. Carbon 2015, 88, 252–261. [Google Scholar] [CrossRef]
- Amato, G. High Temperature Growth of Graphene from Cobalt Volume: Effect on Structural Properties. Materials 2018, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, X.; Sun, X.; Ma, Y. Shape-controlled synthesis of nanocarbons through direct conversion of carbon dioxide. Sci. Rep. 2013, 3, 3534. [Google Scholar] [CrossRef]
- Yan, Q.; Cai, Z. Effect of Solvents on Fe-Lignin Precursors for Production Graphene-Based Nanostructures. Molecules 2020, 25, 2167. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, J.; Shi, P.; Li, M.; Bian, J. Synthesis of a Lignin-Fe/Mn Binary Oxide Blend Nanocomposite and Its Adsorption Capacity for Methylene Blue. ACS Omega 2021, 6, 16837–16846. [Google Scholar] [CrossRef]
- Bakas, N.J.; Neidig, M.L. Additive and Counterion Effects in Iron-Catalyzed Reactions Relevant to C–C Bond Formation. ACS Catal. 2021, 11, 8493–8503. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Heo, Y.-U.; Sasaki, Y. Cementite Formation from Magnetite under High Pressure Conditions. ISIJ Int. 2013, 53, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yan, Q.; Hassan, E.B.; Li, J.; Cai, Z.; Zhang, J. Temperature effects on formation of carbon-based nanomaterials from kraft lignin. Mater. Lett. 2017, 203, 42–45. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. In situ formation of graphene-encapsulated iron nanoparticles in carbon frames through catalytic graphitization of kraft lignin. Nanomater. Nanotechnol. 2018, 8, 1847980418818955. [Google Scholar] [CrossRef] [Green Version]
- Hempelmann, R. Diffusion of hydrogen in metals. J. Less-Common Met. 1984, 101, 69–96. [Google Scholar] [CrossRef]
- Fort, D.; Harris, I.R. The physical properties of some palladium alloy hydrogen diffusion membrane materials. J. Less-Common Met. 1975, 41, 313–327. [Google Scholar] [CrossRef]
- Atsumi, H. Hydrogen bulk retention in graphite and kinetics of diffusion. J. Nucl. Mater. 2002, 307–311, 1466–1470. [Google Scholar] [CrossRef]
- Benac, D.J.; McAndrew, P. Reducing the Risk of High Temperature Hydrogen Attack (HTHA) Failures. J. Fail. Anal. Prev. 2012, 12, 624–627. [Google Scholar] [CrossRef]
