Analyses of Antioxidative Properties of Selected Cyclitols and Their Mixtures with Flavanones and Glutathione
Abstract
:1. Introduction
2. Results and Discussion
2.1. Studies of Antioxidant Properties of Cyclitols by Spectrophotometric Methods
2.2. Ultrafast UHPLC-UV Chromatographic Method for the Determination of Antioxidant Activities of Studied Cyclitols and Their Mixtures with Glutathione and Flavanones
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Isolation and Purification of d-pinitol from Carob (Ceratonia siliqua L.)
3.2.1. Extraction Procedure
3.2.2. GC-FID and GC-MS Analyses of d-pinitol
3.3. The CUPRAC Method Applied to Study the Ox-Red Activity of Cyclitols and Their Mixtures with Flavanones
3.4. Spectrophotometrically DPPH-UV Radical Scavenging Assay
3.5. DPPH-UHPLC-UV Radical Scavenging Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| CUPRAC | Cupric Ion Reducing Antioxidant Capacity |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| UHPLC-UV | ultra-high performance liquid chromatography with ultraviolet detection |
| RSA | radical scavenging activity |
| IC50 | 50% inhibition of the radical scavenging effect |
| GC-FID | high-resolution gas chromatography with flame ionization detection |
| GC-MS | gas chromatography with mass spectrometry detection |
References
- Michell, R.H. Evolution of the diverse biological role of inositols. Biochem. Soc. Symp. 2007, 74, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Obendorf, R.L.; Górecki, R.J. Soluble carbohydrates in legume seeds. Seed Sci. Res. 2012, 22, 219–242. [Google Scholar] [CrossRef]
- Poongothai, G.; Sripathi, S.K. A review on insulinomimetic pinitol from plants. Int. J. Pharm. Bio. Scis. 2013, 4, 992–1009. [Google Scholar]
- Al-Suod, H.; Ligor, M.; Ratiu, I.-A.; Rafińska, K.; Górecki, R.; Buszewski, B. A window on cyclitols: Characterization and analytics of inositols. Phytochem. Lett. 2017, 20, 507–519. [Google Scholar] [CrossRef]
- Gridland, C.; Gillaspy, G. Inositol pyrophosphate pathways and mechanisms: What can we learn from plants? Molecules 2020, 25, 2789. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.-G.; Ahn, H.; Kim, S. Inositol pyrophosphates: Signaling molecules with pleiotropic actions in mammals. Molecules 2020, 25, 2208. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Myo-inositol and beyond—Emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef]
