In Silico Screening and In Vitro Assessment of Natural Products with Anti-Virulence Activity against Helicobacter pylori
Abstract
:1. Introduction
2. Results and Discussion
2.1. Virtual Screening
2.2. MICs
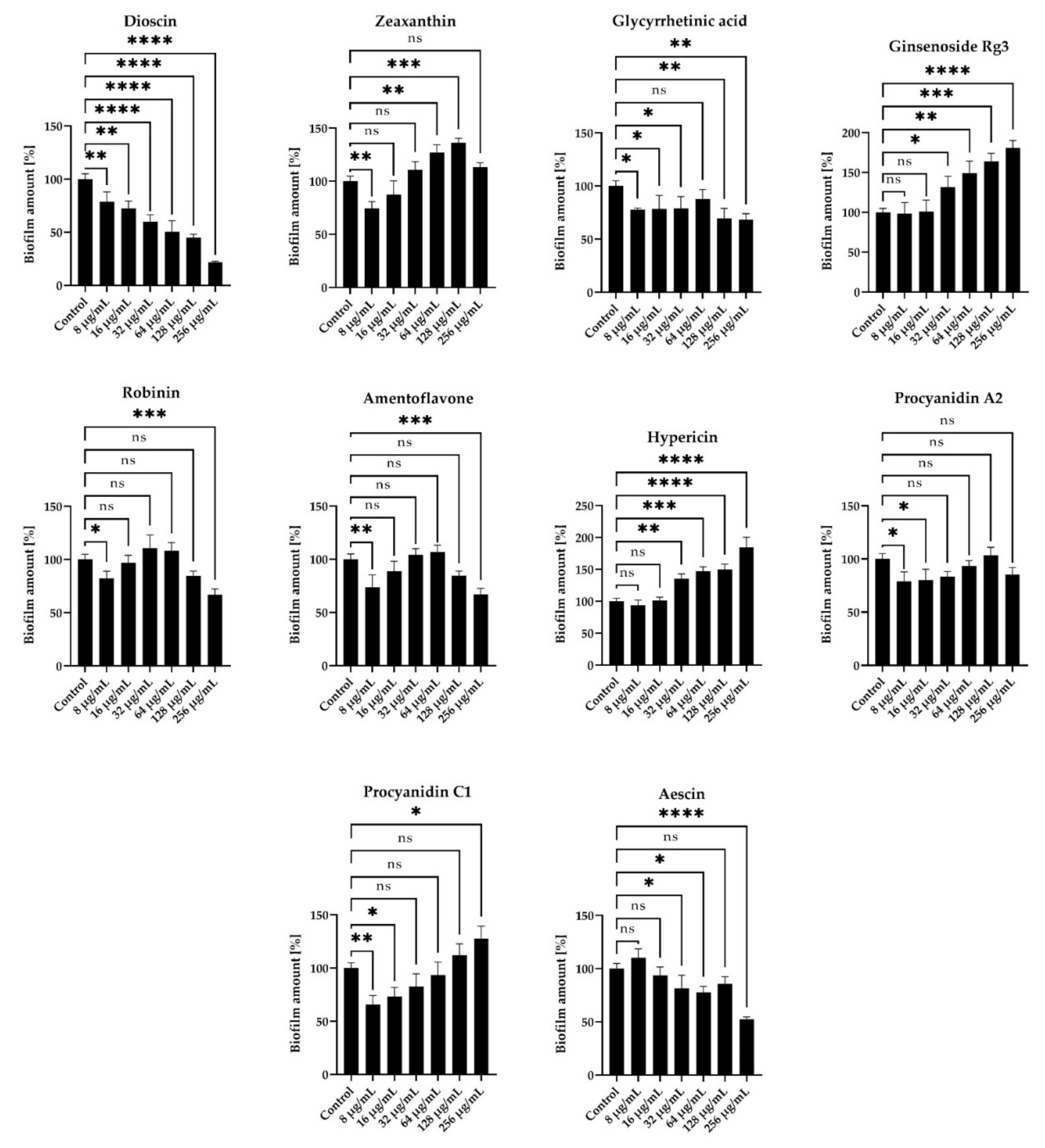
2.3. Anti-Biofilm Activity
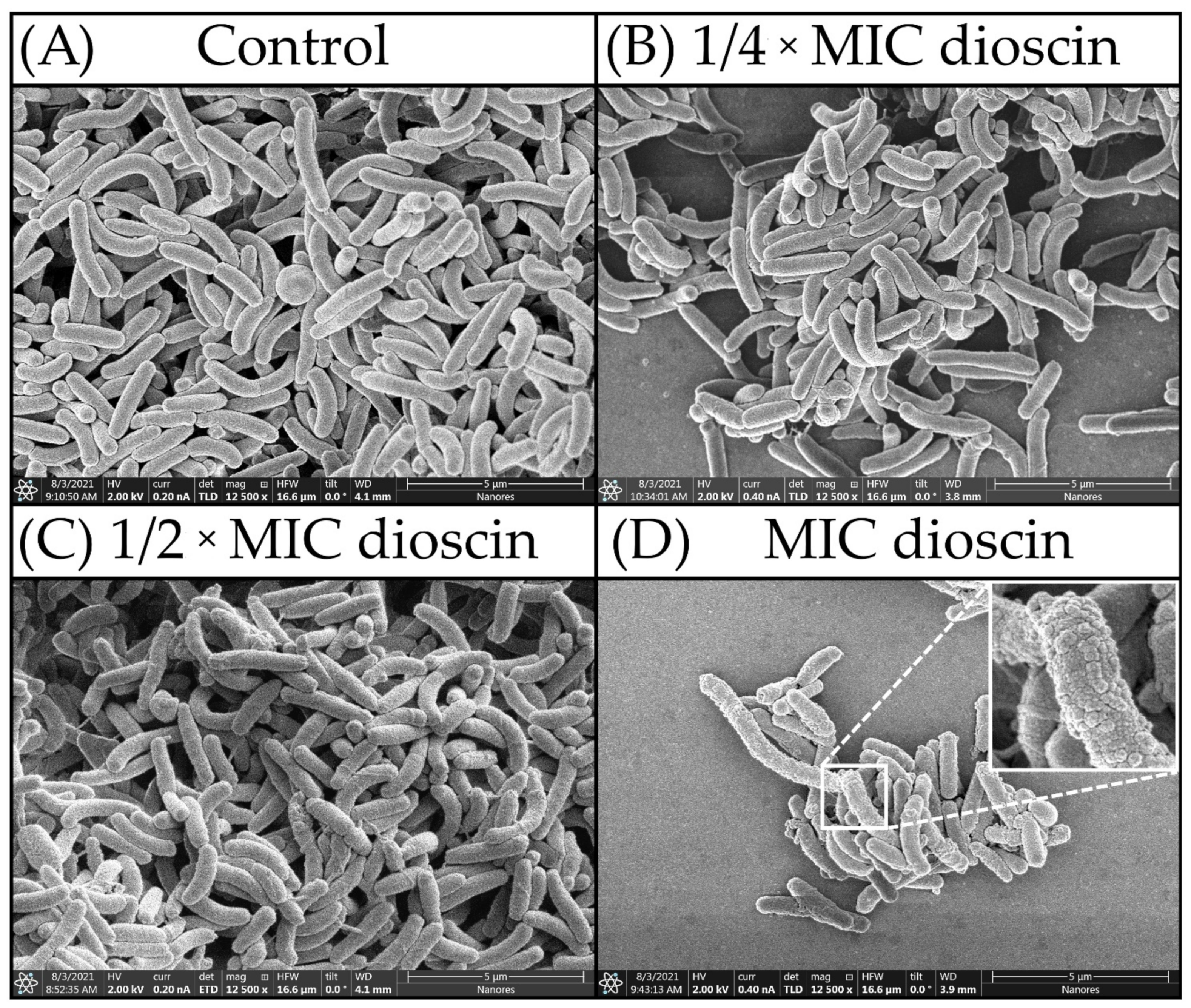
2.4. Morphostructural Analysis of Bacterial Cells
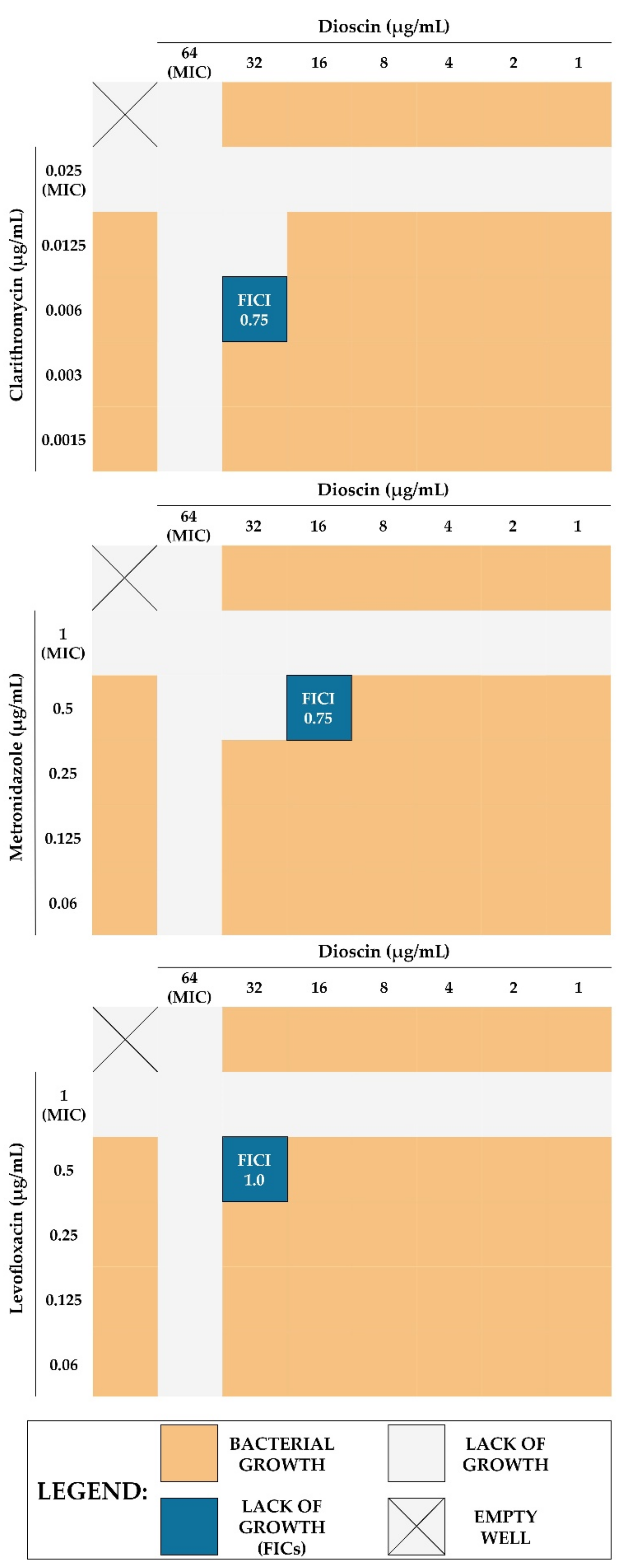
2.5. Checkerboard Assay
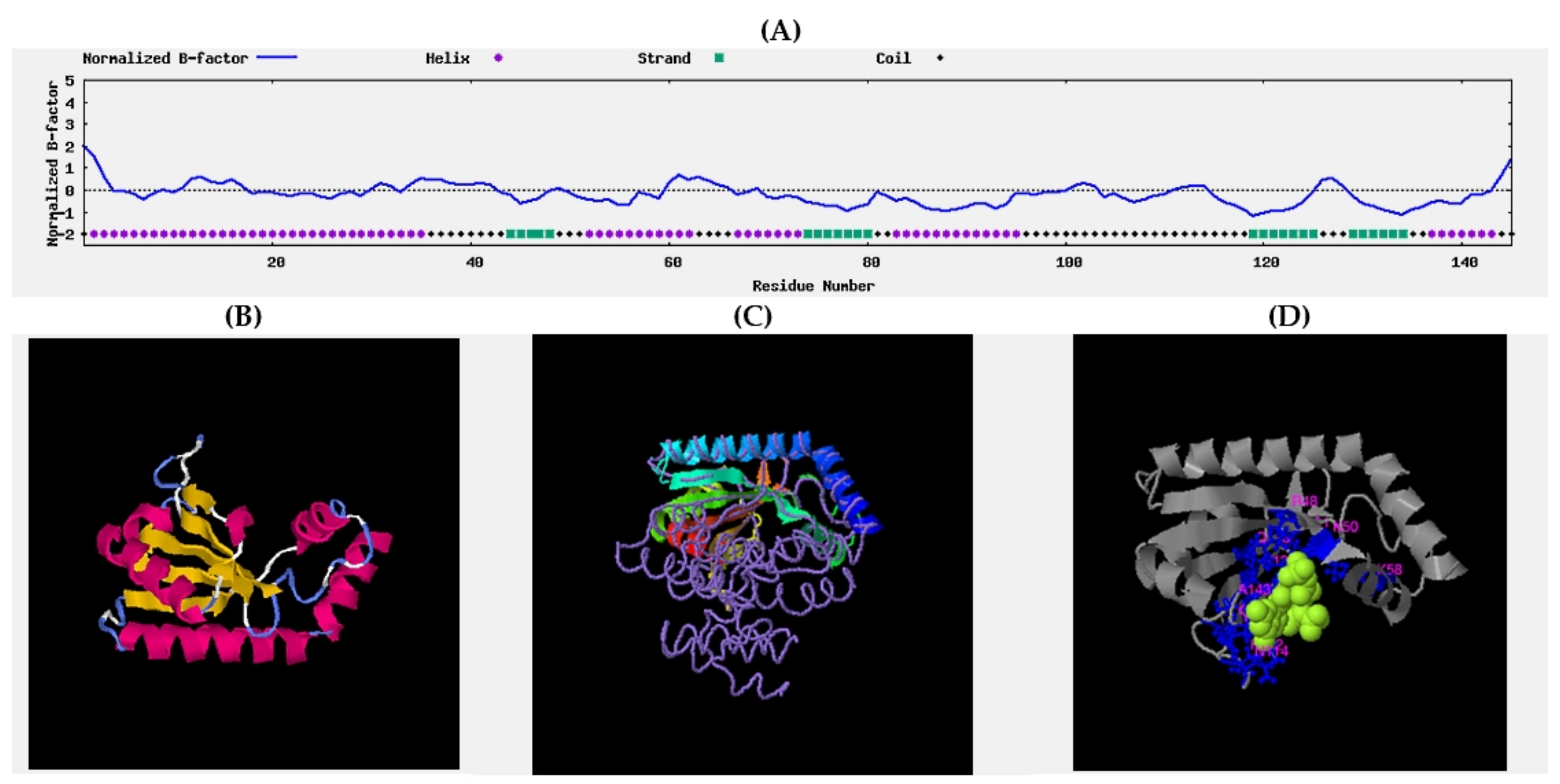
2.6. Concluding Computational Studies
2.7. Limitations and Future Perspectives
3. Materials and Methods
3.1. Computational Analysis
3.1.1. Bioinformatic Protocol
3.1.2. Molecular Dynamics
3.1.3. Virtual Screening
3.2. Laboratory Analysis
3.2.1. Storage and Revival of Bacteria
3.2.2. Antibacterial Activity Determination
3.2.3. Impact on the Morphology and/or Morphostructure
3.2.4. Anti-Biofilm Activity Determination
Activity in Static Conditions
Activity in the Flow Conditions
3.2.5. Checkerboard Assays
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ventola, C.L. The antibiotic Resistance Crisis: Causes and Threats. PTJ 2015, 40, 277–283. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Wall, S. Prevention of Antibiotic Resistance—An Epidemiological Scoping Review to Identify Research Categories and Knowledge Gaps. Glob. Health Action 2019, 12, 1756191. [Google Scholar] [CrossRef]
- Irwin, R. Imagining the Postantibiotic Future: The Visual Culture of a Global Health Threat. Med. Humanit. 2020. ahead of print. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsieh, Y.H.; Powers, Z.M.; Kao, C.Y. Defeating Antibiotic-Resistant Bacteria: Exploring Alternative Therapies for a Post-Antibiotic Era. Int. J. Mol. Sci. 2020, 21, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Schjørring, S.; Krogfelt, K. Assessment of Bacterial Antibiotic Resistance Transfer in the Gut. Int. J. Microbiol. 2011, 2011, 312956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subirats, J.; Domingues, A.; Topp, E. Does Dietary Consumption of Antibiotics by Humans Promote Antibiotic Resistance in the Gut Microbiome? J. Food Prot. 2019, 82, 1636–1642. [Google Scholar] [CrossRef]
- Contreras-Omaña, R.; Escorcia-Saucedo, A.; Velarde-Ruiz Velasco, J. Prevalence and Impact of Antimicrobial Resistance in Gastrointestinal Infections: A Review. Rev. Gastroenterol. Mex. 2021, 86, 265–275. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Haley, K.P.; Gaddy, J.A. Helicobacter pylori: Genomic Insight into the Host-Pathogen Interaction. Int. J. Genomics 2015, 2015, 386905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagoonee, S.; Pellicano, R. Helicobacter pylori: Molecular Basis for Colonization and Survival in Gastric Environment and Resistance to Antibiotics. A Short Review. Infect. Dis. 2019, 51, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Grande, R.; Migdał, P.; Paluch, E.; Gościniak, G. Biofilm Formation as a Complex Result of Virulence and Adaptive Responses of Helicobacter pylori. Pathogens 2020, 9, 1062. [Google Scholar] [CrossRef]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef] [Green Version]
- Yonezawa, H.; Osaki, T.; Kamiya, S. Biofilm Formation by Helicobacter pylori and Its Involvement for Antibiotic Resistance. Biomed Res. Int. 2015, 2015, 914791. [Google Scholar] [CrossRef] [Green Version]
- Krzyżek, P.; Grande, R. Transformation of Helicobacter pylori into Coccoid Forms as a Challenge for Research Determining Activity of Antimicrobial Substances. Pathogens 2020, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Krzyżek, P.; Gościniak, G. Morphology of Helicobacter pylori as a Result of Peptidoglycan and Cytoskeleton Rearrangements. Prz. Gastroenterol. 2018, 13, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Reshetnyak, V.I.; Reshetnyak, T.M. Significance of Dormant Forms of Helicobacter pylori in Ulcerogenesis. World J. Gastroenterol. 2017, 23, 4867–4878. [Google Scholar] [CrossRef]
- Cammarota, G.; Sanguinetti, M.; Gallo, A.; Posteraro, B. Review article: Biofilm Formation by Helicobacter pylori as a Target for Eradication of Resistant Infection. Aliment. Pharmacol. Ther. 2012, 36, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, N.; Taame, M.; Kravtsov, V. Clinical and Laboratory Importance of Detecting Helicobacter pylori Coccoid Forms for the Selection of Treatment. Prz. Gastroenterol. 2020, 15, 294–300. [Google Scholar] [CrossRef]
- Talele, T.; Khedkar, S.; Rigby, A. Successful Applications of Computer Aided Drug Discovery: Moving Drugs from Concept to the Clinic. Curr. Top. Med. Chem. 2010, 10, 127–141. [Google Scholar] [CrossRef]
- Cui, W.; Aouidate, A.; Wang, S.; Yu, Q.; Li, Y.; Yuan, S. Discovering Anti-Cancer Drugs via Computational Methods. Front. Pharmacol. 2020, 11, 733. [Google Scholar] [CrossRef]
- Petrella, C.; Farioli-Vecchioli, S.; Cisale, G.Y.; Strimpakos, G.; Borg, J.J.; Ceccanti, M.; Fiore, M.; Monteleone, G.; Nisticò, R. A Healthy Gut for a Healthy Brain: Preclinical, Clinical and Regulatory Aspects. Curr. Neuropharmacol. 2020, 19, 610–628. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Byrne, R.; Schneider, G.; Yang, S. Concepts of Artificial Intelligence for Computer-Assisted Drug Discovery. Chem. Rev. 2019, 119, 10520–10594. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Krzyżek, P.; Paluch, E.; Gościniak, G. Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori. Antibiotics 2020, 9, 658. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, B.V.; dos Santos Ramos, M.A.; da Silva, P.B.; Bauab, T.M. Antimicrobial Activity of Natural Products against Helicobacter pylori: A Review. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 54. [Google Scholar]