- Cheng, G.; Calizo, I.; Hacker, C.A.; Richter, C.A.; Hight Walker, A.R. Fe-catalyzed etching of exfoliated graphite through carbon hydrogenation. Carbon 2016, 96, 311–315. [Google Scholar] [CrossRef] [Green Version]
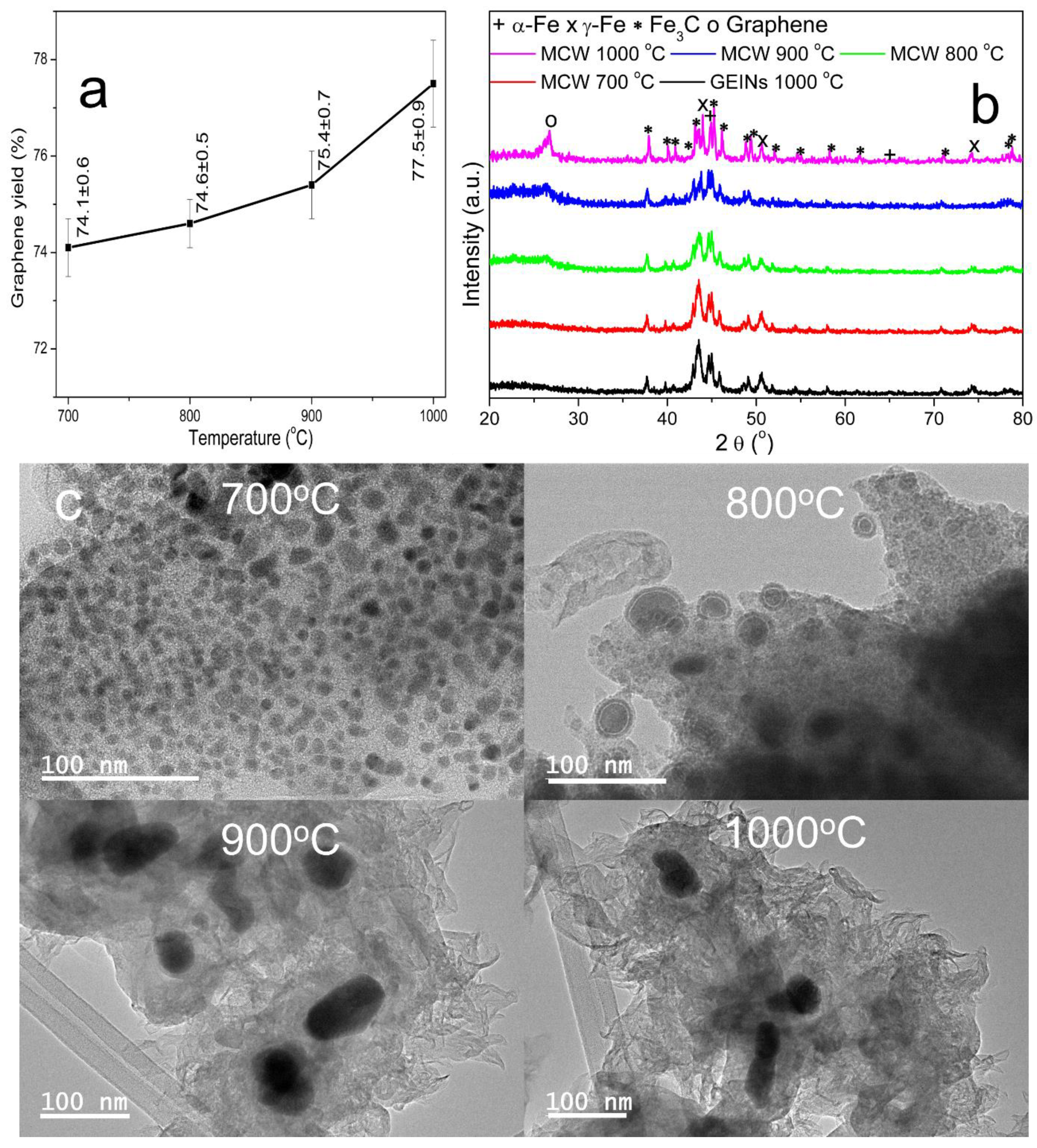
| GEMNs | GEMN Agglomerate Particle Size (µm) | Heating Temperature (°C) | Cracking-Welding Gas (mL/min) | Reaction Time (h) | Graphene Yield (%) | |
|---|---|---|---|---|---|---|
| Ar | CH4 | |||||
| Fe@C | 150–250 | 700 | 50 | 80 | 1 | 74.1 ± 0.6 |
| Fe@C | 150–250 | 800 | 50 | 80 | 1 | 74.6 ± 0.5 |
| Fe@C | 150–250 | 900 | 50 | 80 | 1 | 75.4 ± 0.7 |
| Fe@C | 150–250 | 1000 | 50 | 80 | 1 | 77.5 ± 0.9 |
| Fe@C | 150–250 | 1000 | 50 | 80 | 0.5 | 75.9 ± 0.4 |
| Fe@C | 150–250 | 1000 | 50 | 80 | 2 | 79.9 ± 0.6 |
| Fe@C | 150–250 | 1000 | 50 | 80 | 3 | 81.7 ± 1.0 |
| Fe@C | <50 | 1000 | 50 | 80 | 1 | 78.7 ± 0.7 |
| Fe@C | 50–150 | 1000 | 50 | 80 | 1 | 78.2 ± 0.5 |
| Fe@C | 500–700 | 1000 | 50 | 80 | 1 | 75.3 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Ketelboeter, T.; Cai, Z. A Study of the Key Factors on Production of Graphene Materials from Fe-Lignin Nanocomposites through a Molecular Cracking and Welding (MCW) Method. Molecules 2022, 27, 154. https://doi.org/10.3390/molecules27010154
Yan Q, Ketelboeter T, Cai Z. A Study of the Key Factors on Production of Graphene Materials from Fe-Lignin Nanocomposites through a Molecular Cracking and Welding (MCW) Method. Molecules. 2022; 27(1):154. https://doi.org/10.3390/molecules27010154
Chicago/Turabian StyleYan, Qiangu, Timothy Ketelboeter, and Zhiyong Cai. 2022. "A Study of the Key Factors on Production of Graphene Materials from Fe-Lignin Nanocomposites through a Molecular Cracking and Welding (MCW) Method" Molecules 27, no. 1: 154. https://doi.org/10.3390/molecules27010154
APA StyleYan, Q., Ketelboeter, T., & Cai, Z. (2022). A Study of the Key Factors on Production of Graphene Materials from Fe-Lignin Nanocomposites through a Molecular Cracking and Welding (MCW) Method. Molecules, 27(1), 154. https://doi.org/10.3390/molecules27010154