- Merchant, A.; Richter, A. Polyols as biomarkers and bioindicators for 21 century plant breeding. Funct. Plant Biol. 2011, 38, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [Green Version]
- Ortbauer, M.; Popp, M. Functional role of polyhydroxy compounds on protein structure and thermal stability studied by circular dichroism spectroscopy. Plant Physiol. Biochem. 2008, 46, 428–434. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Lahuta, L.B.; Ligor, M.; Placek, W.; Górecki, R.J.; Buszewski, B. The healing-promoting properties of selected cyclitols—A review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef] [Green Version]
- Antonowski, T.; Osowski, A.; Lahuta, L.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Health-promoting properties of selected cyclitols for metabolic syndrome and diabetes. Nutrients 2019, 11, 2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhetri, D.R. Myo-Inositol and its derivatives: Their emerging role in the treatment of huma diseases. Front. Pharmacol. 2019, 10, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiani, A.K.; Paolacci, S.; Galogero, A.E.; Cannarella, R.; Di Renzo, G.C.; Gerli, S.; Della Morte, C.; Busetto, G.M.; De Berardinis, E.; Del Giudice, F.; et al. From Myo-inositol to D-chiro-inositol molecular pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2390–2402. [Google Scholar]
- Davinelli, S.; Nicolosi, D.; Di Cesare, C.; Scapagnini, G.; Di Marco, R. Targeting metabolic consequences of insulin re-sistance in polycystic ovary syndrome by D-chiro-inositol and emerging nutraceuticals: A focused review. J. Clin. Med. 2020, 9, 987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinetti, F.; Appetecchia, M.; Aragona, C.; Bevilacqua, A.; Bezerra Espinola, M.S.; Bizzarri, M.; D’Anna, R.; Dewailly, D.; Diamanti-Kandarakis, E.; Hernández Marín, I.; et al. Experts’ opinion on inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: A further help for human reproduction and beyond. Expert Opin. Drug Metab. Toxicol. 