The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential
Abstract
:1. Introduction
2. Osteoporosis and miRNAs
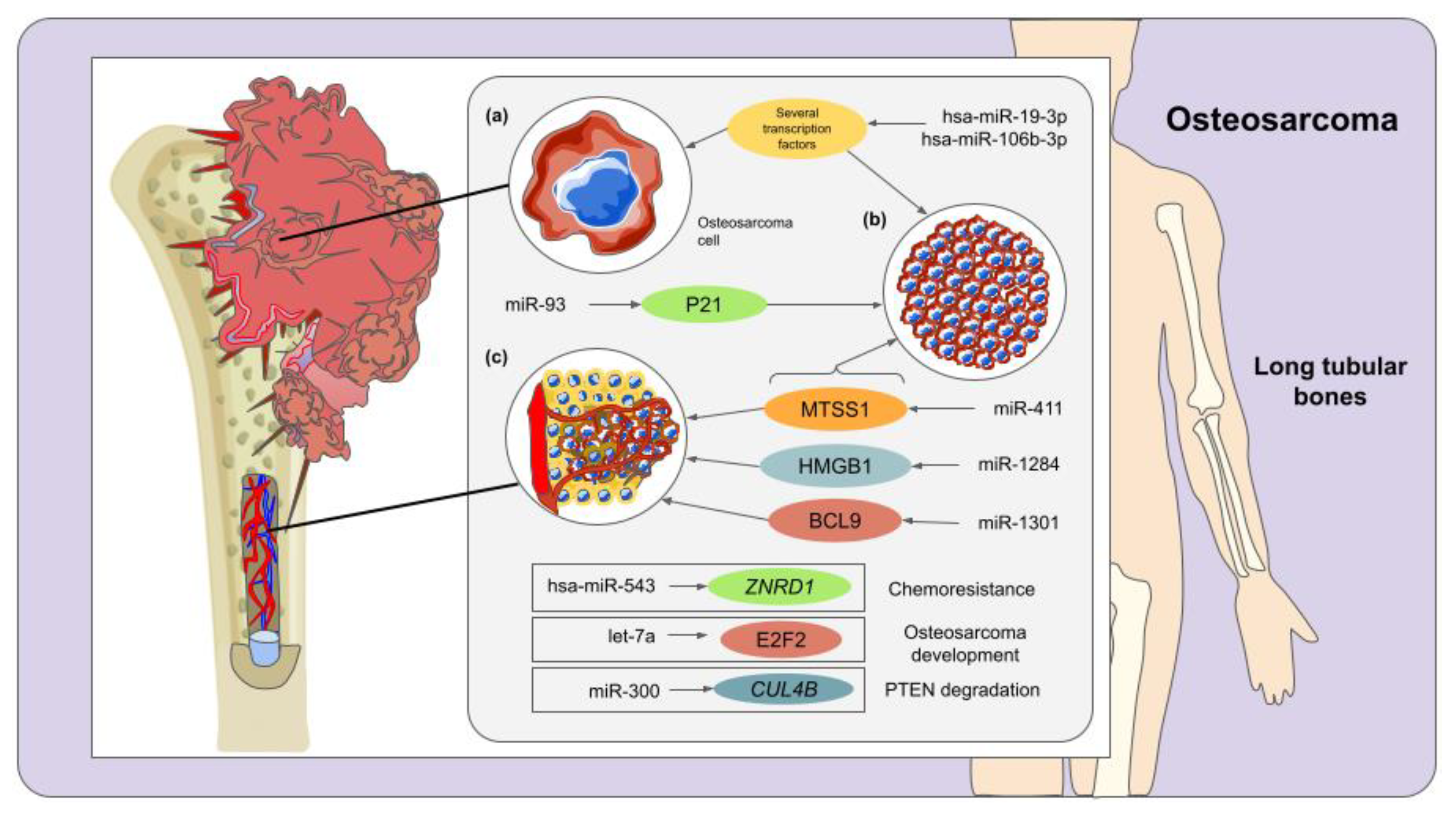
3. Osteosarcoma and miRNAs
4. Osteonecrosis and miRNAs
5. Bone Metastasis and miRNAs
5.1. Prostate Cancer Bone Metastasis
5.2. Breast Cancer Bone Metastasis
5.3. Lung Cancer Bone Metastasis
5.4. Other Bone Metastasis-Related Mechanisms
5.5. Bone Metastasis and Exosomal miRNAs
5.6. Clinical Applications of miRNAs in Bone Metastasis
6. Other Bone Diseases and miRNAs
6.1. Atrophic Non-Union
6.2. Osteogenesis Imperfecta
6.3. Osteomyelitis
6.4. Multiple Myeloma Bone Disease (MMBD)
6.5. Thalassemia Bone Disease (TBD)
6.6. Clinical Applications of miRNAs in Other Bone Diseases
7. Conclusions
8. Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Al-Bari, A.A.; Al Mamun, A. Current advances in regulation of bone homeostasis. FASEB BioAdv. 2020, 2, 668–679. [Google Scholar] [CrossRef]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef]
- Giza, D.E.; Vasilescu, C.; Calin, G.A. Key principles of miRNA involvement in human diseases. Discoveries 2014, 2, e34. [Google Scholar] [CrossRef]
- Ladomery, M.R.; Maddocks, D.G.; Wilson, I.D. MicroRNAs: Their discovery, biogenesis, function and potential use as biomarkers in non-invasive prenatal diagnostics. Int. J. Mol. Epidemiol. Genet. 2011, 2, 253–260. [Google Scholar] [PubMed]
- Almeida, M.I.; Reis, R.M.; Calin, G.A. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. Mol. Mech. Mutagen. 2011, 717, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2020, 21, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic advances of miRNAs: A preclinical and clinical update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef]
- Paul, S.; Bravo Vázquez, L.A.; Pérez Uribe, S.; Manzanero Cárdenas, L.A.; Ruíz Aguilar, M.F.; Chakraborty, S.; Sharma, A. Roles of microRNAs in carbohydrate and lipid metabolism disorders and their therapeutic potential. Biochimie 2021, 187, 83–93. [Google Scholar] [CrossRef]
- Paul, S.; Ruiz-Manriquez, L.M.; Ledesma-Pacheco, S.J.; Benavides-Aguilar, J.A.; Torres-Copado, A.; Morales-Rodríguez, J.I.; De Donato, M.; Srivastava, A. Roles of microRNAs in chronic pediatric diseases and their use as potential biomarkers: A review. Arch. Biochem. Biophys. 2021, 699, 108763. [Google Scholar] [CrossRef]
- Paul, S.; Ruiz-Manriquez, L.M.; Serrano-Cano, F.I.; Estrada-Meza, C.; Solorio-Diaz, K.A.; Srivastava, A. Human microRNAs in host–parasite interaction: A review. 3 Biotech 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Reyes, P.R.; Garza, B.S.; Sharma, A. MicroRNAs and Child Neuropsychiatric Disorders: A Brief Review. Neurochem. Res. 2019, 45, 232–240. [Google Scholar] [CrossRef]
- Paul, S.; Licona-Vázquez, I.; Serrano-Cano, F.I.; Frías-Reid, N.; Pacheco-Dorantes, C.; Pathak, S.; Chakraborty, S.; Srivastava, A. Current insight into the functions of microRNAs in common human hair loss disorders: A mini review. Hum. Cell 2021, 34, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Bravo Vázquez, L.A.; Reyes-Pérez, P.R.; Estrada-Meza, C.; Aponte Alburquerque, R.A.; Pathak, S.; Banerjee, A.; Bandyopadhyay, A.; Chakraborty, S.; Srivastava, A. The role of microRNAs in solving COVID-19 puzzle from infection to therapeutics: A mini-review. Virus Res. 2022, 308, 198631. [Google Scholar] [CrossRef]
- Belaya, Z.E.; Grebennikova, T.A.; Melnichenko, G.A.; Nikitin, A.G.; Solodovnikov, A.G.; Brovkina, O.I.; Grigoriev, A.; Rozhinskaya, L.Y.; Dedov, I.I. Effects of endogenous hypercortisolism on bone mRNA and microRNA expression in humans. Osteoporos. Int. 2018, 29, 211–221. [Google Scholar] [CrossRef]
- Gennari, L.; Bianciardi, S.; Merlotti, D. MicroRNAs in bone diseases. Osteoporos. Int. 2017, 28, 1191–1213. [Google Scholar] [CrossRef]
- Jones, T.L.; Esa, M.S.; Li, K.C.; Krishnan, S.G.; Elgallab, G.M.; Pearce, M.S.; Young, D.A.; Birrell, F.N. Osteoporosis, fracture, osteoarthritis & sarcopenia: A systematic review of circulating microRNA association. Bone 2021, 152, 116068. [Google Scholar] [CrossRef] [PubMed]
- Grillari, J.; Mäkitie, R.E.; Kocijan, R.; Haschka, J.; Vázquez, D.C.; Semmelrock, E.; Hackl, M. Circulating miRNAs in bone health and disease. Bone 2021, 145, 115787. [Google Scholar] [CrossRef]
- Papaioannou, G. miRNAs in Bone Development. Curr. Genom. 2015, 16, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Qiu, M.; Dou, C.; Cao, Z.; Dong, S. MicroRNAs in Bone Balance and Osteoporosis. Drug Dev. Res. 2015, 76, 235–245. [Google Scholar] [CrossRef]
- Moore, B.T.; Xiao, P. MiRNAs in Bone Diseases. MicroRNA 2013, 2, 20–31. [Google Scholar] [CrossRef]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Ojha, R.; Nandani, R.; Pandey, R.K.; Mishra, A.; Prajapati, V.K. Emerging role of circulating microRNA in the diagnosis of human infectious diseases. J. Cell. Physiol. 2019, 234, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-H.; Jin, M.; Li, J.; Kong, X. Identifying circulating miRNA biomarkers for early diagnosis and monitoring of lung cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165847. [Google Scholar] [CrossRef] [PubMed]
- Gherman, A.; Balacescu, L.; Gheorghe-Cetean, S.; Vlad, C.; Balacescu, O.; Irimie, A.; Lisencu, C. Current and New Predictors for Treatment Response in Metastatic Colorectal Cancer. The Role of Circulating miRNAs as Biomarkers. Int. J. Mol. Sci. 2020, 21, 2089. [Google Scholar] [CrossRef] [Green Version]
- Sidorkiewicz, I.; Niemira, M.; Maliszewska, K.; Erol, A.; Bielska, A.; Szalkowska, A.; Adamska-Patruno, E.; Szczerbinski, L.; Gorska, M.; Kretowski, A. Circulating miRNAs as a Predictive Biomarker of the Progression from Prediabetes to Diabetes: Outcomes of a 5-Year Prospective Observational Study. J. Clin. Med. 2020, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, X.; Liu, J. The role of circulating miRNAs in multiple myeloma. Sci. China Life Sci. 2015, 58, 1262–1269. [Google Scholar] [CrossRef] [Green Version]
