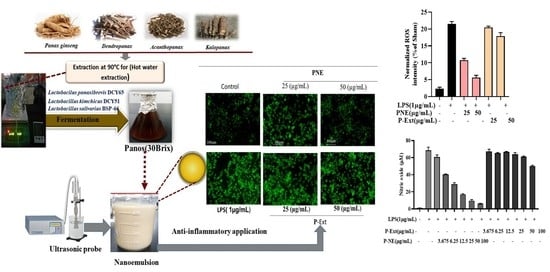
Panos-Fermented Extract-Mediated Nanoemulsion: Preparation, Characterization, and In Vitro Anti-Inflammatory Effects on RAW 264.7 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. P-NE Preparation
2.2. Long-Term Stability Test
2.3. FT-IR Analysis
2.4. FE-TEM Analysis
2.5. Cell Viability Analysis
2.6. Effect of P-NE on NO Production and ROS Generation
2.7. qPCR Analysis on Pro-Inflammatory Mediators
3. Materials and Methods
3.1. P-Ext Preparation
3.2. NE Preparation
3.3. Characterization of NE
3.4. Stability Determination
3.5. Measurement of Nitric Oxide (NO) Production in LPS Induced Raw 264.7 Cell Line
3.6. Quantitative PCR Analysis
3.7. Cell Viability Analysis
3.8. Reactive Oxygen Species (ROS) Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aceituno, V.C.; Ahn, S.; Simu, S.Y.; Wang, C.; Mathiyalagan, R.; Yang, D.C. Silver nanoparticles from Dendropanax morbifera Léveille inhibit cell migration, induce apoptosis, and increase generation of reactive oxygen species in A549 lung cancer cells. Vitr. Cell. Dev. Biol. Anim. 2016, 52, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, R.; Wang, C.; Kim, Y.J.; Castro-Aceituno, V.; Ahn, S.; Subramaniyam, S.; Simu, S.Y.; Jiménez-Pérez, Z.E.; Yang, D.C.; Jung, S.-K. Preparation of polyethylene glycol-ginsenoside Rh1 and Rh2 conjugates and their efficacy against lung cancer and inflammation. Molecules 2019, 24, 4367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.W.; Lee, S.Y.; Kim, S.G.; Heo, Y.R.; Son, M.K. Antimicrobial, Anti-oxidant and Cytotoxic Activities of Dendropanax morbifera Léveille extract for mouthwash and denture cleaning solution. J. Adv. Prosthodont. 2016, 8, 172–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.H.; Nguyen, C.T. Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology 2019, 27, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Seo, S.H.; Kang, E.Y.; Park, S.D.; Park, W.H.; Moon, H.I. Chemical composition and larvicidal effects of essential oil of Dendropanax morbifera against Aedes aegypti L. Biochem. Syst. Ecol. 2009, 37, 470–473. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Wang, C.; Mathiyalagan, R.; Aceituno, V.C.; Singh, P.; Ahn, S.; Wang, D.; Yang, D.C. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. Int. J. Nanomed. 2016, 11, 3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-H.; Kim, D.-W.; Park, S.-E.; Lee, H.-J.; Kim, K.-M.; Kim, K.-J.; Kim, M.-K.; Kim, S.-J.; Kim, S. Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J. Biosci. Bioeng. 2015, 120, 181–186. [Google Scholar] [CrossRef]
- Choo, G.S.; Lim, D.P.; Kim, S.M.; Yoo, E.S.; Kim, S.H.; Kim, C.H.; Woo, J.-S.; Kim, H.-J.; Jung, J.-Y. Anti-inflammatory effects of Dendropanax morbifera in lipopolysaccharide-stimulated RAW264. 7 macrophages and in an animal model of atopic dermatitis. Mol. Med. Rep. 2019, 19, 2087–2096. [Google Scholar]
- Kim, C.G.; Castro-Aceituno, V.; Abbai, R.; Lee, H.A.; Simu, S.Y.; Han, Y.; Hurh, J.; Kim, Y.; Yang, D.C. Caspase-3/MAPK pathways as main regulators of the apoptotic effect of the phyto-mediated synthesized silver nanoparticle from dried stem of Eleutherococcus senticosus in human cancer cells. Biomed. Pharmacother. 2018, 99, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Komaiko, J.; McClements, D.J. Optimization of isothermal low-energy nanoemulsion formation: Hydrocarbon oil, non-ionic surfactant, and water systems. J. Colloid Interface Sci. 2014, 425, 59–66. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Thakkar, H.P.; Khunt, A.; Dhande, R.D.; Patel, A.A. Formulation and evaluation of Itraconazole nanoemulsion for enhanced oral bioavailability. J. Microencapsul. 2015, 32, 559–569. [Google Scholar] [CrossRef]