2020, 16, 255–274. [Google Scholar] [CrossRef] [PubMed]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; de Fonseca, F.R. The biomedical uses of inositols: A nutraceutical approach to metabolic dysfunction in aging and neurodegenerative diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Frej, A.D.; Otto, G.P.; Williams, R.S.B. Tipping the scales: Lessons from simple model systems on inositol imbalance in neurological disorders. Eur. J. Cell Biol. 2017, 96, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Tiwari, M.; Dubey, A.; Dwivedi, A. D-Pinitol–a natural phytomolecule and its pharmacological effect. IJPLS 2020, 11, 6609–6623. [Google Scholar]
- López-Sánchez, J.I.; Moreno, D.A.; García-Viguera, C. D-pinitol, a highly valuable product from carob pods: Health-promoting effects and metabolic pathways of this natural super-food ingredient and its derivatives. AIMS Agric. Food 2018, 3, 41–63. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; León-González, A.J.; Gálvez-Peralta, M.; González-Mauraza, N.H.; Martin-Cordero, C. D-Pinitol: A cyclitol with versatile biological and pharmacological activities. Phytochem. Rev. 2021, 20, 211–224. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Chang, Z.; Kong, D.-X.; Zuo, Z. Quebrachitol: Global status and basic research. Nat. Prod. Bioprospect. 2017, 7, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Yodthong, T.; Kedjarune-Leggat, U.; Smythe, C.; Wititsuwannakul, R.; Pitakpornpreecha, T. L-Quebrachitol promotes the proliferation, differentiation, and mineralization of MC3T3-E1 cells: Involvement of the BMP-2/Runx2/MAPK/Wnt/b-catenin signaling pathway. Molecules 2018, 23, 3086. [Google Scholar] [CrossRef] [Green Version]
- Formoso, G.; Baldassarre, M.P.A.; Ginestra, F.; Carlucci, M.A.; Bucci, I.; Consoli, A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab. Res. Rev. 2019, 35, e3154. [Google Scholar] [CrossRef]
- Orthen, B.; Popp, M.; Smirnoff, N. Hydroxyl radical scavenging properties of cyclitols. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1994, 102, 269–272. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Balasubramanian, M.P.; Nishigaki, I. Free radical scavenging and antioxidant activity of D-pinitol against 7, 12 dimethylbenz (a) anthracene induced breast cancer in sprague dawley rats. Asian Pac. J. Trop. Dis. 2014, 4, 384–390. [Google Scholar] [CrossRef]
