Insights into the Protective Effects of Thymoquinone against Toxicities Induced by Chemotherapeutic Agents
Abstract
:1. Introduction
2. Phytochemicals
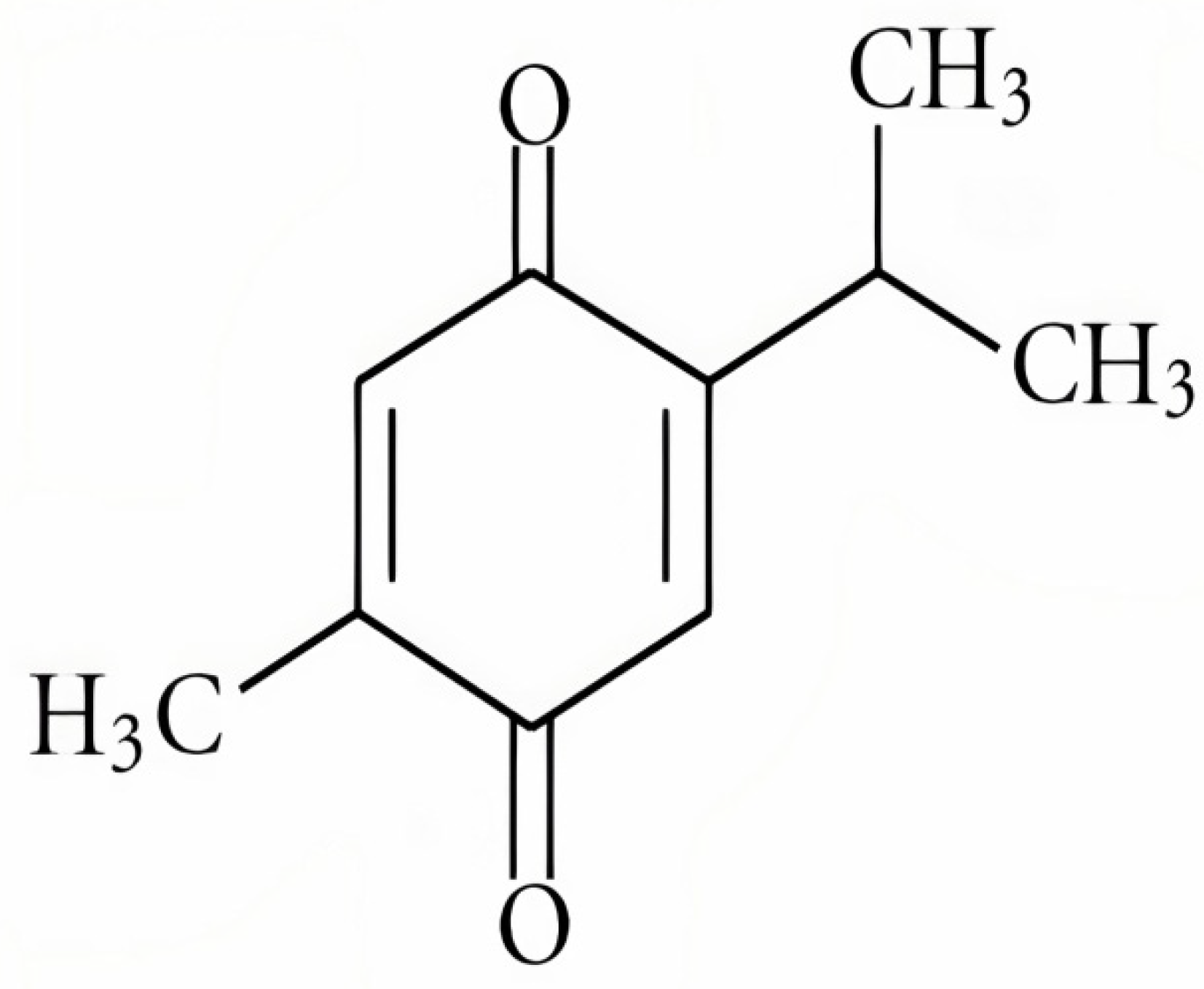
3. Thymoquinone (TQ)
3.1. Role of Thymoquinone in Cancer
3.2. Effect of TQ against ChemotherapyInduced Organ Toxicity
3.2.1. Protective Effect against Cardiotoxicity
3.2.2. Protective Effect against Hepatotoxicity
3.2.3. Protective Effect against Nephrotoxicity
3.2.4. Protective Effect against Intestinal Toxicity
3.2.5. Protective Effect against Urotoxicity
3.2.6. Protective Effect against Ototoxicity
3.2.7. Protective Effect against Testicular Injury
3.2.8. Protective Effect against Pulmonary Toxicity
4. Role of Thymoquinone against Chemotherapy Induced Oxidative Stress
5. New Trends and Directions of Research Related to TQ
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| ABR | Auditory brainstem response |
| ALT | Alanine aminotransferase |
| ANP | Atrial natriuretic peptide |
| AST | Aspartate aminotransferase |
| ATP | Adenosine triphosphate |
| BUN | Blood urea nitrogen |
| CAT | Catalase |
| CK-MB | Creatine kinase myocardial band |
| CPK | Creatine phosphokinase |
| DOX | Doxorubicin |
| DPOE | Distortion product otoacoustic emissions |
| FBPase | Fructose 1.6-bisphosphatase |
| G6Pase | Glucose 6-phosphatase |
| G6PDH | Glucose 6-phosphate dehydrogenase |
| GGTase | γ-Glutamyl transferase |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Reduced glutathione |
| GST | Glutathione S-transferease |
| HK | Hexokinase |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-18 | Interleukin-18 |
| iNOs | Inducible nitric oxide synthase |
| I.P | Intraperitoneally |
| LAP | Leucine aminopeptidase |
| LDH | Lactate dehydrogenase |
| MDA | Malondialdehyde |
| MDH | Malate dehydrogenase |
| ME | Malic enzyme |
| NAG | N-acetyl-b-D-glucosaminidase |
| NO(x) | Total nitrate/nitrite |
| NPSH | Non-protein sulfhydryl |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| P.O | Per os |
| ProBNP | Pro-B type natriuretic peptide |
| SH | Sulfhydryl |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TAS | Tissue anti-oxidant status |
| TC | Total cholesterol |
| TG | Triglycerides |
| TOS | Tissue oxidant status |
| TQ | Thymoquinone |
| TR | Thioredoxin reductase |
References
- Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer#definition (accessed on 13 April 2021).
- Available online: http://www.healthdata.org/results/gbd_summaries/2019/total-cancers-level-2-cause (accessed on 24 April 2021).
- Indian Council of Medical Research; Public Health Foundation of India; Institute for Health Metrics and Evaluation. GBD India Compare Data Visualization. New Delhi: ICMR, PHFI, and IHME. 2017. Available online: http://vizhub.healthdata.org/gbd-compare/india (accessed on 5 April 2021).
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S. Cancer-related symptoms. In Seminars in Radiation Oncology; WB Saunders: Philadelphia, PA, USA, 2000; Volume 10, pp. 175–190. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 10 May 2021).
- Katzke, V.A.; Kaaks, R.; Kühn, T. Lifestyle and cancer risk. Cancer J. 2015, 21, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Wagner, E.; Elliot, K.; Enders, W.; Chobanuk, J.; Tsui, A. BOOK REVIEW: Chemotherapy and Biotherapy Guidelines. Can. Oncol. Nurs. J. Rev. Cancer Soins Infirm. Oncol. 2015, 25, 480. [Google Scholar]