- Donati, S.; Ciuffi, S.; Palmini, G.; Brandi, M.L. Circulating miRNAs: A New Opportunity in Bone Fragility. Biomolecules 2020, 10, 927. [Google Scholar] [CrossRef]
- Bottani, M.; Banfi, G.; Lombardi, G. The Clinical Potential of Circulating miRNAs as Biomarkers: Present and Future Applications for Diagnosis and Prognosis of Age-Associated Bone Diseases. Biomolecules 2020, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Bottani, M.; Banfi, G.; Lombardi, G. Circulating miRNAs as Diagnostic and Prognostic Biomarkers in Common Solid Tumors: Focus on Lung, Breast, Prostate Cancers, and Osteosarcoma. J. Clin. Med. 2019, 8, 1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Chen, S.; Cai, P.; Chen, K.; Li, L.; Yang, X.; Yi, J.; Luo, X.; Du, Y.; Zheng, H. MiRNA-483–5p is involved in the pathogenesis of osteoporosis by promoting osteoclast differentiation. Mol. Cell. Probes 2020, 49, 101479. [Google Scholar] [CrossRef]
- Feng, L.; Xia, B.; Tian, B.-F.; Lu, G.-B. MiR-152 influences osteoporosis through regulation of osteoblast differentiation by targeting RICTOR. Pharm. Biol. 2019, 57, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Rozenberg, S.; Al-Daghri, N.; Aubertin-Leheudre, M.; Brandi, M.-L.; Cano, A.; Collins, P.; Cooper, C.; Genazzani, A.R.; Hillard, T.; Kanis, J.; et al. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos. Int. 2020, 31, 2271–2286. [Google Scholar] [CrossRef]
- Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.; Taraballi, F.; Torreggiani, E.; Rotondo, J.; Otòn-Gonzalez, L.; Mazzoni, E.; Frontini, F.; Bononi, I.; et al. MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2362. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Wei, X.; Sun, Q.; Zhao, X.Q.; Zheng, C.Y.; Bai, C.X.; Du, J.; Zhang, Z.; Zhu, L.G.; Jia, Y.S. MicroRNA-449b-5p promotes the progression of osteoporosis by inhibiting osteogenic differentiation of BMSCs via targeting Satb2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6394–6403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.-R.; Fan, X.-L.; Lin, P.; Yang, H.; Chen, X.-Z.; Xu, X.-D. miR-206 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells by targetting glutaminase. Biosci. Rep. 2019, 39, BSR20181108. [Google Scholar] [CrossRef] [Green Version]
- Kocijan, R.; Weigl, M.; Skalicky, S.; Geiger, E.; Ferguson, J.; Leinfellner, G.; Heimel, P.; Pietschmann, P.; Grillari, J.; Redl, H.; et al. MicroRNA levels in bone and blood change during bisphosphonate and teriparatide therapy in an animal model of postmenopausal osteoporosis. Bone 2020, 131, 115104. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, R.; Yang, W.; Zhou, Z.; Li, C. miR-363-3p is activated by MYB and regulates osteoporosis pathogenesis via PTEN/PI3K/AKT signaling pathway. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 376–386. [Google Scholar] [CrossRef]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; He, X.; Zhang, Y.; Wu, R.; Chen, J.; Lin, Y.; Shen, B. MicroRNA Alterations for Diagnosis, Prognosis, and Treatment of Osteoporosis: A Comprehensive Review and Computational Functional Survey. Front. Genet. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugatani, T.; Hruska, K.A. Down-regulation of miR-21 biogenesis by estrogen action contributes to osteoclastic apoptosis. J. Cell. Biochem. 2013, 114, 1217–1222. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Zhou, H.; Zeng, X.; Feng, S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol. Med. Rep. 2017, 15, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Syal, A.; Aggarwal, N. Postmenopausal hormone therapy and its association with breast cancer. J. Mid-Life Health 2020, 11, 187–195. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, C.; Wang, J.; Xiao, J.; Gatalica, Z.; Recker, R.R.; Xiao, G.G. Let-7 family miRNAs regulate estrogen receptor alpha signaling in estrogen receptor positive breast cancer. Breast Cancer Res. Treat. 2011, 127, 69–80. [Google Scholar] [CrossRef]
- Howard, E.W.; Yang, X. microRNA Regulation in Estrogen Receptor-Positive Breast Cancer and Endocrine Therapy. Biol. Proced. Online 2018, 20, 17. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, B.; Liu, J. MicroRNA-148a inhibition protects against ovariectomy-induced osteoporosis through PI3K/AKT signaling by estrogen receptor α. Mol. Med. Rep. 2018, 17, 7789–7796. [Google Scholar] [CrossRef] [Green Version]
- Mandourah, A.Y.; Ranganath, L.; Barraclough, R.; Vinjamuri, S.; Hof, R.V.; Hamill, S.; Czanner, G.; Dera, A.A.; Wang, D.; Barraclough, D. Circulating microRNAs as potential diagnostic biomarkers for osteoporosis. Sci. Rep. 2018, 8, 8421. [Google Scholar] [CrossRef]
- Wang, C.; He, H.; Wang, L.; Jiang, Y.; Xu, Y. Reduced miR-144-3p expression in serum and bone mediates osteoporosis pathogenesis by targeting RANK. Biochem. Cell Biol. 2018, 96, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, W.; Huang, Y. MiRNA-133a is involved in the regulation of postmenopausal osteoporosis through promoting osteoclast differentiation. Acta Biochim. Biophys. Sin. 2018, 50, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xie, R.-L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Zhang, J.; Yu, T.; Qi, C. Generation of PTEN knockout bone marrow mesenchymal stem cell lines by CRISPR/Cas9-mediated genome editing. Cytotechnology 2018, 70, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.-S.; Ko, J.-Y.; Chen, Y.-S.; Ke, H.-J.; Hsieh, C.-K.; Kuo, C.-W.; Wang, S.-Y.; Huang, B.-W.; Tseng, J.-G.; Wang, F.-S. MicroRNA-29a represses osteoclast formation and protects against osteoporosis by regulating PCAF-mediated RANKL and CXCL12. Cell Death Dis. 2019, 10, 705. [Google Scholar] [CrossRef]
