Tungsten and Molybdenum Heteropolyanions with Different Central Ions—Correlation between Theory and Experiment
Abstract
1. Introduction
2. Results and Discussion
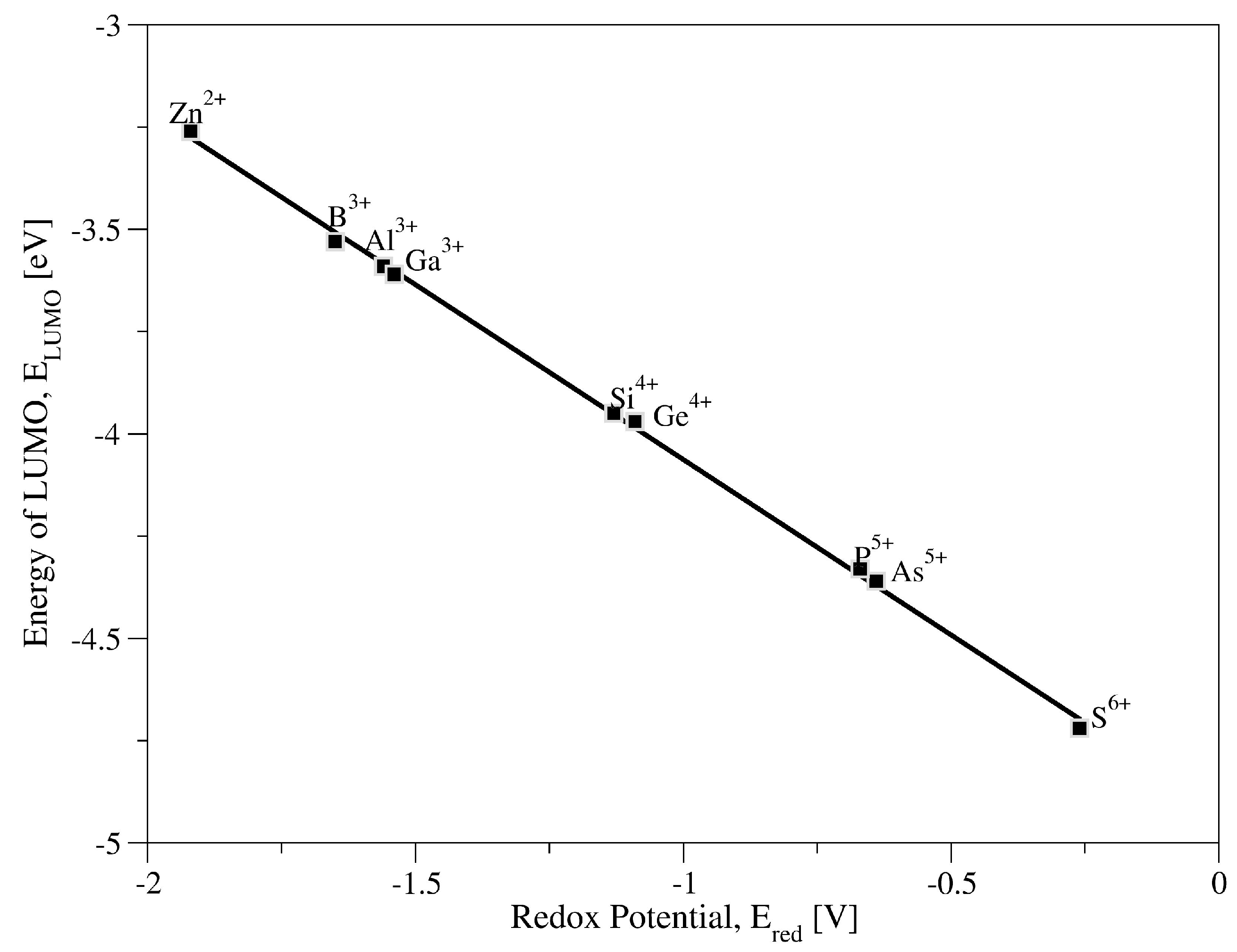
2.1. XW12O40n− Systems
2.2. XMo12O40n− Systems
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikov, I.V. Sustainable heterogeneous acid catalysis by heteropoly acids. J. Mol. Catal. A Chem. 2007, 262, 86–92. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Heterogeneous acid catalysis by heteropoly acids: Approaches to catalyst deactivation. J. Mol. Catal. A Chem. 2009, 305, 104–111. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Matrosova, M.M.; Maksimov, G.M.; Likholobov, V.A. A Study of the Acid Properties of Structurally and Compositionally Different Heteropoly Acids in Acetic Acid. Kinet. Catal. 2001, 42, 785–790. [Google Scholar] [CrossRef]
- Timofeeva, M.N. Acid Catalysis by Heteropoly Acids. Appl. Catal. A Gen. 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Ozaki, A. Acid-Base Properties and Catalytic Activity of Solid Surfaces. J. Catal. 1967, 8, 1–7. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef]
- Muller, F.; Peters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very Large Clusters-Nanoscale Magnets. Chem. Rev. 1998, 98, 239–271. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heterogeneous Catalysis. Chem. Rev. 1998, 98, 199–217. [Google Scholar] [CrossRef]
- Misono, M.; Ono, I.; Koyano, G.; Aoshima, A. Heteropolyacids. Versatile green catalysts usable in a variety of reaction media. Pure Appl. Chem. 2000, 72, 1305–1311. [Google Scholar] [CrossRef]
- Misono, M. Acid catalysts for clean production. Green aspects of heteropolyacid catalysts. C. R. L’académie Sci. Ser. IIC-Chem. 2000, 3, 471–475. [Google Scholar] [CrossRef]
- Klemperer, W.G.; Wall, C.G. Polyoxoanion Chemistry Moves toward the Future: From Solids and Solutions to Surfaces. Chem. Rev. 1998, 98, 297–306. [Google Scholar] [CrossRef]
- Izumia, Y.; Urabe, K.; Onaka, M. Recent advances in liquid-phase organic reactions using heteropolyacid and clay. Catal. Surv. Jpn. 1997, 1, 17–23. [Google Scholar] [CrossRef]
- Heravi, M.M.; Sadjadi, S. Recent Developments in Use of Heteropolyacids, Their Salts and Polyoxometalates in Organic Synthesis. J. Iran. Chem. Soc. 2009, 6, 1–54. [Google Scholar] [CrossRef]
- Coronado, E.; Gomez-Garcia, C.J. Polyoxometalate-Based Molecular Materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef]
- Melsheimer, J.; Mahmoud, S.S.; Mestl, G.; Schlogl, R. In Situ UV-VIS diffuse reflectance spectroscopy of reduction–reoxidation of heteropoly compounds by methanol and ethanol: A correlation between spectroscopic and catalytic data. Catal. Lett. 1999, 60, 103–111. [Google Scholar] [CrossRef]
- Kaba, M.S.; Song, I.K.; Barteau, M.A. Ordered Array Formation and Negative Differential Resistance Behavior of Cation-Exchanged Heteropoly Acids Probed by Scanning Tunneling Microscopy. J. Phys. Chem. 1996, 100, 19577–19581. [Google Scholar] [CrossRef][Green Version]
- Kaba, M.S.; Song, I.K.; Barteau, M.A. Investigation of Framework and Cation Substitutions in Keggin-type Heteropoly Acids Probed by Scanning Tunneling Microscopy and Tunneling Spectroscopy. J. Vac. Sci. Technol. A 1997, 15, 1299–1304. [Google Scholar] [CrossRef]