- Steinchen, W.; Zegarra, V.; Bange, G. (p)ppGpp: Magic Modulators of Bacterial Physiology and Metabolism. Front. Microbiol. 2020, 11, 2072. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.H.; Gaynor, E.C. Helicobacter pylori Initiates the Stringent Response upon Nutrient and pH Downshift. J. Bacteriol. 2006, 188, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouery, K.; Rader, B.A.; Gaynor, E.C.; Guillemin, K. The Stringent Response is Required for Helicobacter pylori Survival of Stationary Phase, Exposure to Acid, and Aerobic Shock. J. Bacteriol. 2006, 188, 5494–5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.; Li, W.; Chen, Z.; Gao, S.; Hong, W.; Ge, X.; Hou, G.; Hu, Z.; Zhou, Y.; Zeng, B.; et al. The Bifunctional Enzyme SpoT Is Involved in the Clarithromycin Tolerance of Helicobacter pylori by Upregulating the Transporters HP0939, HP1017, HP0497, and HP0471. Antimicrob. Agents Chemother. 2017, 61, e02011-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Cai, Y.; Chen, Z.; Gao, S.; Geng, X.; Li, Y.; Li, Y.; Jia, J.; Sun, Y. Bifunctional Enzyme SpoT Is Involved in Biofilm Formation of Helicobacter pylori with Multidrug Resistance by Upregulating Efflux Pump Hp1174 (gluP). Antimicrob. Agents Chemother. 2018, 62, e00957-18. [Google Scholar] [CrossRef] [Green Version]
- Poursina, F.; Fagri, J.; Mirzaei, N.; Safaei, H.G. Overexpression of spoT Gene in Coccoid Forms of Clinical Helicobacter pylori Isolates. Folia Microbiol. 2018, 63, 459–465. [Google Scholar] [CrossRef]
- Yao, H.; Hu, C.; Yin, L.; Tao, X.; Xu, L.; Qi, Y.; Han, X.; Xu, Y.; Zhao, Y.; Wang, C.; et al. Dioscin Reduces Lipopolysaccharide-Induced Inflammatory Liver Injury via Regulating TLR4/MyD88 Signal Pathway. Int. Immunopharmacol. 2016, 36, 132–141. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.; Yang, X. Dioscin Exhibits Anti-inflammatory Effects in IL-1β-Stimulated Human Osteoarthritis Chondrocytes by Activating LXRα. Immunopharmacol. Immunotoxicol. 2020, 42, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, H.; Peng, J.; Wang, C.; Jin, Y.; Liu, K.; Sun, H.; Qin, J. Potent Anti-inflammatory Effect of Dioscin Mediated by Suppression of TNF-α-Induced VCAM-1, ICAM-1 and EL Expression via the NF-κB Pathway. Biochimie 2015, 110, 62–72. [Google Scholar] [CrossRef]