- Lu, X.-D.; Han, W.-X.; Liu, Y.-X. Suppression of miR-451a accelerates osteogenic differentiation and inhibits bone loss via Bmp6 signaling during osteoporosis. Biomed. Pharmacother. 2019, 120, 109378. [Google Scholar] [CrossRef]
- Luo, B.; Yang, J.-F.; Wang, Y.-H.; Qu, G.-B.; Hao, P.-D.; Zeng, Z.-J.; Yuan, J.; Yang, R.; Yuan, Y. MicroRNA-579-3p promotes the progression of osteoporosis by inhibiting osteogenic differentiation of mesenchymal stem cells through regulating Sirt1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6791–6799. [Google Scholar] [CrossRef]
- Lv, R.; Pan, X.; Song, L.; Sun, Q.; Guo, C.; Zou, S.; Zhou, Q. MicroRNA-200a-3p accelerates the progression of osteoporosis by targeting glutaminase to inhibit osteogenic differentiation of bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2019, 116, 108960. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, X.; He, S.; Ding, W.; Wang, F.; Zhao, Y.; Huang, Z. LncRNA MSC-AS1 promotes osteogenic differentiation and alleviates osteoporosis through sponging microRNA-140–5p to upregulate BMP2. Biochem. Biophys. Res. Commun. 2019, 519, 790–796. [Google Scholar] [CrossRef]
- Zhou, J.; Nie, H.; Liu, P.; Wang, Z.; Yao, B.; Yang, L. Down-regulation of miR-339 promotes differentiation of BMSCs and alleviates osteoporosis by targeting DLX5. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 29–36. [Google Scholar] [CrossRef]
- Mao, J.-H.; Sui, Y.-X.; Ao, S.; Wang, Y.; Liu, Y.; Leng, H. miR-140-3p exhibits repressive functions on preosteoblast viability and differentiation by downregulating MCF2L in osteoporosis. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 49–58. [Google Scholar] [CrossRef]
- Mi, B.; Yan, C.; Xue, H.; Chen, L.; Panayi, A.C.; Hu, L.; Hu, Y.; Cao, F.; Sun, Y.; Zhou, W.; et al. Inhibition of Circulating miR-194-5p Reverses Osteoporosis through Wnt5a/β-Catenin-Dependent Induction of Osteogenic Differentiation. Mol. Ther. Nucleic Acids 2020, 21, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.G.; Hua, Y.; Liu, S.W.; Hu, W.Q.; Qian, R.; Xiong, L. MicroRNA-1286 inhibits osteogenic differentiation of mesenchymal stem cells to promote the progression of osteoporosis via regulating FZD4 expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Yi, X.; Tu, S.; Cheng, C.; Luo, J. Kaempferol promotes BMSC osteogenic differentiation and improves osteoporosis by downregulating miR-10a-3p and upregulating CXCL12. Mol. Cell. Endocrinol. 2021, 520, 111074. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; De Luca, A.; Carina, V.; Costa, V.; Raimondi, L.; Salamanna, F.; Alessandro, R.; Fini, M.; Giavaresi, G. Deregulated miRNAs in bone health: Epigenetic roles in osteoporosis. Bone 2019, 122, 52–75. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed. Eng. Online 2021, 20, 24. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, E.; Hornicek, F.J.; Duan, Z. MicroRNA Involvement in Osteosarcoma. Sarcoma 2012, 2012, 359739. [Google Scholar] [CrossRef] [PubMed]
- Sampson, V.B.; Yoo, S.; Kumar, A.; Vetter, N.S.; Kolb, E.A. MicroRNAs and Potential Targets in Osteosarcoma: Review. Front. Pediatr. 2015, 3, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, W.; Jiang, K.; Chen, B.; Wang, K.; Lao, L.; Hou, C.; Wang, F.; Zhang, C.; Shen, H. MicroRNA-300 Regulates the Ubiquitination of PTEN through the CRL4BDCAF13 E3 Ligase in Osteosarcoma Cells. Mol. Ther. Nucleic Acids 2018, 10, 254–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Yu, B. MicroRNA-93 promotes cell proliferation by directly targeting P21 in osteosarcoma cells. Exp. Ther. Med. 2017, 13, 2003–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Yang, W.; Liu, Y.; Yan, F.; Yu, Z. MicroRNA-411 promoted the osteosarcoma progression by suppressing MTSS1 expression. Environ. Sci. Pollut. Res. 2018, 25, 12064–12071. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G. MiR-1284 enhances sensitivity of cervical cancer cells to cisplatin via downregulating HMGB1. Biomed. Pharmacother. 2018, 107, 997–1003. [Google Scholar] [CrossRef]
- Wei, W.; Cao, W.; Zhan, Z.; Yan, L.; Xie, Y.; Xiao, Q. MiR-1284 suppresses gastric cancer progression by targeting EIF4A1. OncoTargets Ther. 2019, 12, 3965–3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, S.; Guan, M. miRNA-1284, a regulator of HMGB1, inhibits cell proliferation and migration in osteosarcoma. Biosci. Rep. 2018, 38, BSR20171675. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Liu, D.; Cao, L.; Wang, D.; Wu, T.; Lin, F.; Su, P.; Niu, Y.; Sun, Y. Diagnostic and prognostic values of blood microRNA-Let7A for osteosarcoma. J. Bone Oncol. 2018, 12, 65–68. [Google Scholar] [CrossRef] [PubMed]
- López-Urrutia, E.; Bustamante Montes, L.P.; Ladrón de Guevara Cervantes, D.; Perez-Plasencia, C.; Campos-Parra, A.D. Crosstalk Between Long Non-coding RNAs, Micro-RNAs and mRNAs: Deciphering Molecular Mechanisms of Master Regulators in Cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef]