- Song, I.K.; Kaba, M.S.; Barteau, M.A.; Lee, W.Y. Investigation of Redox Potential and Negative Differential Resistance Behavior of Heteropolyacids by Scanning Tunneling Microscopy. Catal. Today 1998, 44, 285–291. [Google Scholar] [CrossRef]
- Kinne, M.; Barteau, M.A. STM and TS investigations of silver polyoxometalate monolayers: Model compounds and potential. Surf. Sci. 2000, 447, 105–111. [Google Scholar] [CrossRef]
- Song, I.K.; Barteau, M.A. Bulk redox properties of heteropolyacids determined from surface properties of nanostructured heteropolyacid monolayers. J. Mol. Catal. A Chem. 2002, 182–183, 185–193. [Google Scholar] [CrossRef]
- Song, I.K.; Shnitser, R.B.; Cowan, J.J.; Hill, C.L.; Barteau, M.A. Nanoscale Characterization of Redox and Acid Properties of Keggin-Type Heteropolyacids by Scanning Tunneling Microscopy and Tunneling Spectroscopy: Effect of Heteroatom Substitution. Inorg. Chem. 2002, 41, 1292–1298. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic Chemistry of Heteropoly Compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Grigoriev, V.A.; Hill, C.L.; Weinstock, I.A. Role of Cation Size in the Energy of Electron Transfer to 1:1 Polyoxometalate Ion Pairs {(M+)(Xn+VW11O40)}(8−n)− (M = Li, Na, K). J. Am. Chem. Soc. 2000, 122, 3544–3545. [Google Scholar] [CrossRef]
- Sadakane, M.; Steckhan, E. Electrochemical Properties of Polyoxometalates as Electrocatalysts. Chem. Rev. 1998, 98, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Seiyama, T.; Yamazoe, N.; Katsuki, S.; Taketa, H. Electronic Structures of XMo12O40 Heteropolyanions (X = P, As, Si, and Ge) and Their Reduction Behavior. J. Catal. 1988, 111, 336–344. [Google Scholar] [CrossRef]
- Pope, M.T.; Varga, G.M., Jr. Heteropoly Blues. I. Reduction Stoichiometries and Reduction Potentials of Some 12-Tungstates. Inorg. Chem. 1966, 5, 1249–1254. [Google Scholar]
- Altenau, J.J.; Pope, M.T.; Prados, R.A.; So, H. Models for Heteropoly Blues. Degrees of Valence Trapping in Vanadium (lV)—And Molybdenum(V)—Substituted Keggin Anions. Inorg. Chem. 1975, 14, 417–421. [Google Scholar] [CrossRef]
- Song, I.K.; Barteau, M.A. Redox properties of kegging-type heteropolyacid (HPA) catalysts: Effect of counter-cation, heteroatom, and polyatom substitution. J. Mol. Catal. A Chem. 2004, 212, 229–236. [Google Scholar] [CrossRef]
- Lee, L.; Wang, J.X.; Adzic, R.R.; Robinson, I.K.; Gewirth, A.A. Adsorption configuration and local ordering of silicotungstate anions on Ag(100) electrode surfaces. J. Am. Chem. Soc. 2001, 123, 8838–8843. [Google Scholar] [CrossRef]
- Himeno, S.; Osakai, T.; Saito, A. Voltammetric characterization of alpha and beta-dodecamolybdophosphates in aqueous organic solutions. Bull. Chem. Soc. Jpn. 1989, 62, 1335–1337. [Google Scholar] [CrossRef]
- Kaba, M.S.; Song, I.K.; Wasfi, S.H.; Barteau, M.A. Investigation of Wells-Dawson Heteropoly Oxofluorotungstates by Scanning Tunneling Microscopy and Tunneling Spectroscopy. J. Electrochem. Soc. 2002, 149, E117. [Google Scholar] [CrossRef]
- Lopez, X.; Poblet, J.M. DFT Study on the Five Isomers of PW12O403−: Relative Stabilization upon Reduction. Inorg. Chem. 2004, 43, 6863–6865. [Google Scholar] [CrossRef]