- Ma, T.; Wang, R.; Zou, X. Dioscin Inhibits Gastric Tumor Growth through Regulating the Expression Level of lncRNA HOTAIR. BMC Complement. Altern. Med. 2016, 16, 383. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, C.; Liu, C. Antitumor Effects of Dioscin in A431 Cells via Adjusting ATM/p53-Mediated Cell Apoptosis, DNA Damage and Migration. Oncol. Lett. 2021, 21, 59. [Google Scholar] [CrossRef]
- He, S.; Yang, J.; Hong, S.; Huang, H.; Zhu, Q.; Ye, L.; Li, T.; Zhang, X.; Wei, Y.; Gao, Y. Dioscin Promotes Prostate Cancer Cell Apoptosis and Inhibits Cell Invasion by Increasing SHP1 Phosphorylation and Suppressing the Subsequent MAPK Signaling Pathway. Front. Pharmacol. 2020, 11, 1099. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, M.-J.; An, R.-F.; Zhou, J.; Liu, W.; Morris-Natschke, S.L.; Cheng, Y.-Y.; Lee, K.-H.; Huang, X.-F. Diosgenin Derivatives as Potential Antitumor Agents: Synthesis, Cytotoxicity, and Mechanism of Action. J. Nat. Prod. 2020, 84, 616–629. [Google Scholar] [CrossRef]
- Barros Cota, B.; Batista Carneiro de Oliveira, D.; Carla Borges, T.; Cristina Catto, A.; Valverde Serafim, C.; Rogelis Aquiles Rodrigues, A.; Kohlhoff, M.; Leomar Zani, C.; Assunção Andrade, A. Antifungal Activity of Extracts and Purified Saponins from the Rhizomes of Chamaecostus cuspidatus against Candida and Trichophyton Species. J. Appl. Microbiol. 2021, 130, 61–75. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Zhong, L.; Sui, Y.; Quan, G.; Huang, Y.; Wang, F.; Ma, T. Dioscin Inhibits Virulence Factors of Candida albicans. Biomed Res. Int. 2018, 2018, 4651726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Choi, H.; Lee, J.; Kim, M.S.; Sohn, H.Y.; Lee, D.G. The Antifungal Activity and Membrane-Disruptive Action of Dioscin Extracted from Dioscorea nipponica. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Cong, S.; Tong, Q.; Peng, Q.; Shen, T.; Zhu, X.; Xu, Y.; Qi, S. In Vitro Anti-bacterial Activity of Diosgenin on Porphyromonas gingivalis and Prevotella intermedia. Mol. Med. Rep. 2020, 22, 5392–5398. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, Z.; Muzyka, N.; Lepekhina, E.; Oktyabrsky, O.; Smirnova, G. Medicinal Plant Extracts Can Variously Modify Biofilm Formation in Escherichia coli. Antonie Van Leeuwenhoek 2014, 105, 709–722. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Hupková, H.; Grančai, D. Rosmarinic Acid Interaction with Planktonic and Biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8, 1747–1750. [Google Scholar] [CrossRef] [Green Version]