- Zhu, C.; Cheng, D.; Qiu, X.; Zhuang, M.; Liu, Z. Long Noncoding RNA SNHG16 Promotes Cell Proliferation by Sponging MicroRNA-205 and Upregulating ZEB1 Expression in Osteosarcoma. Cell. Physiol. Biochem. 2018, 51, 429–440. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, K.; Chao, Y. MicroRNA-1301 inhibits migration and invasion of osteosarcoma cells by targeting BCL9. Gene 2018, 679, 100–107. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Q.; Ma, S.; Sun, Y.; Vadamootoo, A.S.; Jin, C. A four serum-miRNA panel serves as a potential diagnostic biomarker of osteosarcoma. Int. J. Clin. Oncol. 2019, 24, 976–982. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Si, H.; Shen, B. Integrated bioinformatics analysis of miRNA expression in osteosarcoma. Artif. Cells Nanomed. Biotechnol. 2016, 45, 936–943. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Li, Y.; Zhao, R.; Xu, Y.; Wu, Y.; Wang, J.; Xia, D.; Han, W.; Chen, D. Identification of Key Genes and miRNAs in Osteosarcoma Patients with Chemoresistance by Bioinformatics Analysis. BioMed Res. Int. 2018, 2018, 4761064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram Kumar, R.M.; Boro, A.; Fuchs, B. Involvement and Clinical Aspects of MicroRNA in Osteosarcoma. Int. J. Mol. Sci. 2016, 17, 877. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Chen, C.; Kong, N.; Tian, R.; Li, Y.; Li, Z.; Wang, K.; Yang, P. Identification of potential miRNA biomarkers for traumatic osteonecrosis of femoral head. J. Cell. Physiol. 2020, 235, 8129–8140. [Google Scholar] [CrossRef]
- Wang, A.; Ren, M.; Song, Y.; Wang, X.; Wang, Q.; Yang, Q.; Liu, H.; Du, Z.; Zhang, G.; Wang, J. MicroRNA Expression Profiling of Bone Marrow Mesenchymal Stem Cells in Steroid-Induced Osteonecrosis of the Femoral Head Associated with Osteogenesis. Med. Sci. Monit. 2018, 24, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Hu, J.-Z.; Shi, Z.-Y. MiR-181d promotes steroid-induced osteonecrosis of the femoral head by targeting SMAD3 to inhibit osteogenic differentiation of hBMSCs. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4053–4062. [Google Scholar] [CrossRef]
- Xu, W.; Li, J.; Tian, H.; Wang, R.; Feng, Y.; Tang, J.; Jia, J. MicroRNA-186-5p mediates osteoblastic differentiation and cell viability by targeting CXCL13 in non-traumatic osteonecrosis. Mol. Med. Rep. 2019, 20, 4594–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.; Park, J.; Choi, S.H.; Kim, G. Traumatic and Non-traumatic Osteonecrosis in the Femoral Head of a Rabbit Model. Lab. Anim. Res. 2011, 27, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seamon, J.; Keller, T.; Saleh, J.; Cui, Q. The Pathogenesis of Nontraumatic Osteonecrosis. Arthritis 2012, 2012, 601763. [Google Scholar] [CrossRef] [Green Version]
- Bergman, J.; Nordström, A.; Nordström, P. Epidemiology of osteonecrosis among older adults in Sweden. Osteoporos. Int. 2019, 30, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.N.; Racine, J.; Jones, L.C.; Aaron, R.K. Pathophysiology and risk factors for osteonecrosis. Curr. Rev. Musculoskelet. Med. 2015, 8, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zhai, P.; Ye, Z.; Deng, P.; Fan, Y.; Zeng, Y.; Pang, Z.; Zeng, J.; Li, J.; Feng, W. Differential expression of miR-195-5p in collapse of steroid-induced osteonecrosis of the femoral head. Oncotarget 2017, 8, 42638–42647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, R.S. Glucocorticoid-induced osteonecrosis. Endocrine 2012, 41, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Jin, Y.; Zheng, J.; Liu, K.; Zhao, J.; Zhang, S.; Wu, F.; Sun, Z. MiR-217 promotes cell proliferation and osteogenic differentiation of BMSCs by targeting DKK1 in steroid-associated osteonecrosis. Biomed. Pharmacother. 2019, 109, 1112–1119. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Ling, S.; Wang, Y.; Wang, X.; Meng, H.; Li, Y.; Yuan, X.; Li, J.; Liu, R.; et al. AAV-Anti-miR-214 Prevents Collapse of the Femoral Head in Osteonecrosis by Regulating Osteoblast and Osteoclast Activities. Mol. Ther. Nucleic Acids 2019, 18, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Ding, L.; Hou, Y.; Jiang, H.; Zhang, J.; Dai, Z.; Zhang, G. Upregulating MicroRNA-410 or Downregulating Wnt-11 Increases Osteoblasts and Reduces Osteoclasts to Alleviate Osteonecrosis of the Femoral Head. Nanoscale Res. Lett. 2019, 14, 383. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Lu, C.; Zhang, B.; Xu, Z.; Guo, H.; Zhang, G. Identifying the Potential Differentially Expressed miRNAs and mRNAs in Osteonecrosis of the Femoral Head Based on Integrated Analysis. Clin. Interv. Aging 2021, 16, 187–202. [Google Scholar] [CrossRef]
- Yue, J.; Yu, H.; Liu, P.; Wen, P.; Zhang, H.; Guo, W.; Zhang, Q. Preliminary study of icariin indicating prevention of steroid-induced osteonecrosis of femoral head by regulating abnormal expression of miRNA-335 and protecting the functions of bone microvascular endothelial cells in rats. Gene 2021, 766, 145128. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Weng, X.; Tse, G.; Chan, M.T.V.; Wu, W.K.K. Emerging roles of MicroRNAs in osteonecrosis of the femoral head. Cell Prolif. 2018, 51, e12405. [Google Scholar] [CrossRef] [Green Version]
- Käkönen, S.M.; Mundy, G.R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 97, 834–839. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, M.; Guise, T.; Kang, Y. The Biology of Bone Metastasis. Cold Spring Harb. Perspect. Med. 2018, 8, a031252. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, L.A.; Fournier, P.G.J.; Chirgwin, J.M.; Guise, T.A. Molecular Biology of Bone Metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Body, J.-J.; Quinn, G.; Talbot, S.; Booth, E.; Demonty, G.; Taylor, A.; Amelio, J. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit. Rev. Oncol. Hematol. 2017, 115, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Oster, G.; Lamerato, L.; Glass, A.G.; Richert-Boe, K.E.; Lopez, A.; Chung, K.; Richhariya, A.; Dodge, T.; Wolff, G.G.; Balakumaran, A.; et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: A 15-year study in two large US health systems. Support. Care Cancer 2013, 21, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Puppo, M.; Taipaleenmäki, H.; Hesse, E.; Clézardin, P. Non-coding RNAs in bone remodelling and bone metastasis: Mechanisms of action and translational relevance. Br. J. Pharmacol. 2021, 178, 1936–1954. [Google Scholar] [CrossRef]