- Tarver, T. Cancer Facts & Figures 2012; American Cancer Society(ACS): Atlanta, GA, USA, 2012; p. 66. [Google Scholar]
- Brenner, G.M.; Stevens, C.W. Antineoplastic Drugs, Text Book of Pharmacology, 3rd ed.; Saunders Elsevier: Philadelphia, PA, USA, 2010; pp. 493–511. [Google Scholar]
- Rang, H.P.; Dale, M.M.; Ritter, J.M. Anticancer Drugs, Text Book of Pharmacology, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 673–687. [Google Scholar]
- Remesh, A. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Fennell, C.W.; Lindsey, K.L.; McGaw, L.J.; Sparg, S.G.; Stafford, G.I.; Elgorashi, E.E.; Grace, O.M.; VanStaden, J. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J. Ethnopharmacol. 2004, 94, 205–217. [Google Scholar] [CrossRef]
- Doughari, J.H.; Human, I.S.; Bennade, S.; Ndakidemi, P.A. Phytochemicals as chemotherapeutic agents and antioxidants: Possible solution to the control of antibiotic resistant verocytotoxin producing bacteria. J. Med. Plant Res. 2009, 3, 839–848. [Google Scholar]
- Doughari, J.H.; Obidah, J.S. Antibacterial potentials of stem bark extracts of Leptadenia lancifoli against some pathogenic bacteria. Pharmacologyonline 2008, 3, 172–180. [Google Scholar]
- Rao, V. (Ed.) Phytochemicals: A Global Perspective of Their Role in Nutrition and Health; BoD–Books on Demand; Intechopen: London, UK, 2012. [Google Scholar]
- Singh, S.; Jarial, R.; Kanwar, S.S. Therapeutic effect of herbal medicines on obesity: Herbal pancreatic lipase inhibitors. Wudpecker J. Med. Plants 2013, 2, 53–65. [Google Scholar]
- Rahman, M.; Beg, S.; Verma, A.; Al Abbasi, F.A.; Anwar, F.; Saini, S.; Akhter, S.; Kumar, V. Phytoconstituents as pharmacotherapeutics in rheumatoid arthritis: Challenges and scope of nano/submicromedicine in its effective delivery. J. Pharm. Pharmacol. 2017, 69, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.Y.Y.; Yuen, A.C.Y.; Chan, R.Y.K.; Chan, S.W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phyther Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Davinelli, S.; Sapere, N.; Zella, D.; Bracale, R.; Intrieri, M.; Scapagnini, G. Pleiotropic protective effects of phytochemicals in Alzheimer’s disease. Oxid Med Cell Longev. 2012, 2012, 386527. [Google Scholar] [CrossRef]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Ogle, W.O.; Speisman, R.B.; Ormerod, B.K. Potential of treating age-related depression and cognitive decline with nutraceutical approaches: A mini-review. Gerontology 2012, 59, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Edwards, D.; Hamernig, I.; Jian, L.; James, A.P.; Johnson, S.K.; Tapsell, L.C. Vegetables containing phytochemicals with potential anti-obesity properties: A review. Food Res. Int. 2013, 52, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.F.; Khan, G.; Safhi, M.M.; Alshahrani, S.; Siddiqui, R.; Sivagurunathan, M.S.; Anwer, T. Thymoquinone ameliorates doxorubicin-induced cardiotoxicity in swiss albino mice by modulating oxidative damage and cellular inflammation. Cardiol. Res. Pract. 2018, 2018, 1483041. [Google Scholar] [CrossRef] [Green Version]