- Lemos, T.L.G.; Machado, L.L.; Souza, J.S.N.; Fonseca, A.M.; Maia, J.L.; Pessoa, O.D.L. Antioxidant, ichtyotoxicity and brine shrimp lethality tests of Magnolia glabrata. Fitoterapia 2006, 77, 443–445. [Google Scholar] [CrossRef]
- Mo, E.J.; Ahn, J.H.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Inositol derivatives and phenolic compounds from the roots of Taraxacum coreanum. Molecules 2017, 22, 1349. [Google Scholar] [CrossRef] [Green Version]
- Tewari, R.; Gupta, M.; Ahmad, F.; Rout, P.K.; Misra, L.; Patwardhan, A.; Vasudeva, R. Extraction, quantification and antioxidant activities of flavonoids, polyphenols and pinitol from wild and cultivated Saraca asoca bark using RP-HPLC-PDA-RI method. Ind. Crops Prod. 2017, 103, 73–80. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Zechmann, B. Subcellular roles of glutathione in mediating plant defense during biotic stress. Plants 2020, 9, 1067. [Google Scholar] [CrossRef]
- Marí, M.; de Gregorio, E.; de Dios, C.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants 2020, 9, 909. [Google Scholar] [CrossRef]
- Sikora, E.; Cieślik, E.; Topolska, K. The sources of natural antioxidants. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–17. [Google Scholar]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment, and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glovera, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–77. [Google Scholar] [CrossRef]
- Rains, J.; Jain, S.K. Oxidative stress, insulin signaling and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Lassere, A.M.; Marti–Soler, H.; Strippoli, M.P.; Vaucher, J.; Glaus, J.; Vandeleur, C.L.; Castelao, E.; Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; et al. Clinical and course characteristic of depression and all—Cause mortality. A prospective population based study. J. Affect Disord. 2016, 189, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Saki, K.; Bahmani, M.; Rafieian–Kopaei, M. The effect of most important medicinal plants on two important psychiatric disorders (anxiety and depression)—A review. Asian Pac. J. Trop. Med. 2014, 7, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Nones, J.; de Sampaio e Spohr, T.C.L.; Gomes, F.C.A. Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem. Res. 2011, 36, 1776–1784. [Google Scholar] [CrossRef]