- Colden, M.; Dar, A.A.; Saini, S.; Dahiya, P.V.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; Stein, G.; Dahiya, R.; Majid, S. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2018, 8, e2572. [Google Scholar] [CrossRef]
- Wa, Q.; Li, L.; Lin, H.; Peng, X.; Ren, D.; Huang, Y.; He, P.; Huang, S. Downregulation of miR-19a-3p promotes invasion, migration and bone metastasis via activating TGF-β signaling in prostate cancer. Oncol. Rep. 2018, 39, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zou, C.; Tang, Y.; Wa, Q.; Peng, X.; Chen, X.; Yang, C.; Ren, D.; Huang, Y.; Liao, Z.; et al. miR-582-3p and miR-582-5p Suppress Prostate Cancer Metastasis to Bone by Repressing TGF-β Signaling. Mol. Ther. Nucleic Acids 2019, 16, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Voss, G.; Haflidadóttir, B.S.; Järemo, H.; Persson, M.; Catela Ivkovic, T.; Wikström, P.; Ceder, Y. Regulation of cell–cell adhesion in prostate cancer cells by microRNA-96 through upregulation of E-Cadherin and EpCAM. Carcinogenesis 2020, 41, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef] [Green Version]
- Massoner, P.; Thomm, T.; Mack, B.; Untergasser, G.; Martowicz, A.; Bobowski, K.; Klocker, H.; Gires, O.; Puhr, M. EpCAM is overexpressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200c/205. Br. J. Cancer 2014, 111, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, D.; Dang, L.; Liang, C.; Guo, B.; Lu, C.; He, X.; Cheung, H.Y.S.; He, B.; Liu, B.; et al. Osteoclastic miR-214 targets TRAF3 to contribute to osteolytic bone metastasis of breast cancer. Sci. Rep. 2017, 7, 40487. [Google Scholar] [CrossRef]
- Cai, W.-L.; Huang, W.-D.; Li, B.; Chen, T.-R.; Li, Z.-X.; Zhao, C.-L.; Li, H.-Y.; Wu, Y.-M.; Yan, W.-J.; Xiao, J.-R. microRNA-124 inhibits bone metastasis of breast cancer by repressing Interleukin-11. Mol. Cancer 2018, 17, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Yang, F.; Liu, R.; Li, X.; Fan, H.; Liu, J.; Wei, S.; Chen, G.; Chen, J.; Da, Y. Serum microRNA-139-5p is downregulated in lung cancer patients with lytic bone metastasis. Oncol. Rep. 2018, 39, 2376–2384. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhu, N.; Tsoi, H.; Zhao, Z.; Wu, C.W.; Wang, K.; Zheng, S.; Ng, S.S.; Chan, F.K.; et al. microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol. Cancer 2014, 13, 124. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Zhao, Y.-K.; Liu, Y.; Zhao, J.-F. MicroRNA-34a inhibits tumor invasion and metastasis in osteosarcoma partly by effecting C-IAP2 and Bcl-2. Tumor Biol. 2017, 39, 1010428317705761. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y.; Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA 2018, 115, 2204–2209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, J. The Potential Roles of Exosomal miR-214 in Bone Metastasis of Lung Adenocarcinoma. Front. Oncol. 2021, 10, 611054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Zhu, Y.; Li, L.; Zhou, S.; Yin, G.; Yu, G.; Cui, H. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 2019, 8, 5687–5701. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-R.; Pi, C.; Yu, R.; Fan, X.-J.; Peng, X.-X.; Zhang, X.-C.; Chen, Z.-H.; Wu, X.; Shao, Y.; Wu, Y.-L.; et al. Correlation of exosomal microRNA clusters with bone metastasis in non-small cell lung cancer. Clin. Exp. Metastasis 2021, 38, 109–117. [Google Scholar] [CrossRef]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef]
- Wu, K.; Feng, J.; Lyu, F.; Xing, F.; Sharma, S.; Liu, Y.; Wu, S.-Y.; Zhao, D.; Tyagi, A.; Deshpande, R.P.; et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 2021, 12, 5196. [Google Scholar] [CrossRef]
- Zoni, E.; van der Pluijm, G. The role of microRNAs in bone metastasis. J. Bone Oncol. 2016, 5, 104–108. [Google Scholar] [CrossRef]
- Chen, H.; Ji, X.; She, F.; Gao, Y.; Tang, P. miR-628-3p regulates osteoblast differentiation by targeting RUNX2: Possible role in atrophic non-union. Int. J. Mol. Med. 2017, 39, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.K. Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malays. Orthop. J. 2019, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Lee, S.Y.; Niikura, T.; Iwakura, T.; Dogaki, Y.; Okumachi, E.; Kuroda, R.; Kurosaka, M. Profiling microRNA expression in fracture nonunions: Potential role of microRNAs in nonunion formation studied in a rat model. Bone Jt. J. 2015, 97-B, 1144–1151. [Google Scholar] [CrossRef]
- Long, H.; Zhu, Y.; Lin, Z.; Wan, J.; Cheng, L.; Zeng, M.; Tang, Y.; Zhao, R. miR-381 modulates human bone mesenchymal stromal cells (BMSCs) osteogenesis via suppressing Wnt signaling pathway during atrophic nonunion development. Cell Death Dis. 2019, 10, 470. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Liu, M.; Jin, Y.; Lin, H.; Zhang, Y.; Zheng, S. miR-1323 suppresses bone mesenchymal stromal cell osteogenesis and fracture healing via inhibiting BMP4/SMAD4 signaling. J. Orthop. Surg. Res. 2020, 15, 237. [Google Scholar] [CrossRef]
- Wei, J.; Chen, H.; Fu, Y.; Zhang, B.; Zhang, L.; Tao, S.; Lin, F. Experimental study of expression profile and specific role of human microRNAs in regulating atrophic bone nonunion. Medicine 2020, 99, e21653. [Google Scholar] [CrossRef]