- Singh, K.K.; Vingkar, S.K. Formulation, antimalarial activity and biodistribution of oral lipid nanoemulsion of primaquine. Int. J. Pharm. 2008, 347, 136–143. [Google Scholar] [CrossRef]
- Kumar, S. Role of nanoemulsion in pharmaceutical sciences-a review. AJRPSB 2014, 2, 1–15. [Google Scholar]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsko, N.; Zimmermann, A.M.; Vojnikovic, E.; Valenta, C. Enhancement of stability and skin permeation by sucrose stearate and cyclodextrins in progesrerone nanoemulsions. Int. J. Pharm. 2010, 60, 393. [Google Scholar]
- Yilmaz, E.; Borchert, H.-H. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema—An in vivo study. Int. J. Pharm. 2006, 307, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Juráňová, J.; Franková, J.; Ulrichová, J. The role of keratinocytes in inflammation. J. Appl. Biomed. 2017, 15, 169–179. [Google Scholar] [CrossRef]
- Taupitz, T.; Dressman, J.B.; Klein, S. New formulation approaches to improve solubility and drug release from fixed dose combinations: Case examples pioglitazone/glimepiride and ezetimibe/simvastatin. Eur. J. Pharm. Biopharm. 2013, 84, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Rupa, E.J.; Li, J.F.; Arif, M.H.; Yaxi, H.; Puja, A.M.; Chan, A.J.; Hoang, V.; Kaliraj, L.; Yang, D.C.; Kang, S.C. Cordyceps militaris Fungus Extracts-Mediated Nanoemulsion for Improvement Antioxidant, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2020, 25, 5733. [Google Scholar] [CrossRef]
- Sonneville-Aubrun, O.; Simonnet, J.-T.; L’alloret, F. Nanoemulsions: A new vehicle for skincare products. Adv. Colloid Interface Sci. 2004, 108, 145–149. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochemistry 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Larmo, P.; Alin, J.; Salminen, E.; Kallio, H.; Tahvonen, R. Effects of sea buckthorn berries on infections and inflammation: A double-blind, randomized, placebo-controlled trial. Eur. J. Clin. Nutr. 2008, 62, 1123–1130. [Google Scholar] [CrossRef]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Ullah, S. Sea buckthorn seed oil protects against the oxidative stress produced by thermally oxidized lipids. Food Chem. 2015, 186, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.S.X.; Basri, M.; Zakaria, M.R.S.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Rahman, M.B.A. A potential tocopherol acetate loaded palm oil esters-in-water nanoemulsions for nanocosmeceuticals. J. Nanobiotechnol. 2010, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, E.C.V.; Boock, K.P.; Maruno, M.; Rocha-Filho, P.A. Accelerated stability and moisturizing capacity of emulsions presenting lamellar gel phase obtained from brazilian natural raw material. J. Dispers. Sci. Technol. 2011, 32, 1135–1139. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Jiang, Y.; Shen, T.; You, L.; Sun, X.; Xu, X.; Hu, W.; Wu, H.; Wang, G. Anti-inflammatory effects of chloranthalactone B in LPS-stimulated RAW264. 7 cells. Int. J. Mol. Sci. 2016, 17, 1938. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.O.; Choi, E.; Shin, K.K.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Hossain, M.A.; Kim, H.S.; Yi, Y.-S.; Kim, D. Compound K, a ginsenoside metabolite, plays an anti-inflammatory role in macrophages by targeting the AKT1-mediated signaling pathway. J. Ginseng Res. 2019, 43, 154–160. [Google Scholar] [CrossRef]