- Lopez, X.; Fernandez, J.A.; Poblet, J.M. Redox properties of polyoxometalates: New insights on the anion charge effect. Dalton Trans. 2006, 9, 1162–1167. [Google Scholar] [CrossRef]
- Lopez, X.; Fernandez, J.A.; Romo, S.; Paul, J.F.; Kazansky, L.; Poblet, J.M. Are the solvent effects critical in the modeling of polyoxoanions? J. Comput. Chem. 2004, 25, 1542–1549. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: New York, NY, USA, 1983. [Google Scholar]
- Mbomekalle, I.-M.; Lopez, X.; Poblet, J.M. Influence of the Heteroatom Size on the Redox Potentials of Selected Polyoxoanions. Inorg. Chem. 2010, 49, 7001–7006. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid organic-inorganic polyoxometalate compounds: From structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal-organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- He, W.-W.; Li, S.-L.; Zang, H.-Y.; Yang, G.-S.; Zhang, S.-R.; Su, Z.-M.; Lan, Y.-Q. Entangled structures in polyoxometalate-based coordination polymers. Coord. Chem. Rev. 2014, 279, 141–160. [Google Scholar] [CrossRef]
- Wang, X.; Tian, A.; Wang, X. Architectural chemistry of polyoxometalate-based coordination frameworks constructed from flexible N-donor ligands. RSC Adv. 2005, 5, 41155–41168. [Google Scholar] [CrossRef]
- Gong, P.; Li, Y.; Luo, J.; Chen, L.; Zhao, J. Syntheses, structures and properties of two copper-2-picolinic-acid germanomolybdate hybrids with mixed organic components. Inorg. Chem. Commun. 2016, 71, 113–118. [Google Scholar] [CrossRef]
- Li, L.; Cheng, M.; Bai, Y.; An, B.; Dang, D. A polyoxometalate-based inorganic–organic hybrid polymer constructed from silver-Schiff base building block and Keggin-type cluster: Synthesis, crystal structure and photocatalytic performance for the degradation of rhodamine B. Spectrochim. Acta Part A Mol. Biomol. Spect. 2015, 150, 846–854. [Google Scholar] [CrossRef]
- Tian, A.; Ning, Y.; Ying, J.; Zhang, J.; Hou, X.; Li, T.; Wang, X. Highly efficient usage of the hydrothermal technique through the one-pot method to construct four Keggin-based compounds containing pendent ligands. Dalton Trans. 2015, 44, 10499–10507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, T.; Tian, A.; Xu, N.; Zhang, R. Introduction of secondary pyridyl-1H-tetrazole derivatives into Keggin–Ag–(1,10-phenanthroline) system for tuning dimensionalities and architectures: Assembly and properties. J. Coord. Chem. 2016, 69, 2532–2544. [Google Scholar] [CrossRef]
- Zhao, C.; Li, S.; Ma, H.; Zhang, C.; Pang, H.; Yu, Y.; Zhang, Z. The factors affecting the assembly of Keggin–metal–bimb systems: Charge/polarity of Keggin polyanions and coordination modes of metal cations. CrystEngComm 2016, 18, 6233–6244. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.-W.; Sha, J.-Q.; Li, G.-M.; Yan, P.-F.; Wang, C.; Yu, L. Structure refinement and photocatalytic properties of porous POMCPs by selecting the isomerous PYTTZ. Dalton Trans. 2015, 44, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.-F.; Zhou, W.-Z.; Zang, H.-Y.; Tan, H.-Q.; Qi, Y.-F.; Wang, Y.-H.; Li, Y.-G. Keggin-Type Polyoxometalate-Based Metal–Organic Networks for Photocatalytic Dye Degradation. Chem. Asian J. 2015, 10, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, S.; Yan, J.; Li, L.; Wu, G.; Shi, W.; Yang, G.; Guan, N.; Cheng, P. Polyoxometalate-based metal–organic frameworks as visible-light-induced photocatalysts. Inorg. Chem. 2018, 57, 5030–5037. [Google Scholar] [CrossRef]