- Aras, S.; Gerin, F.; Aydin, B.; Ustunsoy, S.; Sener, U.; Turan, B.C.; Armutcu, F. Effects of sodium arsenite on the some laboratory signs and therapeutic role of thymoquinone in the rats. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 658–663. [Google Scholar]
- Alrashedi, M.G. The Protective Role of Thymoquinone against Drugs Toxicity: A Review. J. Pharm. Res. Int. 2018, 24, 1–11. [Google Scholar] [CrossRef]
- Available online: https://commons.wikimedia.org/wiki/File:Nigella_sativa_002.JPG (accessed on 20 November 2021).
- Melissa, P. “black cumin”. Encyclopedia Britannica. 2018. Available online: https://www.britannica.com/plant/black-cumin (accessed on 18 November 2021).
- Taborsky, J.; Kunt, M.; Kloucek, P.; Lachman, J.; Zeleny, V.; Kokoska, L. Identification of potential sources of thymoquinone and related compounds in Asteraceae, Cupressaceae, Lamiaceae, and Ranunculaceae families. Cent. Eur. J. Chem. 2012, 10, 1899–1906. [Google Scholar] [CrossRef] [Green Version]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95, 138–158. [Google Scholar] [CrossRef]
- Abukhader, M.M. The effect of route of administration in thymoquinone toxicity in male and female rats. Indian J. Pharm. Sci. 2012, 74, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Taha, M.M.; Sheikh, B.Y.; Salim, L.Z.; Mohan, S.; Khan, A.; Kamalidehghan, B.; Ahmadipour, F.; Abdelwahab, S.I. Thymoquinone induces apoptosis and increase ROS in ovarian cancer cell line. Cell Mol. Biol. 2016, 62, 97–101. [Google Scholar]
- Wilson, A.J.; Saskowski, J.; Barham, W.; Yull, F.; Khabele, D. Thymoquinone enhances cisplatin-response through direct tu-mor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Yıldırım, I.H.; Azzawri, A.A.; Duran, T. Thymoquinone induces apoptosis via targeting the Bax/BAD and Bcl-2 pathway in breast cancer cells. Dicle Tıp Derg. 2019, 46, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Rajput, S.; Kumar, B.N.; Dey, K.K.; Pal, I.; Parekh, A.; Mandal, M. Molecular targeting of Akt by thymoquinone promotes G(1) arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013, 93, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv. Prev. Med. 2016, 2016, 341. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. Journal of cellular physiology. J. Cell Physiol. 2019, 234, 10421–10431. [Google Scholar] [CrossRef]
- Hussein, S.A.; Abdel-Aal, S.A.; Amin, A.; Khalaf, H.A. Caspase-3, Bcl-2, p53, CYP1A1 and COX-2 as a potential target in chemoprevention of Benzo (a) pyrene-induced lung carcinogenesis in mice: Role of thymoquinone. Nat. Sci. 2016, 4, 430–441. [Google Scholar]
- Badary, O.A.; Gamal El-Din, A.M. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcomatumorigenesis. Cancer Detect Prev. 2001, 25, 362–368. [Google Scholar]
- Arumugam, P.; Subramanian, R.; Priyadharsini, J.V.; Gopalswamy, J. Thymoquinone inhibits the migration of mouse neuroblastoma (Neuro-2a) cells by down-regulating MMP-2 and MMP-9. Chin. J. Nat. Med. 2016, 14, 904–912. [Google Scholar] [CrossRef]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.; Yang, S.; Ying, X.; Shen, W. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2013, 5, 634–648. [Google Scholar] [CrossRef] [Green Version]