- Vacheva, A.; Mustafa, B.; Staneva, J.; Marhova, M.; Kostadinova, S.; Todorova, M.; Ivanova, R.; Stoitsova, S. Effects of Extracts from Medicinal Plants on Biofilm Formation by Escherichia coli Urinary Tract Isolates. Biotechnol. Biotechnol. Equip. 2014, 25, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Luganini, A.; Terlizzi, M.E.; Catucci, G.; Gilardi, G.; Maffei, M.E.; Gribaudo, G. The Cranberry Extract Oximacro® Exerts In Vitro Virucidal Activity against Influenza Virus by Interfering with Hemagglutinin. Front. Microbiol. 2018, 9, 1826. [Google Scholar] [CrossRef]
- Liu, D.; Deng, J.; Joshi, S.; Liu, P.; Zhang, C.; Yu, Y.; Zhang, R.; Fan, D.; Yang, H.; D’Souza, D.H. Monomeric Catechin and Dimeric Procyanidin B2 against Human Norovirus Surrogates and their Physicochemical Interactions. Food Microbiol. 2018, 76, 353. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial Activity of Resveratrol-Derived Monomers and Dimers against Foodborne Pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Huiszoon, R.C.; Chu, S.; Bentley, W.E.; Ghodssi, R. Microsystems for Biofilm Characterization and Sensing—A Review. Biofilm 2020, 2, 100015. [Google Scholar] [CrossRef]
- Tao, R.; Tong, Z.; Lin, Y.; Xue, Y.; Wang, W.; Kuang, R.; Wang, P.; Tian, Y.; Ni, L. Antimicrobial and Antibiofilm Activity of Pleurocidin against Cariogenic Microorganisms. Peptides 2011, 32, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Díez-Aguilar, M.; Morosini, M.I.; Köksal, E.; Oliver, A.; Ekkelenkamp, M.; Cantón, R. Use of Calgary and Microfluidic BioFlux Systems To Test the Activity of Fosfomycin and Tobramycin Alone and in Combination against Cystic Fibrosis Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2017, 62, e01650-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Xu, C.; Fang, R.; Cao, J.; Zhang, X.; Zhao, Y.; Dong, G.; Sun, Y.; Zhou, T. Resistance and Heteroresistance to Colistin in Pseudomonas aeruginosa Isolates from Wenzhou, China. Antimicrob. Agents Chemother. 2019, 63, e00556-19. [Google Scholar] [CrossRef] [Green Version]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 619. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chanda, W.; Zhong, M. The Relationship between Biofilm and Outer Membrane Vesicles: A Novel Therapy Overview. FEMS Microbiol. Lett. 2015, 362, fnv117. [Google Scholar] [CrossRef]
- Mozaheb, N.; Mingeot-Leclercq, M.-P. Membrane Vesicle Production as a Bacterial Defense Against Stress. Front. Microbiol. 2020, 11, 600221. [Google Scholar] [CrossRef]
- Murray, B.O.; Dawson, R.A.; Alsharaf, L.M.; Winter, J.A. Protective Effects of Helicobacter pylori Membrane Vesicles against Stress and Antimicrobial Agents. Microbiology 2020, 166, 751–758. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori Infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [Green Version]
- ATCC. Helicobacter pylori (Marshall et al.) Goodwin et al. Available online: https://www.atcc.org/products/700824 (accessed on 21 October 2021).