- Tian, A.-X.; Hou, X.; Ying, J.; Liu, G.-C.; Yang, Y.; Ning, Y.-L.; Li, T.-J.; Wang, X.-L. A series of polyoxometalate-based compounds including infinite Ag belts and circles constructed by two tolyl-1H-tetrazole isomers. RSC Adv. 2015, 5, 53757–53765. [Google Scholar] [CrossRef]
- Zhou, E.-L.; Qin, C.; Wang, X.-L.; Shao, K.-Z.; Su, Z.-M. Assembly of two novel 3D organic–inorganic hybrids based on Keggin-type polyoxometalates: Syntheses, crystal structures and properties. CrystEngComm 2016, 18, 6370–6377. [Google Scholar] [CrossRef]
- Li, M.-T.; Sha, J.-Q.; Zong, X.-M.; Sun, J.-W.; Yan, P.-F.; Li, L.; Yang, X.-N. Assembly of Polyoxometalate-Based Hybrids with Different Helical Channels upon Subtle Ligand Variation. Cryst. Growth Des. 2014, 14, 2794–2802. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, H.; Pang, H.; Li, S.; Zhang, Z.; Yu, Y. A new POMOF consisting of [VW12]4− clusters and metal-organic nanotubes: Synthesis, structure, electrocatalytic and luminescent properties. Inorg. Chem. Commun. 2016, 69, 57–61. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Sun, L.-J.; Zhu, P.-P.; Jiang, J. The first two-fold interpenetrating polyoxometalate-based coordination polymer with helical channels: Structure and catalytic activities. CrystEngComm 2016, 18, 283–289. [Google Scholar] [CrossRef]
- Khenkin, A.M.; Efremenko, I.; Weiner, L.; Martin, J.M.L.; Neumann, R. Photochemical Reduction of Carbon Diooxide Catalyzed by a Ruthenium-Substituted Polyoxometalate. Chem. Eur. J. 2010, 16, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Song, G.; Lin, H.-Y.; Wang, X.; Liu, G.-C.; Rong, X. Polyoxometalate-induced different metal–organic frameworks based on isonicotinic acid and AgI ion: Syntheses, structures and properties. Inorg. Chem. Commun. 2017, 84, 168–173. [Google Scholar] [CrossRef]
- Chai, D.-F.; Wang, M.; Zhang, C.; Ning, F.; Xu, W.; Pang, H.; Ma, H. A novel 3D POMOF based on dinuclear copper (II)-oxalate complexes and Keggin polyoxoanions with excellent photocatalytic activity. Inorg. Chem. Commun. 2017, 83, 16–19. [Google Scholar] [CrossRef]
- Sha, J.; Yang, X.; Zhu, P.; Lan, Y.; Sheng, N. Two new silver triazole frameworks with polyoxometalate templates. RSC Adv. 2016, 6, 108328–108334. [Google Scholar] [CrossRef]
- Li, Y.-W.; Guo, L.-Y.; Su, H.-F.; Jagodic, M.; Luo, M.; Zhou, X.-Q.; Zeng, S.-Y.; Tung, C.-H.; Sun, D.; Zheng, L.-S. Two Unprecedented POM-Based Inorganic–Organic Hybrids with Concomitant Heteropolytungstate and Molybdate. Inorg. Chem. 2017, 56, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Lu, X.-X.; Zhang, H. An ultrastable, flexible POM-based coordination polymer with redox properties. CrystEngComm 2014, 16, 7865–7868. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, J.; Zhang, Z.; Shi, Z.; Khan, S.U.; Liu, H. Assembly of hybrids based on polyoxotungstates and Co-tris(imidazolyl) complexes with bifunctional electrocatalytic activities. RSC Adv. 2015, 5, 35753–35759. [Google Scholar] [CrossRef]