- Abdelfadil, E.; Cheng, Y.H.; Bau, D.T.; Ting, W.J.; Chen, L.M.; Hsu, H.H.; Lin, Y.M.; Chen, R.J.; Tsai, F.J.; Tsai, C.H.; et al. Thymoquinone induces apoptosis in oral cancer cells through p38β inhibition. Am. J. Chin. Med. 2013, 41, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Choi, B.Y.; Jeong, C.H.; Kundu, J.K.; Chun, K.S. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Src mediated phosphorylation of EGF receptor tyrosine kinase. Oncol. Rep. 2014, 32, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Hsieh, D.J.Y.; Lin, Y.M.; Chen, L.M.; Kuo, W.W.; Huang, C.Y. Thymoquinone Induces Caspase-Independent, Autophagic Cell Death in CPT-11- Resistant LoVo Colon Cancer via Mitochondrial Dysfunction and Activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Apata, T.; Gordetsky, J.B.; Singh, R. Docetaxel Combined with Thymoquinone Induces Apoptosis in Prostate Cancer Cells via Inhibition of the PI3K/AKT Signaling Pathway. Cancers 2019, 11, 1390. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Dey, K.K.; Dey, G.; Pal, I.; Majumder, A.; Choudhury, S.M.; Kundu, S.C.; Mandal, M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgeninin squamous cell carcinoma. PLoS ONE 2012, 7, e46641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.M.; Wang, X.F.; Huang, Q.X. Thymoquinone induces cytotoxicity and reprogramming of EMT ingastric cancer cells by targeting PI3K/Akt/mTOR pathway. J. Biosci. 2017, 42, 547–554. [Google Scholar] [CrossRef]
- Musalli, M.G.; Hassan, M.A.; Sheikh, R.A.; Kalantan, A.A.; Halwani, M.A.; Zeyadi, M.; Hosawi, S.; Alhosin, M. Thymoquinone induces cell proliferation inhibition and apoptosis in acute myeloid leukemia cells: Role of apoptosis-related WT1 and BCL2 genes. Eur. J. Cell Sci. 2019, 1, 2–9. [Google Scholar] [CrossRef]
- Butt, A.S.; Nisar, N.; Ghani, N.; Altaf, I.; Mughal, T.A. Isolation of thymoquinone from Nigella sativa L. and Thymus vulgaris L., and its anti-proliferative effect on HeLa cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Meral, I.; Pala, M.; Akbas, F.; Ustunova, S.; Yildiz, C.; Demirel, M. Effects of thymoquinone on liver miRNAs and oxidative stress in Ehrlich acid mouse solid tumor model. Biotech. Histochem. 2018, 93, 301–308. [Google Scholar] [CrossRef]
- Bashir, A.O.; El-Mesery, M.E.; Anwar, R.; Eissa, L.A. Thymoquinone potentiates miR-16 and miR-375 expressions in hepato-cellular carcinoma. Life Sci. 2020, 254, 117794. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, D.-H.; Ha, E.; Choi, S.M.; Choi, J.-S.; Chun, K.S.; Joo, S.H. Thymoquinone induces apoptosis of human epi-dermoid carcinoma A431 cells through ROS-mediated suppression of STAT3. Chem. Biol. Interact. 2019, 312, 108799. [Google Scholar] [CrossRef] [PubMed]
- Nagi, M.N.; Mansour, M.A. Protective effect of thymoquinone against doxorubicin–induced cardiotoxicity in rats: A possible mechanism of protection. Pharmacol. Res. 2000, 41, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagi, M.N.; Al-Shabanah, O.A.; Hafez, M.M.; SayedAhmed, M.M. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2011, 25, 135–142. [Google Scholar] [CrossRef]