- Consortium, T.U.; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; et al. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- HP_0775—Penta-Phosphate Guanosine-3′-pyrophosphohydrolase (SpoT)—Helicobacter pylori (strain ATCC 700392/26695)—_0775 Gene & Protein. Available online: https://www.uniprot.org/uniprot/O25466 (accessed on 21 October 2021).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Henikoff, S.; Henikoff, J.G. Amino Acid Substitution Matrices from Protein Blocks. Proc. Natl. Acad. Sci. USA 1992, 89, 10915–10919. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Li, R.; Shi, Q.; Xue, Z.; Zhang, Y. ThreaDomEx: A Unified Platform for Predicting Continuous and Discontinuous Protein Domains by Multiple-Threading and Segment Assembly. Nucleic Acids Res. 2017, 45, W400–W407. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A Unified Platform for Automated Protein Structure and Function Prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein Structure and Function Prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, Y. I-TASSER Server: New Development for Protein Structure and Function Predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y. I-TASSER Server for Protein 3D Structure Prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Moult, J.; Pedersen, J.T.; Judson, R.; Fidelis, K. A Large-Scale Experiment to Assess Protein Structure Prediction Methods. Proteins Struct. Funct. Bioinform. 1995, 23, 2–4. [Google Scholar] [CrossRef]
- Johnson, T.W.; Gallego, R.A.; Brooun, A.; Gehlhaar, D.; McTigue, M. Reviving B-Factors: Retrospective Normalized B-Factor Analysis of c-ros Oncogene 1 Receptor Tyrosine Kinase and Anaplastic Lymphoma Kinase L1196M with Crizotinib and Lorlatinib. ACS Med. Chem. Lett. 2018, 9, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Zhang, Y. ResQ: An Approach to Unified Estimation of B-Factor and Residue-Specific Error in Protein Structure Prediction. J. Mol. Biol. 2016, 428, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Páll, S.; Abraham, M.J.; Kutzner, C.; Hess, B.; Lindahl, E. Tackling Exascale Software Challenges in Molecular Dynamics Simulations with GROMACS. Lect. Notes Comput. Sci. 2014, 8759, 3–27. [Google Scholar]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [Green Version]
- Kroese, D.P.; Brereton, T.; Taimre, T.; Botev, Z.I. Why the Monte Carlo Method Is So Important Today. Wiley Interdiscip. Rev. Comput. Stat. 2014, 6, 386–392. [Google Scholar] [CrossRef]
- Nosé, S. A Molecular Dynamics Method for Simulations in the Canonical Ensemble. Mol. Phys. 2006, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1998, 52, 7182. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N⋅log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1998, 98, 10089. [Google Scholar] [CrossRef] [Green Version]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for Molecular Simulations. J. Comput. Chem. 1997, 1463–1472. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano–Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Hogg, T.; Mechold, U.; Malke, H.; Cashel, M.; Hilgenfeld, R. Conformational Antagonism between Opposing Active Sites in a Bifunctional RelA/SpoT Homolog Modulates (p)ppGpp Metabolism during the Stringent Response. Cell 2004, 117, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Allouche, A.-R. Gabedit—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02. Available online: https://gaussian.com/g09citation/ (accessed on 21 October 2021).
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate ab initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyżek, P.; Franiczek, R.; Krzyżanowska, B.; Łaczmański, Ł.; Migdał, P.; Gościniak, G. In Vitro Activity of 3-Bromopyruvate, an Anti-Cancer Compound, Against Antibiotic-Susceptible and Antibiotic-Resistant Helicobacter pylori Strains. Cancers 2019, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Krzyżek, P.; Franiczek, R.; Krzyżanowska, B.; Łaczmański, Ł.; Migdał, P.; Gościniak, G. In Vitro Activity of Sertraline, an Antidepressant, Against Antibiotic-Susceptible and Antibiotic-Resistant Helicobacter pylori Strains. Pathogens 2019, 8, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyżek, P.; Biernat, M.M.; Gościniak, G. Intensive Formation of Coccoid Forms as a Feature Strongly Associated with Highly Pathogenic Helicobacter pylori Strains. Folia Microbiol. 2019, 64, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Migdał, P.; Paluch, E.; Karwańska, M.; Wieliczko, A.; Gościniak, G. Myricetin as an Antivirulence Compound Interfering with a Morphological Transformation into Coccoid Forms and Potentiating Activity of Antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef]
- Yang, L.; Ren, S.; Xu, F.; Ma, Z.; Liu, X.; Wang, L. Recent Advances in the Pharmacological Activities of Dioscin. Biomed Res. Int. 2019, 2019, 5763602. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.B.; Ryu, J.; Cho, Y.; Choi, S.Z.; Son, M.; Sung, S.H. Combined Application of UHPLC-QTOF/MS, HPLC-ELSD and 1H–NMR Spectroscopy for Quality Assessment of DA-9801, A Standardised Dioscorea Extract. Phytochem. Anal. 2017, 28, 185–194. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as Potential Leads for New Therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [Green Version]
- Schempp, C.M.; Winghofer, B.; Langheinrich, M.; Schöpf, E.; Simon, J.C. Hypericin Levels in Human Serum and Interstitial Skin Blister Fluid after Oral Single-Dose and Steady-State Administration of Hypericum perforatum Extract (St. John’s Wort). Skin Pharmacol. Physiol. 1999, 12, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Rizk, Y.S.; Santos-Pereira, S.; Gervazoni, L.; de Hardoim, D.J.; de Cardoso, F.O.; de Souza, C.D.S.F.; Pelajo-Machado, M.; Carollo, C.A.; de Arruda, C.C.P.; Almeida-Amaral, E.E.; et al. Amentoflavone as an Ally in the Treatment of Cutaneous Leishmaniasis: Analysis of Its Antioxidant/Prooxidant Mechanisms. Front. Cell. Infect. Microbiol. 2021, 11, 615814. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, S.V.; Kulkarni, Y.A. Toxicity of Escin-Triterpene Saponins from Aesculus. Toxicol. Environ. Chem. 2021. ahead of print. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review Expert Panel Final Report on the Safety Assessment of Glycyrrhetinic Acid, Potassium Glycyrrhetinate, Disodium Succinoyl Glycyrrhetinate, Glyceryl Glycyrrhetinate, Glycyrrhetinyl Stearate, Stearyl Glycyrrhetinate, Glycyrrhizic Acid, Ammonium Glycyrrhizate, Dipotassium Glycyrrhizate, Disodium Glycyrrhizate, Trisodium Glycyrrhizate, Methyl Glycyrrhizate, and Potassium Glycyrrhizinate. Int. J. Toxicol. 2007, 26 (Suppl. 2), 79–112.