- Wang, X.-I.; Hu, H.-I.; Tian, A.-X. Influence of Transition Metal Coordination Nature on the Assembly of Multinuclear Subunits in Polyoxometalates-Based Compounds. Cryst. Growth Des. 2010, 10, 4786–4794. [Google Scholar] [CrossRef]
- Wang, X.-L.; Gao, Q.; Tian, A.-X.; Hu, H.-L.; Liu, G.-C. Assembly of a series of Keggin-based multi- and mono-nuclear structures by tuning the bis(tetrazole)-functionalized thioether ligands. Inorg. Chim. Acta 2012, 384, 62–68. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, H.; Ma, H.; Li, S.; Zhao, C. pH-Directed assembly of four polyoxometalate-based supramolecular hybrids by using tritopic bridging ligand 1, 3, 5-tris-(1-imidazolyl)-benzene: Structures and electrocatalytic properties. Solid State Sci. 2018, 75, 1–8. [Google Scholar] [CrossRef]
- Tian, A.; Tian, Y.; Ning, Y.; Hou, X.; Ni, H.; Ji, X.; Liu, G.; Ying, J. A series of Keggin-based compounds constructed by conjugate ring-rich pyrazine and quinoxaline derivatives. Dalton Trans. 2016, 45, 13925–13936. [Google Scholar] [CrossRef]
- Cao, Y.; Lv, J.; Yu, K.; Wang, C.; Su, Z.; Wang, L.; Zhou, B. Synthesis and photo-/electro-catalytic properties of Keggin polyoxometalate inorganic–organic hybrid layers based on d10 metal and rigid benzo-diazole/-triazole ligands. New J. Chem. 2017, 41, 12459–12469. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wang, T.-T.; Lan, Q.; Yao, S.; Wu, H.-L.; Zhou, Y.-Y.; Zhang, Z.-M.; Wang, E.-B. Polyoxometalate-based supramolecular architecture constructed from a purely inorganic 1D chain and a metal–organic layer with efficient catalytic activity. RSC Adv. 2016, 6, 15513–15517. [Google Scholar] [CrossRef]
- Jiao, Y.-Q.; Qin, C.; Zang, H.-Y.; Chen, W.-C.; Wang, C.-G.; Zheng, T.-T.; Shao, K.-Z.; Su, Z.-M. Assembly of organic–inorganic hybrid materials constructed from polyoxometalate and metal–1,2,4-triazole units: Synthesis, structures, magnetic, electrochemical and photocatalytic properties. CrystEngComm 2015, 17, 2176–2189. [Google Scholar] [CrossRef]
- Wang, W.; Xu, L.; Gao, G.; Liu, L.; Liu, X. The first ε-Keggin core of molybdogermanate in extended architectures of nickel(II) with N-donor ligands: Syntheses, crystal structures and magnetic properties. CrystEngComm 2009, 11, 2488–2493. [Google Scholar] [CrossRef]
- Reinoso, S.; Vitoria, P.; San Felices, L.; Montero, A.; Lezama, L.; Gutiérrez-Zorrilla, J.M. Tetrahydroxy-p-benzoquinone as a Source of Polydentate O-Donor Ligands. Synthesis, Crystal Structure, and Magnetic Properties of the [Cu(bpy)(dhmal)]2 Dimer and the Two-Dimensional [{SiW12O40}{Cu2(bpy)2(H2O)(ox)}2]·16H2O Inorganic−Metalorganic Hybrid. Inorg. Chem. 2007, 46, 1237–1249. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Zhang, Q.-K.; Kuang, X.-F.; Yang, W.; Yu, R.-M.; Lu, C.-Z. Two hybrid polyoxometalate-pillared metal–organic frameworks. Dalton Trans. 2012, 41, 11783–11787. [Google Scholar] [CrossRef] [PubMed]
- Darling, K.; Smith, T.M.; Vargas, J.; O’Connor, C.J.; Zubieta, J. Polyoxometalate clusters as building blocks for oxide materials: Synthesis and structure of a three-dimensional copper-pyrazinetetrazolate/Keggin assembly. Inorg. Chem. Commun. 2013, 32, 1–4. [Google Scholar] [CrossRef]