- Kaneto, C.M.; Lima, P.S.; Zanette, D.L.; Prata, K.L.; Pina Neto, J.M.; de Paula, F.J.; Silva Jr, W.A. COL1A1 and miR-29b show lower expression levels during osteoblast differentiation of bone marrow stromal cells from Osteogenesis Imperfecta patients. BMC Med. Genet. 2014, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Grafe, I.; Alexander, S.; Lee, B. Genetic causes and mechanisms of Osteogenesis Imperfecta. Bone 2017, 102, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wang, J.; Liu, F.; Ji, Z.; Guo, Z.; Zhang, C.; Yao, M. Ossotide promotes cell differentiation of human osteoblasts from osteogenesis imperfecta patients by up-regulating miR-145. Biomed. Pharmacother. 2016, 83, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y.; Zhang, X.; Ren, X.; Wang, Y.; Li, Z.; Xu, C.; Han, J. Serum microRNA is a promising biomarker for osteogenesis imperfecta. Intractable Rare Dis. Res. 2012, 1, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuru, S.; Gordon, P.L.; Shimono, K.; Jethva, R.; Marino, R.; Phillips, C.L.; Hofmann, T.J.; Veronesi, E.; Dominici, M.; Iwamoto, M.; et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012, 120, 1933–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuru, S.; Desbourdes, L.; Guess, A.J.; Hofmann, T.J.; Relation, T.; Kaito, T.; Dominici, M.; Iwamoto, M.; Horwitz, E.M. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy 2018, 20, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2011, 84, 1027–1033. [Google Scholar]
- Birt, M.; Anderson, D.W.; Bruce Toby, E.; Wang, J. Osteomyelitis: Recent advances in pathophysiology and therapeutic strategies. J. Orthop. 2017, 14, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lu, Y.; He, Q.X.; Wang, H.; Li, B.F.; Zhu, L.Y.; Xu, Q.Y. The Role of MicroRNA, miR-24, and Its Target CHI3L1 in Osteomyelitis Caused by Staphylococcus aureus. J. Cell. Biochem. 2015, 116, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cao, F.; Zhou, W.; Wang, G.; Liu, G.; Xia, T.; Liu, M.; Mi, B.; Liu, Y. Long Noncoding RNA FAM83H-AS1 Modulates SpA-Inhibited Osteogenic Differentiation in Human Bone Mesenchymal Stem Cells. Mol. Cell. Biol. 2020, 40, e00362-19. [Google Scholar] [CrossRef]
- Bourebaba, L.; Michalak, I.; Baouche, M.; Kucharczyk, K.; Fal, A.M.; Marycz, K. Cladophora glomerata enriched by biosorption with Mn(II) ions alleviates lipopolysaccharide-induced osteomyelitis-like model in MC3T3-E1, and 4B12 osteoclastogenesis. J. Cell. Mol. Med. 2020, 24, 7282–7300. [Google Scholar] [CrossRef]
- Ma, X.; Xia, W.; Zong, Y.; Jiang, C.; Shan, H.; Lin, Y.; Yin, F.; Wang, N.; Zhou, L.; Wen, G.; et al. Tumor necrosis factor-α promotes Staphylococcus aureus-induced osteomyelitis through downregulating endothelial nitric oxide synthase. J. Microbiol. Immunol. Infect. 2021, 54, 1018–1027. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin. Proc. 2016, 91, 101–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Handa, H.; Murakami, Y.; Ishihara, R.; Kimura-Masuda, K.; Masuda, Y. The Role and Function of microRNA in the Pathogenesis of Multiple Myeloma. Cancers 2019, 11, 1738. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-K.; Wang, H.; Leng, Y.; Li, Z.-L.; Yang, Y.-F.; Xiao, F.-J.; Li, Q.-F.; Chen, X.-Q.; Wang, L.-S. Overexpression of microRNA-29b induces apoptosis of multiple myeloma cells through down regulating Mcl-1. Biochem. Biophys. Res. Commun. 2011, 414, 233–239. [Google Scholar] [CrossRef]
- Cosco, D.; Cilurzo, F.; Maiuolo, J.; Federico, C.; Di Martino, M.T.; Cristiano, M.C.; Tassone, P.; Fresta, M.; Paolino, D. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci. Rep. 2015, 5, 17579. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Kumar, R.; Seth, T.; Garg, B.; Sati, H.C.; Sharma, A. Clinical significance of circulatory microRNA-203 in serum as novel potential diagnostic marker for multiple myeloma. J. Cancer Res. Clin. 2019, 145, 1601–1611. [Google Scholar] [CrossRef]
- Yu, M.; Yu, J.; Zhang, Y.; Sun, X.; Sun, R.; Xia, M.; Li, S.; Cui, X. A novel circRNA-miRNA-mRNA network revealed exosomal circ-ATP10A as a biomarker for multiple myeloma angiogenesis. Bioengineered 2021. [Google Scholar] [CrossRef]
- Eltaweel, N.H.; ElKamah, G.Y.; Khairat, R.; Atia, H.A.E.; Amr, K.S. Epigenetic effects toward new insights as potential therapeutic target in B-thalassemia. J. Genet. Eng. Biotechnol. 2021, 19, 51. [Google Scholar] [CrossRef]
- Das, S.S.; Das, S.; Byram, P.K.; Rahaman, M.; Dolai, T.K.; Chatterjee, A.; Chakravorty, N. MicroRNA expression patterns in HbE/β-thalassemia patients: The passwords to unlock fetal hemoglobin expression in β-hemoglobinopathies. Blood Cells Mol. Dis. 2021, 87, 102523. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, M.A.; Asadi, M.; Naderi, M.; Azarkeivan, A.; Soleimani, M.; Atashi, A. miR-30a regulates γ-globin expression in erythoid precursors of intermedia thalassemia through targeting BCL11A. Mol. Biol. Rep. 2020, 47, 3909–3918. [Google Scholar] [CrossRef]
- Wang, F.; Ling, L.; Yu, D. MicroRNAs in β-thalassemia. Am. J. Med. Sci. 2021, 362, 5–12. [Google Scholar] [CrossRef]
- Kuno, S.; Penglong, T.; Srinoun, K. Anemia Severity in β-Thalassemia Correlates with Elevated Levels of microRNA-125b in Activated Phagocytic Monocytes. Hemoglobin 2019, 43, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Koromani, F.; Trajanoska, K.; Rivadeneira, F.; Oei, L. Recent Advances in the Genetics of Fractures in Osteoporosis. Front. Endocrinol. 2019, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Hannan, F.M.; Newey, P.J.; Whyte, M.P.; Thakker, R.V. Genetic approaches to metabolic bone diseases. Br. J. Clin. Pharmacol. 2019, 85, 1147–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley, A.P.; McAlinden, A. The role of microRNAs in bone development. Bone 2021, 143, 115760. [Google Scholar] [CrossRef]
- van Driel, M.; van Leeuwen, J.P.T.M. Vitamin D endocrinology of bone mineralization. Mol. Cell. Endocrinol. 2017, 453, 46–51. [Google Scholar] [CrossRef]
- Lisse, T.S.; Adams, J.S.; Hewison, M. Vitamin D and microRNAs in bone. Crit. Rev. Eukaryot. Gene Expr. 2013, 23, 195–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Liu, Y.; Liao, W.; Liu, R.; Shi, P.; Wang, L. miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J. Bone Oncol. 2016, 5, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Kelch, S.; Balmayor, E.R.; Seeliger, C.; Vester, H.; Kirschke, J.S.; van Griensven, M. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci. Rep. 2017, 7, 15861. [Google Scholar] [CrossRef]