- Baranowska, I.; Bajkacz, S. A new HPLC-MS/MS method for the determination of flavonoids in supplements and DPPH-UHPLC-UV method for the evaluation the radical scavenging activity of flavonoids. Food Chem. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Cho, J. Antioxidant and neuroprotective effects of hesperitin and its aglycone hesperetin. Arch. Pharm. Res. 2006, 29, 699–706. [Google Scholar] [CrossRef]
- Gu, S.-F.; Wang, L.-Y.; Tian, Y.-J.; Zhou, Z.-X.; Tang, J.-B.; Liu, X.-R.; Jiang, H.-P.; Shen, Y.-Q. Enhanced water solubility antioxidant activity and oral absorption of hesperetin by D-α-tocoferyl polyethylene glycol 1000 succinate and phosphatidyl-choline. J. Zhejiang Univ. Sci. B 2019, 20, 273–281. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Wang, Y.; Luo, K.; Wang, Y.; Cai, Y. Liguiritigenin inhibits tumor growth and vascularization in a mouse model Hela cells. Molecules 2012, 17, 7206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanez, J.A.; Remsberg, C.M.; Miranda, N.D.; Vega-Villa, K.R.; Andrews, P.K.; Davies, N.M. Pharmacokinetics of selected chiral flavonoids: Hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm. Drug Dispos. 2008, 29, 63–82. [Google Scholar] [CrossRef]
- Erlund, J. Review of flavonoids quercetin, hesperetin and naringenin. Dietary sources, bioactivities, bioavailability and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Asatsuma-Okumura, T.; Ito, T.; Handa, H. Molecular mechanisms of the teratogenic effects of thalidomide. Pharmaceuticals 2020, 13, 95. [Google Scholar] [CrossRef]
- Valencia, E.; Martin, A.; Hardy, G. Glutathione—Nutritional and pharmacologic viewpoints. Part II. Nutrition 2001, 17, 696–697. [Google Scholar] [CrossRef]