- Tripuramallu, B.K.; Das, S.K. Hydrothermal Synthesis and Structural Characterization of Metal Organophosphonate Oxide Materials: Role of Metal-Oxo Clusters in the Self Assembly of Metal Phosphonate Architectures. Cryst. Growth Des. 2013, 13, 2426–2434. [Google Scholar] [CrossRef]
- Wang, X.-L.; Rong, X.; Liu, D.-N.; Lin, H.-Y.; Liu, G.-C.; Wang, X.; Song, G. Diverse polyoxometalate-based metal–organic complexes constructed by a tetrazole- and pyridyl-containing asymmetric amide ligand or its in situ transformed ligand. CrystEngComm 2016, 18, 5101–5109. [Google Scholar] [CrossRef]
- Li, S.; Ma, H.; Pang, H.; Zhang, Z.; Yu, Y.; Liu, H.; Yu, T. Tuning the dimension of POM-based inorganic–organic hybrids from 3D self-penetrating framework to 1D poly-pendant chain via changing POM clusters and introducing secondary spacers. CrystEngComm 2014, 16, 2045–2055. [Google Scholar] [CrossRef]
- Wang, D.-B.; Gao, H.; Bai, Y.; Hu, X.-F.; Yang, F.; Chen, Y.; Niu, J.-Y. A 2D helical coordination polymer based on Keggin-type polyoxoanion: Syntehesis, crysta structure and luminescent properties. Inorg. Chem. Commun. 2010, 13, 37–41. [Google Scholar] [CrossRef]
- Han, Z.; Hao, Q.; Zhai, X.; Xie, J. Multiple supported Keggin-type polyoxometalate polymer built upon weak copper–oxygen interaction. Inorg. Chim. Acta 2012, 382, 105–110. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, J.; Pang, H.-J.; Zhang, P.-P.; Chen, Y.; Wang, D.-D.; Liu, M.-G.; Wang, Y.-H. Polyoxometalate immobilization in CuI/Ag–pz porous coordination polymers: The influences of them on the structural properties of frameworks. J. Solid State Chem. 2011, 184, 1070–1078. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Alimaje, K.; Shi, Z.-Y. Keggin POM-based 3D framework tuned by silver polymeric motifs: Structural influences of tetrazolate functional groups. CrystEngComm 2012, 14, 8509–8514. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Zhang, C.-J.; Chen, Y.-G.; Liu, S.-X. A new 3D hybrid architecture containing Keggin-type tungstosilicate and Cu4(EGTA)2 metallamacrocyclic cation. Inorg. Chem. Commun. 2011, 14, 1465–1468. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Pang, H.-J.; Tang, Q.; Chen, Y.-G. A feasible route to approach 3D POM-based hybrids: Utilizing substituted or reduced Keggin anions with high charge density. Dalton Trans. 2012, 41, 9365–9372. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Wu, H.; Yang, J.; Liu, Y.-Y.; Ma, J.-F.; Bai, H.-Y. Solvothermal Assembly of a Series of Organic–Inorganic Hybrid Materials Constructed from Keggin Polyoxometalate Clusters and Copper(I)–Organic Frameworks. Cryst. Growth Des. 2011, 11, 1786–1797. [Google Scholar] [CrossRef]
- Nakajima, K.; Eda, K.; Himeno, S. Effect of the Central Oxoanion Size on the Voltammetric Properties of Keggin-Type [XW12O40]n− (n = 2–6) Complexes. Inorg. Chem. 2010, 49, 5212–5215. [Google Scholar] [CrossRef]
- Maeda, K.; Himeno, S.; Osakai, T.; Saito, A. A voltammetric study of Keggin-type heteropolymolybdate anions. J. Electroanal. Chem. 1994, 364, 149–154. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- TURBOMOLE V6.4 2012, a Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, Since 2007. Available online: http://www.turbomole.com (accessed on 2 July 2021).