- Yang, Y.; Yujiao, W.; Fang, W.; Linhui, Y.; Ziqi, G.; Zhichen, W.; Zirui, W.; Shengwang, W. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol. Res. 2020, 53, 40. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tavakkoli Avval, S.; Rahmani, S.; Shoorei, H.; Taheri, M.; Samadian, M. Contribution of miRNAs and lncRNAs in osteogenesis and related disorders. Biomed. Pharmacother. 2021, 142, 111942. [Google Scholar] [CrossRef] [PubMed]
- Bhansali, A. Metabolic bone disease: Newer perspectives. Indian J. Endocrinol. Metab. 2012, 16, S140–S141. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [Green Version]
- van der Ree, M.H.; van der Meer, A.J.; van Nuenen, A.C.; de Bruijne, J.; Ottosen, S.; Janssen, H.L.; Kootstra, N.A.; Reesink, H.W. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment. Pharmacol. Ther. 2016, 43, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Campbell, F.; Han, K.; Theodore, D.; Deeg, M.; Huang, M.; Hamatake, R.; Lahiri, S.; Chen, S.; Horvath, G.; et al. Randomized clinical trials towards a single-visit cure for chronic hepatitis C: Oral GSK2878175 and injectable RG-101 in chronic hepatitis C patients and long-acting injectable GSK2878175 in healthy participants. J. Viral Hepat. 2020, 27, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, H.; Yheskel, M.; Patel, V. Modulation of polycystic kidney disease by non-coding RNAs. Cell. Signal. 2020, 71, 109548. [Google Scholar] [CrossRef]
- Lee, E.C.; Valencia, T.; Allerson, C.; Schairer, A.; Flaten, A.; Yheskel, M.; Kersjes, K.; Li, J.; Gatto, S.; Takhar, M.; et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat. Commun. 2019, 10, 4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, B.D.; Parsons, C.; Walker, L.; Zhang, W.C.; Slack, F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017, 127, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Leimena, C.; Qiu, H. Non-Coding RNA in the Pathogenesis, Progression and Treatment of Hypertension. Int. J. Mol. Sci. 2018, 19, 927. [Google Scholar] [CrossRef] [Green Version]
- Arabian, M.; Azad, F.M.; Maleki, M.; Malakootian, M. Insights into role of microRNAs in cardiac development, cardiac diseases, and developing novel therapies. Iran. J. Basic Med. Sci. 2020, 23, 961–969. [Google Scholar] [CrossRef] [PubMed]



| Bone Disease | miRNA | miRNA Regulation | Target | Biological Implication | Reference |
|---|---|---|---|---|---|
| Osteoporosis | miR-148a | Upregulated | ER-α | Inhibition of osteoblast cell growth and osteoblast apoptosis | [49] |
| miR-122-5p | Downregulated | ER-α | Development of osteoporosis | [50] | |
| miR-144-3p | Downregulated | RANK | Osteoclastogenesis alteration | [51] | |
| miR-133a | Upregulated | RUNX2 | Osteoclast differentiation and loss of bone density | [52] | |
| miR-363-3p | Upregulated | PTEN | Osteoclastogenesis promotion and inhibition of osteogenic differentiation | [41] | |
| miR-29a | Downregulated | RANKL | Osteoclastogenic differentiation | [55] | |
| miR-152 | Upregulated | RICTOR | Inhibition of osteoblast differentiation | [35] | |
| miR-579-3p | Upregulated | SIRT1 | Inhibition of osteogenic differentiation | [57] | |
| miR-200a-3p | Upregulated | GLS | Inhibition of osteogenic differentiation | [58] | |
| miR-140-5p | Downregulated | BMP2 | Enhancement of osteogenic differentiation | [59] | |
| miR-339 | Downregulated | DLX5 | Enhancement of osteogenic differentiation | [60] | |
| miR-140-3p | Upregulated | MCF2L | Inhibition of preosteoblast viability and induction of preosteoblast apoptosis | [61] | |
| miR-194-5p | Upregulated | WNT5A | Inhibition of bone formation and osteoblast/osteogenic differentiation | [62] | |
| miR-1286 | Upregulated | FZD4 | Inhibition of osteogenic differentiation | [63] | |
| miR-483-5p | Upregulated | IGF2 | Promotion of osteoclast differentiation | [34] | |
| miR-203a | Upregulated | DLX5 | Osteogenic differentiation delay and bone loss | [40] | |
| miR-10a-3p | Downregulated (by a kaempferol treatment) | CXCL12 | Promotion of osteogenic differentiation | [64] | |
| miR-300 | Downregulated | CUL4B | Degradation of PTEN (tumor suppressor) | [71] | |
| miR-93 | Upregulated | P21 | Proliferation of osteosarcoma cells | [72] | |
| miR-411 | Upregulated | MTSS1 | Osteosarcoma cell migration and proliferation | [73] | |
| miR-1284 | Downregulated | HMGB1 | Osteosarcoma cell migration and proliferation | [76] | |
| let-7a | Downregulated | E2F2 | Osteosarcoma development | [77] | |
| miR-1301 | Downregulated | BCL9 | Cell proliferation, invasion, and migration | [81] | |
| miR-487a | Upregulated | - | - | [82] | |
| miR-493-5p | Upregulated | - | - | ||
| miR-501-3p | Upregulated | - | - | ||
| miR-502-5p | Upregulated | - | - | ||
| hsa-miR-19-3p | Upregulated | Several transcription factors | Cell proliferation and carcinogenesis | [83] | |
| hsa-miR-106b-3p | Upregulated | ||||
| hsa-miR-543 | Downregulated | ZNRD1 | Osteosarcoma chemoresistance | [84] | |
| Osteonecrosis | hsa-miR-195-5p | Downregulated | 157 different genes | Osteoblast dissemination disruption, accelerated cell apoptosis, and collapse of the femoral head | [94] |
| hsa-miR-601 | Upregulated | 238 different genes | Adipogenic and osteogenic differentiation | [87] | |
| hsa-miR-452-3p | Upregulated | Adipogenic and osteogenic differentiation | |||
| hsa-miR-647 | Upregulated | Adipogenic and osteogenic differentiation | |||
| hsa-miR-516b-5p | Upregulated | Adipogenic and osteogenic differentiation | |||