- Pan, L.; Zhang, T.; Sun, H.; Liu, G. Ginsenoside Rg3 (Shenyi Capsule) Combined with Chemotherapy for Digestive System Cancer in China: A Meta-Analysis and Systematic Review. Evid. Based. Complement. Alternat. Med. 2019, 2019, 2417418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Lahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Tsiklauri, L.; Švík, K.; Chrastina, M.; Poništ, S.; Dráfi, F.; Slovák, L.; Alania, M.; Kemertelidze, E.; Bauerova, K. Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis. Nutrients 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.A. Zeaxanthin: Review of Toxicological Data and Acceptable Daily Intake. J. Ophthalmol. 2016, 2016, 3690140. [Google Scholar] [CrossRef] [Green Version]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef] [Green Version]
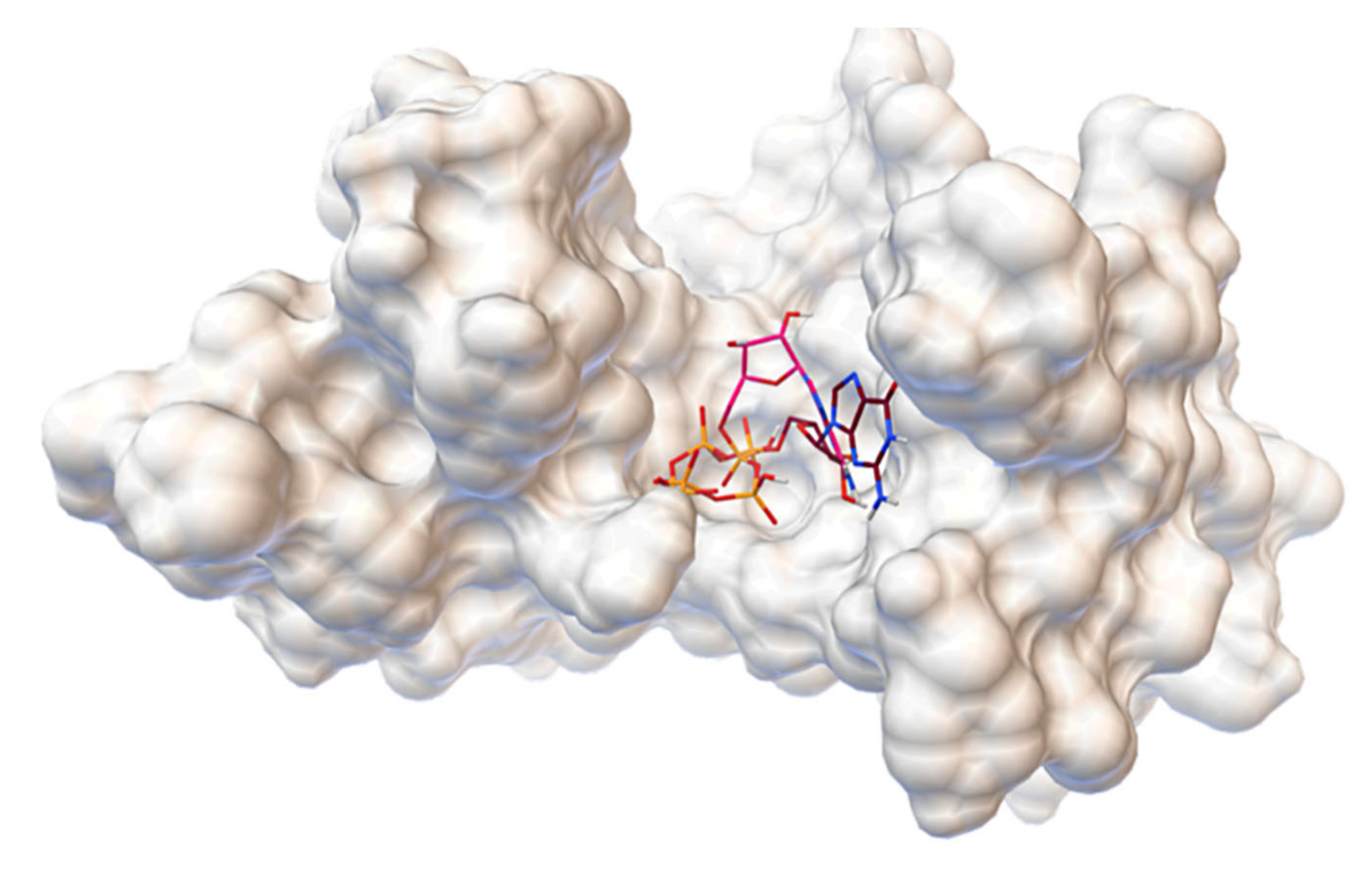
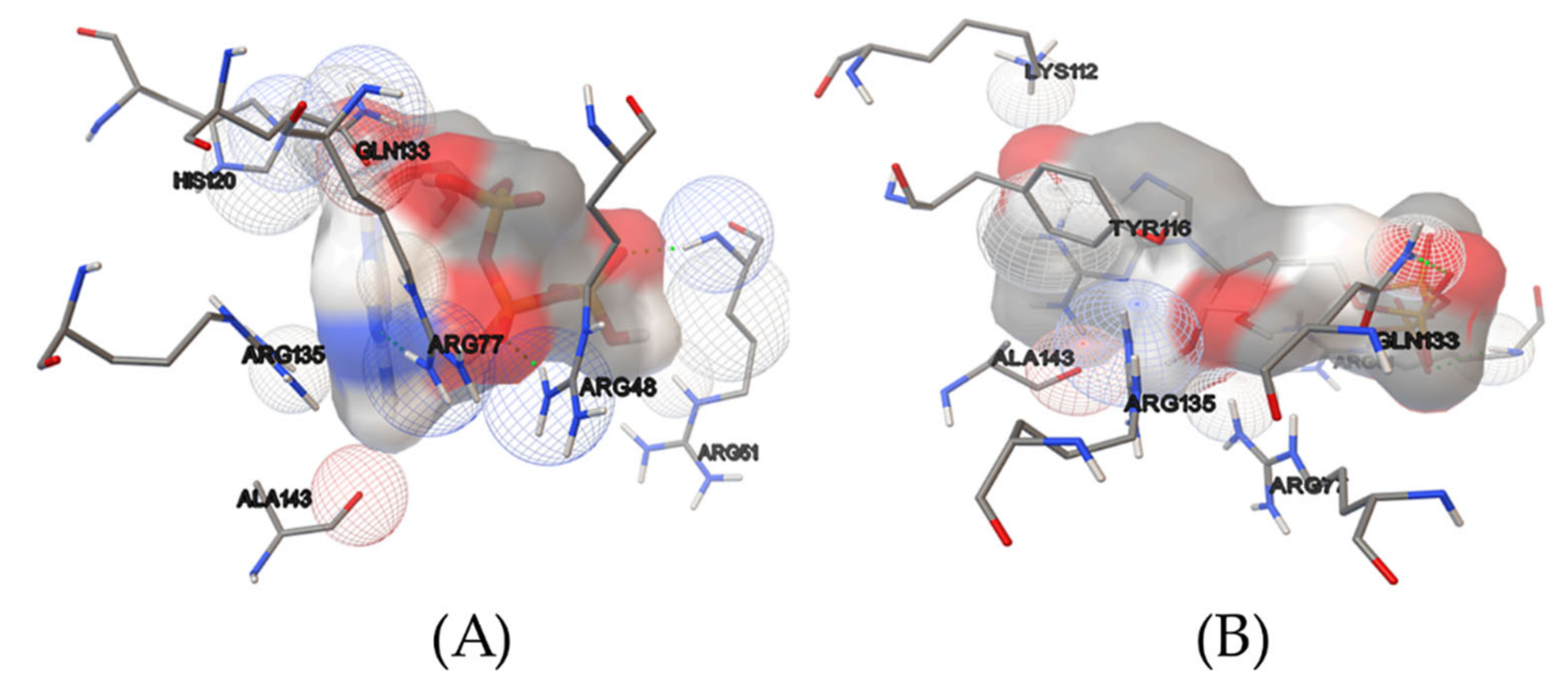



| Compound | Class | ΔG [kcal/mol] | Ki [μM] |
|---|---|---|---|
| Dioscin | Saponine glycoside | −9.8 | 0.122 |
| Zeaxanthin | Carotenoid | −8.9 | 0.527 |
| Glycyrrhizic acid | Saponine glycoside | −8.8 | 0.620 |
| Ginsenoside Rg3 | Saponine glycoside | −8.6 | 0.858 |
| Robinin | Flavone glycoside | −8.6 | 0.858 |
| Amentoflavone | Biflavonoid | −8.5 | 1.009 |
| Hypericin | Naphthodianthrone | −8.5 | 1.009 |
| Procyanidin A2 | Proanthocyanidin | −8.5 | 1.009 |
| Procyanidin C1 | Proanthocyanidin | −8.2 | 1.642 |
| Aescin | Saponine glycoside | −8.2 | 1.642 |
| Tested Compounds | MIC [µg/mL] |