- Christiansen, P.A.; Ermler, W.C.; Pitze, K.S. Relativistic Effects in Chemical Systems. Annu. Rev. Phys. Chem. 1985, 36, 407–432. [Google Scholar] [CrossRef][Green Version]
- Broyden, C.G. The Convergence of a Class of Double-rank Minimization Algorithms: 2. The New Algorithm. J. Inst. Math. Appl. 1970, 6, 222–231. [Google Scholar] [CrossRef]
- Fletcher, R. A new approach to variable metric algorithms. Comput. J. 1970, 13, 317–322. [Google Scholar] [CrossRef]
- Goldfarb, D. A family of variable-metric methods derived by variational means. Math. Comput. 1970, 24, 23–26. [Google Scholar] [CrossRef]
- Shanno, D.F. Conditioning of quasi-Newton methods for function minimization. Math. Comput. 1970, 24, 647–656. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Morokuma, K. Molecular Orbital Studies of Hydrogen Bonds. III. C=O···H–O Hydrogen Bond in H2CO···H2O and H2CO···2H2O. J. Chem. Phys. 1971, 55, 1236. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the calculation of bonding energies by Hartree Fock Slater method. Theor. Chim. Acta 1977, 46, 1–10. [Google Scholar] [CrossRef]






| X | Zn2+ | B3+ | Al3+ | Ga3+ | Si4+ | Ge4+ | P5+ | As5+ | S6+ |
|---|---|---|---|---|---|---|---|---|---|
| Vacuum | |||||||||
| EHOMO | 8.23 | 5.99 | 5.29 | 5.28 | 2.59 | 2.57 | −0.15 | −0.16 | −2.89 |
| ELUMO | 10.52 | 7.99 | 7.87 | 7.84 | 5.17 | 5.13 | 2.41 | 2.37 | −0.38 |
| Gap | 2.29 | 2.00 | 2.58 | 2.56 | 2.58 | 2.56 | 2.56 | 2.53 | 2.51 |
| Water (H2O) | |||||||||
| EHOMO | −5.78 | −5.71 | −6.43 | −6.43 | −6.74 | −6.73 | −7.04 | −7.03 | −7.33 |
| ELUMO | −3.55 | −3.77 | −3.83 | −3.84 | −4.14 | −4.16 | −4.47 | −4.49 | −4.81 |
| Gap | 2.23 | 1.94 | 2.60 | 2.59 | 2.60 | 2.57 | 2.57 | 2.54 | 2.52 |
| Acetonitrile (CH3CN) | |||||||||
| EHOMO | −5.50 | −5.48 | −6.20 | −6.19 | −6.55 | −6.55 | −6.89 | −6.90 | −7.24 |
| ELUMO | −3.26 | −3.53 | −3.59 | −3.61 | −3.95 | −3.97 | −4.33 | −4.36 | −4.72 |
| Gap | 2.24 | 1.95 | 2.61 | 2.58 | 2.60 | 2.58 | 2.56 | 2.54 | 2.52 |
| ΔEint [a.u.] | −1.122 | −0.907 | −0.808 | −0.804 | −0.570 | −0.571 | −0.345 | −0.348 | −0.145 |
| Ered [eV] | −1.92 | −1.65 | −1.56 | −1.54 | −1.13 | −1.09 | −0.64 | −0.67 | −0.26 |
| X | B3+ | Al3+ | Si4+ | P5+ | S6+ | Zn2+ | Ga3+ | Ge4+ | As5+ |
|---|---|---|---|---|---|---|---|---|---|
| HOMO | |||||||||
| X | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 12.25 | 0.02 | 0.00 | 0.00 |
| Oa | 77.43 | 0.39 | 0.03 | 0.01 | 0.01 | 50.56 | 0.93 | 0.09 | 0.02 |
| W | 4.16 | 0.61 | 0.70 | 0.84 | 1.05 | 2.04 | 0.64 | 0.74 | 0.88 |
| Ob | 1.67 | 44.78 | 46.47 | 47.26 | 47.71 | 2.21 | 43.81 | 46.03 | 46.92 |
| Oc | 6.72 | 52.08 | 51.46 | 50.88 | 50.49 | 26.01 | 51.84 | 51.56 | 50.99 |
| Od | 10.03 | 2.12 | 1.34 | 1.01 | 0.75 | 6.94 | 2.76 | 1.57 | 1.19 |
| LUMO | |||||||||
| X | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Oa | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.00 |
| W | 75.39 | 75.28 | 74.99 | 74.71 | 74.48 | 75.60 | 75.29 | 74.99 | 74.73 |
| Ob | 11.62 | 11.69 | 11.79 | 11.93 | 12.04 | 11.68 | 11.69 | 11.78 | 11.88 |
| Oc | 12.80 | 12.89 | 13.08 | 13.23 | 13.35 | 12.61 | 12.91 | 13.11 | 13.28 |
| Od | 0.19 | 0.15 | 0.14 | 0.13 | 0.11 | 0.12 | 0.13 | 0.13 | 0.12 |
| X | Zn2+ | B3+ | Al3+ | Ga3+ | Si4+ | Ge4+ | P5+ | As5+ | S6+ |