| hsa-miR-127-5p | Upregulated | Adipogenic and osteogenic differentiation | |||
| hsa-miR-122-3p | Downregulated | Adipogenic and osteogenic differentiation | |||
| miR-181d | Upregulated | SMAD3 | Inhibition of osteogenic differentiation | [88] | |
| miR-217 | Downregulated | DKK1 | Inhibition of cell proliferation and osteogenic differentiation | [96] | |
| miR-214 | Upregulated | ATF4 and PTEN | Inhibition of osteoblast differentiation and promotion of osteoclast function | [97] | |
| miR-186-5p | Upregulated | CXCL13 | Alteration of cell viability and osteoblastic differentiation | [89] | |
| miR-410 | Downregulated | Wnt-11 | High levels of osteoclasts and low levels of osteoblasts. Low bone mineral density | [98] | |
| miR-93-5p | Upregulated | - | - | [86] | |
| miR-320a | Upregulated | - | - | ||
| hsa-miR-378-c | Upregulated | WNT3A, DACT1 and CSF1 | Bone remodeling and angiogenesis during ONFH | [99] | |
| hsa-let-7a-5p | Upregulated | RCAN2 and IL9R | Progression of ONFH | ||
| hsa-miR-3200-5p | Upregulated | RELN | Progression of ONFH | ||
| hsa-miR-28-5p | Upregulated | RELA | Cartilage degeneration | ||
| hsa-miR-532-5p | Upregulated | CLDN18 and CLDN10 | Bone loss | ||
| Bone Metastasis | miR-466 | Downregulated | RUNX2 | Inhibition of apoptosis and cell cycle arrest. Cell migration, proliferation, and invasion | [109] |
| miR-19a-3p | Downregulated | SMAD2 and SMAD4 | Cell migration, invasion, and bone metastasis | [110] | |
| miR-582-3p | Downregulated | SMAD2, SMAD4, and TGFBR1 | Cell migration, invasion, and metastasis | [111] | |
| miR-582-5p | Downregulated | SMAD2, TGFBR1 and TGFBR2 | |||
| miR-96 | Upregulated | E-Cadherin and EpCAM | Cancer cell metastasis within bone microenvironment and tumor development | [113] | |
| miR-214-3p | Upregulated | TRAF3 | Osteolytic bone metastasis and elevated bone resorption | [116] | |
| miR-124 | Downregulated | IL-11 | Bone metastasis of breast cancer cells | [117] | |
| miR-139-5p | Downregulated | NOTCH1 | Osteogenic differentiation and lytic bone disease in lung cancer | [118] | |
| miR-34a | Downregulated | C-IAP2 and Bcl-2 | Tumor invasion and metastasis | [120] | |
| hsa-miR-940 | Upregulated | ARHGAP1 and FAM134A | Osteogenic differentiation and induction of osteoblastic lesions in tumors | [121] | |
| Atrophic non-union | miR-31a-3p | Upregulated | FGF3 | Osteogenesis, chondrogenesis, and impairment of fracture healing | [131] |
| miR-31a-5p | Upregulated | SATB2, Osterix, RUNX2, BMPR2, and NIK | Osteogenic differentiation | ||
| miR-146a-5p | Upregulated | TRAF6, IRAK1, CXCR4, and SDF-1 | Development of non-union | ||
| miR-146b-5p | Upregulated | TRAF6 and IRAK1 | Development of non-union | ||
| miR-223-3p | Upregulated | STAT3 and IGF1R | Development of non-union | ||
| miR-628-3p | Upregulated | RUNX2 | Inhibition of osteoblast differentiation | [129] | |
| miR-381 | Upregulated | WNT5A and FZD3 | Inhibition of osteogenic differentiation | [132] | |
| miR-1323 | Upregulated | BMP4 and SMAD4 | Inhibition of osteogenic differentiation and development of atrophic non-union | [133] | |
| hsa-miR-149* | Upregulated | ALPL | Development of atrophic non-union | [134] | |
| hsa-miR-221 | Upregulated | PDGFA | Development of atrophic non-union | ||
| hsa-miR-654-5p | Upregulated | BMP2 | Development of atrophic non-union | ||
| Osteogenesis imperfecta | miR-29b | Downregulated | - | Altered regulation of collagen protein accumulation | [135] |
| miR-145 | Upregulated (by an ossotide treatment) | RUNX2 and OSX | Enhancement of osteoblast cell differentiation and proliferation | [137] | |
| Osteomyelitis | miR-24 | Downregulated | CHI3L1 | Inhibition of cell proliferation, blockage of both bone formation and mineralization, and osteoblast apoptosis | [143] |
| miR-129-5p | Upregulated | eNOS | Occurrence of mineralization defect and progression of osteomyelitis | [146] | |
| Multiple myeloma | miR-29b | Downregulated | Mcl-1 | Survival ofmyeloma cells | [150] |
| miR-143 | Downregulated | Versican | Myeloma-associated parameters | [152] | |
| miR-144 | Downregulated | ||||
| miR-199 | Downregulated | ||||
| miR-203 | Downregulated | ||||
| Thalassemia | miR-30a | Upregulated | BCL11A | Increased expression levels of HbF and a decreased expression levels of ferritin | [156] |
| miR-15a | Upregulated | MAF proteins and MYB | HbF induction | [154] | |
| miR-486-3p | Upregulated | MAFK, BCL11A, MTA1, and NR2F2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo Vázquez, L.A.; Moreno Becerril, M.Y.; Mora Hernández, E.O.; León Carmona, G.G.d.; Aguirre Padilla, M.E.; Chakraborty, S.; Bandyopadhyay, A.; Paul, S. The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential. Molecules 2022, 27, 211. https://doi.org/10.3390/molecules27010211
Bravo Vázquez LA, Moreno Becerril MY, Mora Hernández EO, León Carmona GGd, Aguirre Padilla ME, Chakraborty S, Bandyopadhyay A, Paul S. The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential. Molecules. 2022; 27(1):211. https://doi.org/10.3390/molecules27010211
Chicago/Turabian StyleBravo Vázquez, Luis Alberto, Mariana Yunuen Moreno Becerril, Erick Octavio Mora Hernández, Gabriela García de León Carmona, María Emilia Aguirre Padilla, Samik Chakraborty, Anindya Bandyopadhyay, and Sujay Paul. 2022. "The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential" Molecules 27, no. 1: 211. https://doi.org/10.3390/molecules27010211
APA StyleBravo Vázquez, L. A., Moreno Becerril, M. Y., Mora Hernández, E. O., León Carmona, G. G. d., Aguirre Padilla, M. E., Chakraborty, S., Bandyopadhyay, A., & Paul, S. (2022). The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential. Molecules, 27(1), 211. https://doi.org/10.3390/molecules27010211