- Pereira, R.B.; Sousa, C.; Costa, A.; Andrade, P.B.; Valentao, P. Glutathione and the antioxidant potential of binary mixtures with flavonoids: Synergism and antagonism. Molecules 2013, 18, 8858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiera, S.; Baranowska, I.; Lautenszleger, A. UHPLC–UV method for the determination of flavonoids in dietary supplements and for evaluation of their antioxidant activities. J. Pharm. Biomed. Anal. 2015, 102, 468–475. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Ramos, L.; Soria, A.C.; Sanz, M.L. Optimisation of a biotechnological procedure for selective fractionation of bioactive inositols in edible legume extracts. J. Sci. Food Agric. 2013, 93, 2797–2803. [Google Scholar] [CrossRef]
- Lahuta, L.B. Biosynthesis of raffinose family oligosaccharides and galactosyl pinitols in developing and maturing seeds of winter vetch (Vicia villosa Roth.). Acta Soc. Bot. Pol. 2006, 75, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314–153324. [Google Scholar] [CrossRef] [PubMed]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
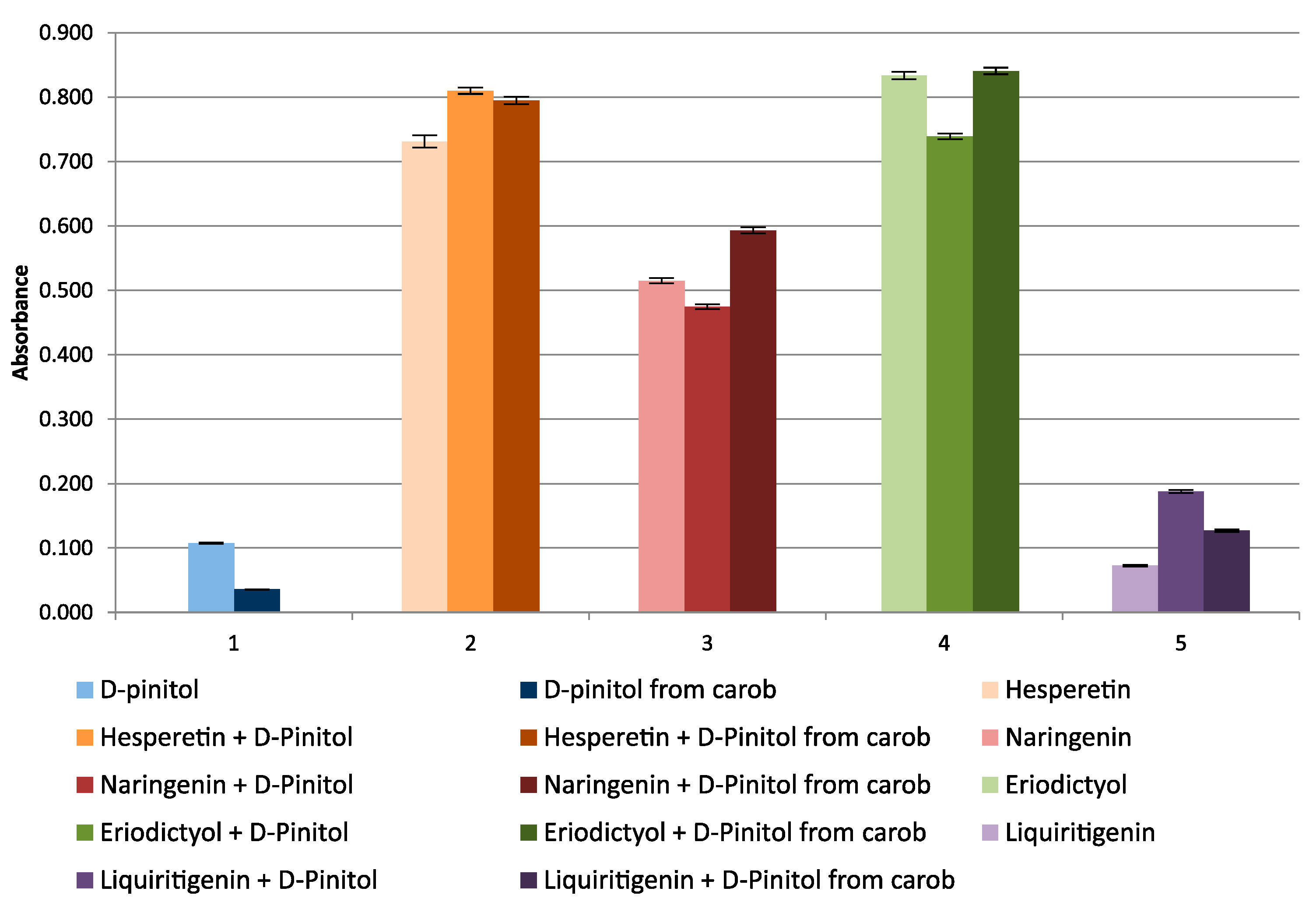



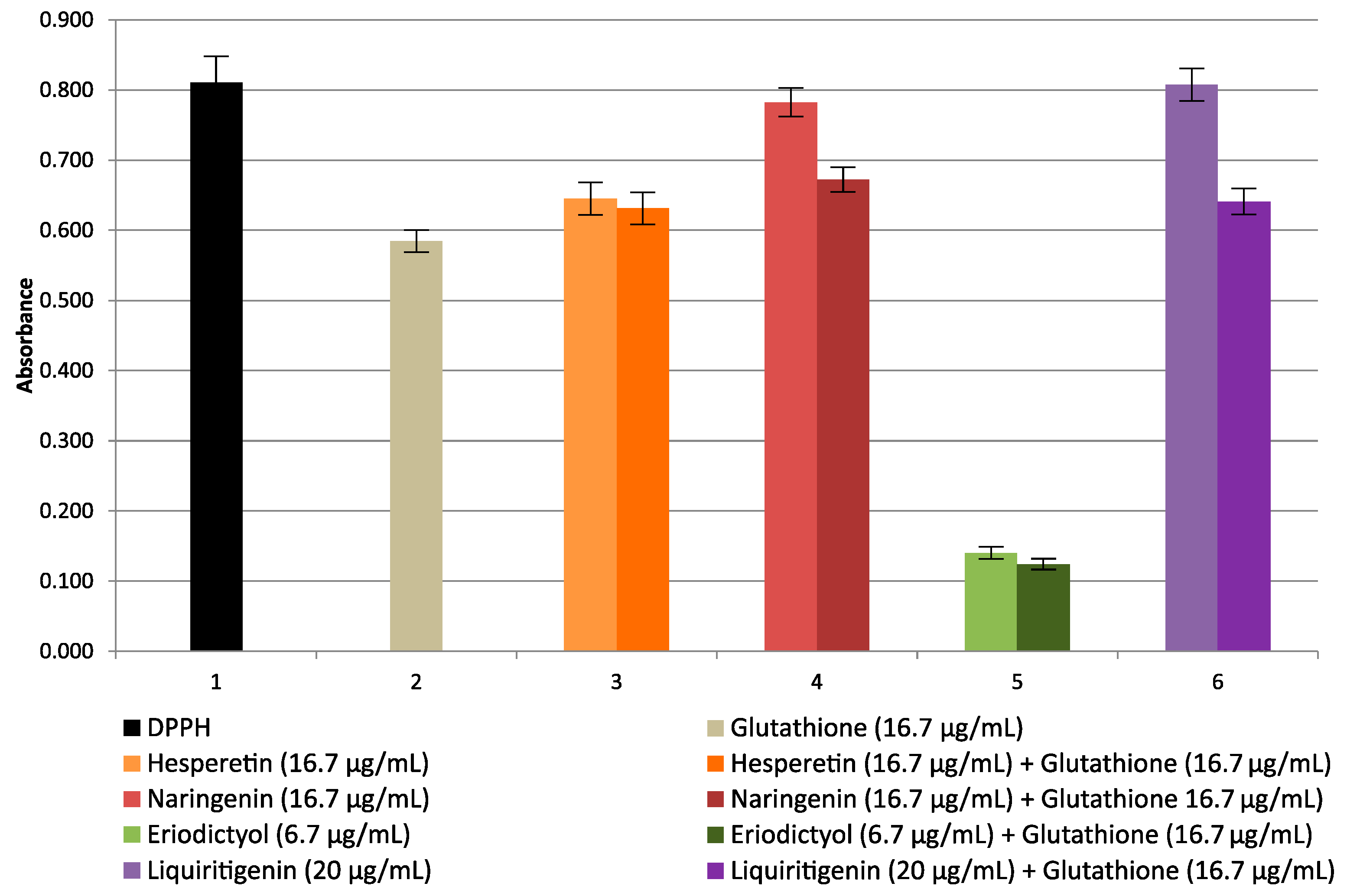
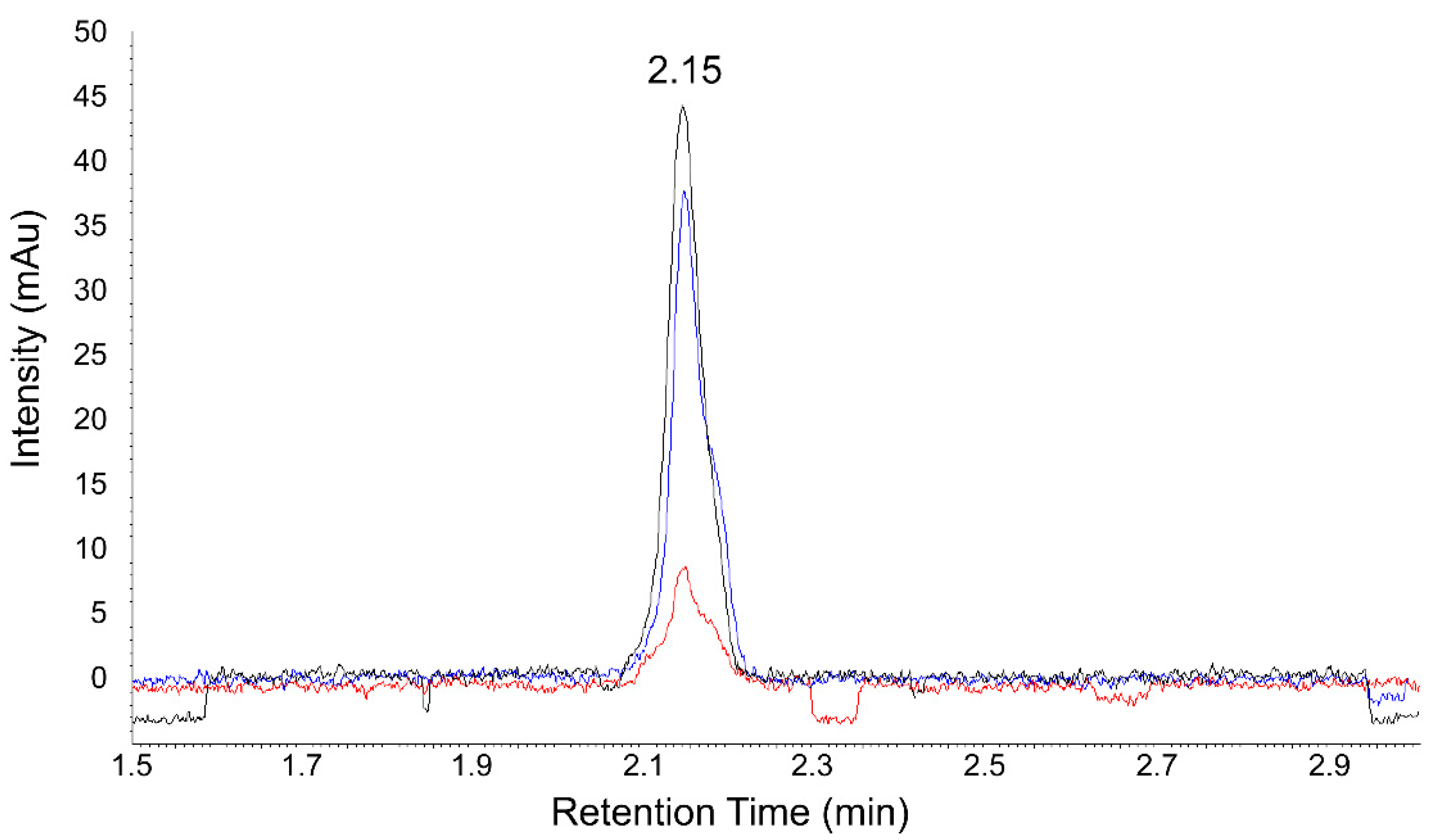
| Cyclitol | RSA% | Antioxidant | RSA% | Theoretical RSA% | Experimental RSA% | Change of RSA% |
|---|---|---|---|---|---|---|
| d-Pinitol (10 mg/mL) | 19.3 | Hesperetin (50 μg/mL) | 43.6 | 62.9 | 36.4 | ↓↓ |
| Naringenin (100 μg/mL) | 25.8 | 45.1 | 21.2 | ↓↓ | ||
| Eriodictyol (2 μg/mL) | 41.9 | 61.2 | 37.0 | ↓↓ | ||
| Liquiritigenin (20 μg/mL) | 22.6 | 41.9 | 21.5 | ↓↓ | ||
| Glutathione (10 μg/mL) | 30.7 | 50.0 | 54.0 | ↔ | ||
| d-Pinitol from carob (10 mg/mL) | 6.6 | Hesperetin (50 μg/mL) | 43.6 | 50.2 | 37.9 | ↓↓ |
| Naringenin (100 μg/mL) | 25.8 | 32.4 | 16.9 | ↓↓ | ||
| Eriodictyol (2 μg/mL) | 41.9 | 48.5 | 34.9 | ↓ | ||
| Liquiritigenin (20 μg/mL) | 22.6 | 29.2 | 21.6 | ↔ | ||
| Glutathione (10 μg/mL) | 30.7 | 37.3 | 79.9 | ↑↑ | ||
| l-Quebrachitol (10 mg/mL) | 14.0 | Hesperetin (50 μg/mL) | 43.6 | 57.6 | 58.1 | ↔ |
| Naringenin (100 μg/mL) | 25.8 | 39.8 | 18.1 | ↓↓ | ||
| Eriodictyol (2 μg/mL) | 41.9 | 55.9 | 34.1 | ↓↓ | ||
| Liquiritigenin (20 μg/mL) | 22.6 | 36.6 | 20.5 | ↓ | ||
| Glutathione (10 μg/mL) | 30.7 | 44.7 | 42.7 | ↔ | ||
| l-chiro-Inositol (10 mg/mL) | 9.8 | Hesperetin (50 μg/mL) | 43.6 | 53.7 | 38.8 | ↓ |
| Naringenin (100 μg/mL) | 25.8 | 35.6 | 20.2 | ↓ | ||