|---|---|---|---|---|---|---|---|---|---|
| Vacuum | |||||||||
| EHOMO | 8.20 | 6.18 | 5.47 | 5.45 | 2.81 | 2.78 | 0.10 | 0.07 | −2.65 |
| ELUMO | 10.73 | 8.00 | 7.96 | 7.95 | 5.14 | 5.13 | 2.30 | 2.29 | −0.55 |
| Gap | 2.53 | 1.82 | 2.49 | 2.50 | 2.33 | 2.35 | 2.20 | 2.22 | 2.10 |
| Water (H2O) | |||||||||
| EHOMO | −5.87 | −5.61 | −6.32 | −6.33 | −6.59 | −6.61 | −6.90 | −6.88 | −7.19 |
| ELUMO | −3.38 | −3.84 | −3.82 | −3.81 | −4.25 | −4.24 | −4.68 | −4.67 | −5.08 |
| Gap | 2.49 | 1.77 | 2.50 | 2.52 | 2.34 | 2.37 | 2.22 | 2.21 | 2.11 |
| Acetonitrile (CH3CN) | |||||||||
| EHOMO | −5.87 | −5.37 | −6.08 | −6.09 | −6.40 | −6.42 | −6.74 | −6.76 | −7.10 |
| ELUMO | −3.38 | −3.60 | −3.58 | −3.57 | −4.06 | −4.05 | −4.54 | −4.53 | −4.99 |
| Gap | 2.48 | 1.77 | 2.50 | 2.52 | 2.34 | 2.37 | 2.20 | 2.23 | 2.10 |
| ΔEint (a.u.) | −1.248 | −0.900 | −0.796 | −0.795 | −0.660 | −0.643 | −0.443 | −0.448 | −0.254 |
| X | B3+ | Al3+ | Si4+ | P5+ | S6+ | Zn2+ | Ga3+ | Ge4+ | As5+ |
|---|---|---|---|---|---|---|---|---|---|
| HOMO | |||||||||
| X | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 10.82 | 0.00 | 0.00 | 0.00 |
| Oa | 76.12 | 0.12 | 0.02 | 0.01 | 0.01 | 49.90 | 0.23 | 0.11 | 0.01 |
| W | 5.79 | 1.65 | 1.70 | 1.89 | 2.19 | 2.01 | 1.70 | 1.74 | 1.96 |
| Ob | 1.89 | 41.47 | 43.51 | 44.86 | 45.80 | 2.52 | 40.82 | 45.47 | 44.25 |
| Oc | 5.82 | 55.13 | 53.54 | 52.27 | 51.24 | 24.20 | 55.46 | 51.21 | 52.72 |
| Od | 10.39 | 1.63 | 1.23 | 0.98 | 0.76 | 10.56 | 1.78 | 1.47 | 1.05 |
| LUMO | |||||||||
| X | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Oa | 0.06 | 0.03 | 0.02 | 0.01 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 |
| W | 70.29 | 69.74 | 70.10 | 70.34 | 70.44 | 69.33 | 69.62 | 71.83 | 70.16 |
| Ob | 14.45 | 15.04 | 14.64 | 14.34 | 14.13 | 15.46 | 15.11 | 12.92 | 14.51 |
| Oc | 14.14 | 14.14 | 14.44 | 14.73 | 14.99 | 13.99 | 14.19 | 15.01 | 14.72 |
| Od | 1.05 | 1.05 | 1.23 | 0.58 | 0.44 | 1.20 | 1.06 | 0.27 | 0.61 |
| X. | Al3+ | Ga3+ | Si4+ | Ge4+ | P5+ | As5+ | S6+ |
|---|---|---|---|---|---|---|---|
| CH3CN | |||||||
| Model | −1.56 | −1.54 | −1.10 | −1.08 | −0.63 | −0.64 | −0.21 |
| Ered (eV) | - | - | −1.10 | −1.14 | −0.64 | −0.66 | −0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemiec, P.; Tokarz-Sobieraj, R.; Witko, M. Tungsten and Molybdenum Heteropolyanions with Different Central Ions—Correlation between Theory and Experiment. Molecules 2022, 27, 187. https://doi.org/10.3390/molecules27010187
Niemiec P, Tokarz-Sobieraj R, Witko M. Tungsten and Molybdenum Heteropolyanions with Different Central Ions—Correlation between Theory and Experiment. Molecules. 2022; 27(1):187. https://doi.org/10.3390/molecules27010187
Chicago/Turabian StyleNiemiec, Piotr, Renata Tokarz-Sobieraj, and Małgorzata Witko. 2022. "Tungsten and Molybdenum Heteropolyanions with Different Central Ions—Correlation between Theory and Experiment" Molecules 27, no. 1: 187. https://doi.org/10.3390/molecules27010187
APA StyleNiemiec, P., Tokarz-Sobieraj, R., & Witko, M. (2022). Tungsten and Molybdenum Heteropolyanions with Different Central Ions—Correlation between Theory and Experiment. Molecules, 27(1), 187. https://doi.org/10.3390/molecules27010187