|---|---|
| Dioscin | 64 |
| Hypericin | 128 |
| Amentoflavone | 128 |
| Aescin | 256 |
| Glycyrrhetinic acid | >256 |
| Ginsenoside Rg3 | >256 |
| Procyanidin A2 | >256 |
| Procyanidin C1 | >256 |
| Robinin | >256 |
| Zeaxanthin | >256 |
| Tested Combination | MIC [µg/mL] | FICI (Outcome) | |||||
|---|---|---|---|---|---|---|---|
| Dioscin | Antibiotic | ||||||
| Alone | Combination | Fold Change | Alone | Combination | Fold Change | ||
| Dioscin + CLR | 64 | 32 | 2 | 0.025 | 0.006 | 4 | 0.75 (additive) |
| Dioscin + MTZ | 64 | 16 | 4 | 1 | 0.5 | 2 | 0.75 (additive) |
| Dioscin + LEV | 64 | 32 | 2 | 1 | 0.5 | 2 | 1.0 (additive) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiegel, M.; Krzyżek, P.; Dworniczek, E.; Adamski, R.; Sroka, Z. In Silico Screening and In Vitro Assessment of Natural Products with Anti-Virulence Activity against Helicobacter pylori. Molecules 2022, 27, 20. https://doi.org/10.3390/molecules27010020
Spiegel M, Krzyżek P, Dworniczek E, Adamski R, Sroka Z. In Silico Screening and In Vitro Assessment of Natural Products with Anti-Virulence Activity against Helicobacter pylori. Molecules. 2022; 27(1):20. https://doi.org/10.3390/molecules27010020
Chicago/Turabian StyleSpiegel, Maciej, Paweł Krzyżek, Ewa Dworniczek, Ryszard Adamski, and Zbigniew Sroka. 2022. "In Silico Screening and In Vitro Assessment of Natural Products with Anti-Virulence Activity against Helicobacter pylori" Molecules 27, no. 1: 20. https://doi.org/10.3390/molecules27010020