- Kuo, F.; Subramanian, B.; Kotyla, T.; Wilson, T.A.; Yoganathan, S.; Nicolosi, R.J. Nanoemulsions of an anti-oxidant synergy formulation containing gamma tocopherol have enhanced bioavailability and anti-inflammatory properties. Int. J. Pharm. 2008, 363, 206–213. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Cervi, V.F.; Gehrcke, M.; Barbieri, A.V.; Zborowski, V.A.; Beck, R.C.R.; Nogueira, C.W.; Cruz, L. Pomegranate seed oil nanoemulsions improve the photostability and in vivo antinociceptive effect of a non-steroidal anti-inflammatory drug. Colloids Surf. B Biointerfaces 2016, 144, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Bist, G.; Pun, N.T.; Magar, T.B.T.; Shrestha, A.; Oh, H.J.; Khakurel, A.; Park, P.-H.; Lee, E.-S. Inhibition of LPS-stimulated ROS production by fluorinated and hydroxylated chalcones in RAW 264.7 macrophages with structure-activity relationship study. Bioorganic Med. Chem. Lett. 2017, 27, 1205–1209. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, M.O.; Lee, H.; Kim, Y.; Kim, E.; Kim, J.S. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem. 2013, 141, 1947–1955. [Google Scholar] [CrossRef]
- Hossen, M.J.; Hong, Y.D.; Baek, K.S.; Yoo, S.; Hong, Y.H.; Kim, J.H.; Lee, J.-O.; Kim, D.; Park, J.; Cho, J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Surfactant (Mixed) | Composition | HLB Value |
|---|---|---|
| Tween 80: Span 80 | 9:1 | 13:93 |
| Tween 80: Span 80 | 8:2 | 12:86 |
| Tween 80: Span 80 | 7:3 | 11:79 |
| Tween 80: Span 80 | 6:3 | 10:2 |
| Scheme 2. | P-Ext (% H2O) | Surfactant | Oil |
|---|---|---|---|
| S1 | 5 g (86% H2O) | 6% | 8% |
| S2 | 10 g (87% H2O) | 5% | 8% |
| S3 | 15 g (92% H2O) | 3% | 5% |
| S4 | 30 g (87% H2O) | 7% | 6% |
| No. of Parameter | 24 h | 90 Days (After) | ||
|---|---|---|---|---|
| Temperature (°C) | Room temp | 4 °C | 10 °C | 60 °C |
| Size (nm) | 83.0 ± 2.1 | 88.1 ± 3 | 117.5 ± 2 | 147.8 |
| PDI value | 0.078 ± 0.023 | 0.100 ± 0.010 | 0.102 ± 0.02 | 0.115 ± 0.02 |
| Zeta potential (mV) | −28.20 ± 2 | −27.14 ± 0.7 | −21.81 ± 0.5 | −14 ± 1.2 |
| pH value | 6.43 ± 0.22 | 6.34 ± 0.23 | 6.49 ± 0.07 | 5.23 ± 0.06 |
| Primer | Primer Sequence | |
|---|---|---|
| GAPDH | Forward | 5′-ACCACAGTCCATGCCATCAC-3 |
| Reverse | 5′-CCACCACCCTGTTGCTGTAG-3 | |
| IL-6 | Forward | 5′-AGCCCACGTCGTAGCAAACCACCAA-3′ |
| Reverse | 5′-AACACCCATTCCCTTCACAGAGCAAT-3′ | |
| IL-1β | Forward | 5′-TGCAGAGTTCCCCAACTGGTACATC-3′ |
| Reverse | 5′-GTGCTGCCTAATGTCCCCTTGAATC-3′ | |
| NF-κB | Forward | 5′-TATTTCAACCACAGATGGCACTGC-3 |
| Reverse | 5′-CAGATTTTGACCTGAGGGTAAGAC-3 | |
| iNOS | Forward | 5′-AATGGCAACATCAGGTCGGCCATCACT-3 |
| Reverse | 5′-GCTGTGTGTCACGAAGTCTCGAACTC-3 | |
| TNF-α | Forward | 5′-AGCCCACGTCGTAGCAAACCACCAA-3′ |
| Reverse | 5′-AACACCCATTCCCTTCACAGAGCAAT-3′ | |
| IKKα | Forward | 5′-GGCCTGTGATGTCCTGAAGAATT-3 |
| Reverse | 5′-TCGAATCCCAGACCCTATATCACT-3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Rupa, E.J.; Zheng, S.; Nahar, J.; Yang, D.C.; Kang, S.C.; Wang, Y. Panos-Fermented Extract-Mediated Nanoemulsion: Preparation, Characterization, and In Vitro Anti-Inflammatory Effects on RAW 264.7 Cells. Molecules 2022, 27, 218. https://doi.org/10.3390/molecules27010218
Zhang R, Rupa EJ, Zheng S, Nahar J, Yang DC, Kang SC, Wang Y. Panos-Fermented Extract-Mediated Nanoemulsion: Preparation, Characterization, and In Vitro Anti-Inflammatory Effects on RAW 264.7 Cells. Molecules. 2022; 27(1):218. https://doi.org/10.3390/molecules27010218
Chicago/Turabian StyleZhang, Rui, Esrat Jahan Rupa, Siwen Zheng, Jinnatun Nahar, Deok Chun Yang, Se Chan Kang, and Yingping Wang. 2022. "Panos-Fermented Extract-Mediated Nanoemulsion: Preparation, Characterization, and In Vitro Anti-Inflammatory Effects on RAW 264.7 Cells" Molecules 27, no. 1: 218. https://doi.org/10.3390/molecules27010218
APA StyleZhang, R., Rupa, E. J., Zheng, S., Nahar, J., Yang, D. C., Kang, S. C., & Wang, Y. (2022). Panos-Fermented Extract-Mediated Nanoemulsion: Preparation, Characterization, and In Vitro Anti-Inflammatory Effects on RAW 264.7 Cells. Molecules, 27(1), 218. https://doi.org/10.3390/molecules27010218