| Eriodictyol (2 μg/mL) | 41.9 | 51.7 | 31.3 | ↓↓ | ||
| Liquiritigenin (20 μg/mL) | 22.6 | 32.4 | 16.2 | ↓↓ | ||
| Glutathione (10 μg/mL) | 30.7 | 40.5 | 53.9 | ↑↑ | ||
| d-chiro-Inositol (10 mg/mL) | 18.5 | Hesperetin (50 μg/mL) | 43.6 | 62.1 | 44.2 | ↓ |
| Naringenin (100 μg/mL) | 25.8 | 44.3 | 12.9 | ↓↓ | ||
| Eriodictyol (2 μg/mL) | 41.9 | 60.4 | 36.3 | ↓↓ | ||
| Liquiritigenin (20 μg/mL) | 22.6 | 41.1 | 22.2 | ↓↓ | ||
| Glutathione (10 μg/mL) | 30.7 | 49.2 | 75.6 | ↑↑ | ||
| myo-Inositol (10 mg/mL) | 16.5 | Hesperetin (50 μg/mL) | 43.6 | 60.1 | 42.1 | ↓ |
| Naringenin (100 μg/mL) | 25.8 | 42.3 | 24.3 | ↓↓ | ||
| Eriodictyol (2 μg/mL) | 41.9 | 58.4 | 25.8 | ↓↓ | ||
| Liquiritigenin (20 μg/mL) | 22.6 | 39.1 | 13.4 | ↓↓ | ||
| Glutathione (10 μg/mL) | 30.7 | 47.2 | 31.8 | ↓ |
| Compound | IC50 | Scavenging Activity |
|---|---|---|
| Myo-Inositol | 117.3 mg/mL | very weak |
| d-Pinitol | 14.3 mg/mL | very weak |
| Eriodictyol | 2.8 μg/mL | very strong |
| Hesperetin | 30.8 μg/mL | strong |
| Naringenin | 210.0 μg/mL | weak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płonka, J.; Szablińska-Piernik, J.; Buszewski, B.; Baranowska, I.; Lahuta, L.B. Analyses of Antioxidative Properties of Selected Cyclitols and Their Mixtures with Flavanones and Glutathione. Molecules 2022, 27, 158. https://doi.org/10.3390/molecules27010158
Płonka J, Szablińska-Piernik J, Buszewski B, Baranowska I, Lahuta LB. Analyses of Antioxidative Properties of Selected Cyclitols and Their Mixtures with Flavanones and Glutathione. Molecules. 2022; 27(1):158. https://doi.org/10.3390/molecules27010158
Chicago/Turabian StylePłonka, Joanna, Joanna Szablińska-Piernik, Bogusław Buszewski, Irena Baranowska, and Lesław B. Lahuta. 2022. "Analyses of Antioxidative Properties of Selected Cyclitols and Their Mixtures with Flavanones and Glutathione" Molecules 27, no. 1: 158. https://doi.org/10.3390/molecules27010158
APA StylePłonka, J., Szablińska-Piernik, J., Buszewski, B., Baranowska, I., & Lahuta, L. B. (2022). Analyses of Antioxidative Properties of Selected Cyclitols and Their Mixtures with Flavanones and Glutathione. Molecules, 27(1), 158. https://doi.org/10.3390/molecules27010158