- Adalı, F.; Gonul, Y.; Kocak, A.; Yuksel, Y.; Ozkececi, G.; Ozdemir, C.; Tunay, K.; Bozkurt, M.F.; Sen, O.G. Effects of thymoquinone against cisplatin-induced cardiac injury in rats. Acta Cir. Bras. 2016, 4, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Suddek, G.M. Protective role of thymoquinone against liver damage induced by tamoxifen in female rats. Can. J. Physiol. Pharmacol. 2014, 92, 640–644. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; Sayed, A.A. Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC Complement. Altern. Med. 2014, 14, 282. [Google Scholar] [CrossRef] [Green Version]
- Alenzi, F.Q.; El-Bolkiny, Y.E.; Salem, M.L. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br. J. Biomed. Sci. 2010, 67, 20–28. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Morsy, M.A.; Abdalla, A.M.; Hamouda, A.H.; Alhaider, I.A. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediat. Inflamm. 2015, 2015, 859383. [Google Scholar] [CrossRef] [Green Version]
- Badary, O.A.; Abdel-Naim, A.B.; Abdel-Wahab, M.H.; Hamada, F.M. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology 2000, 143, 219–226. [Google Scholar] [CrossRef]
- Badary, O.A.; Nagi, M.N.; Al-Shabanah, O.A.; Al-Sawaf, H.A.; Al-Sohaibani, M.O.; Al-Bekairi, A.M. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can. J. Physiol. Pharmacol. 1997, 75, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; El-Sherbiny, M. Thymoquinone attenuates Doxorubicin-inuced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chem. Biol. Interact. 2014, 223, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J. Ethnopharmacol. 1999, 67, 135–142. [Google Scholar] [CrossRef]
- El-Sheik, A.A.; Morsy, M.A.; Hamouda, A.H. Protective mechanisms of thymoquinone on methotrexate-induced intestinal toxicity in rats. Pharmacogn. Mag. 2016, 12, 76–81. [Google Scholar]
- Shahid, F.; Farooqui, Z.; Khan, A.A.; Khan, F. Oral Nigella sativa oil and thymoquinone administration ameliorates the effect of long-term cisplatin treatment on the enzymes of carbohydrate metabolism, brush border membrane and antioxidant defense in rat intestine. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Gore, P.R.; Prajapati, C.P.; Mahajan, U.B.; Goyal, S.N.; Belemkar, S.; Ojha, S.; Patil, C.R. Protective effect of thymoquinone against cyclophosphamide-induced hemorrhagic cystitis through inhibiting DNA damage and upregulation of Nrf2 expression. Int. J. Biol. Sci. 2016, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Sagit, M.; Korkmaz, F.; Akcadag, A.; Somdas, M.A. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, A.; Oktar, S.; Koc, A.; Yonden, Z. Protective effects of thymoquinone against methotrexate-induced testicular injury. Hum. Exp. Toxicol. 2011, 30, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Suddek, G.M.; Ashry, N.A.; Gameil, N.M. Thymoquinone attenuates cyclophosphamide-induced pulmonary injury in rats. Inflammopharmacology 2013, 21, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, D.Y.; Durdağı, G. Effects of Thymoquinone on Blood Parameters in Doxorubicin Cardiotoxicity. Exp. Appl. Med. Sci. 2020, 1, 7–16. [Google Scholar]
- Karabulut, D.; Ozturk, E.; Kaymak, E.; Akin, A.T.; Yakan, B. Thymoquinone attenuates doxorubicin-cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22618. [Google Scholar] [CrossRef]
- Dunn, B.K.; Wickerham, D.L.; Ford, L.G. Prevention of hormone-related cancers: Breast cancer. J. Clin. Oncol. 2005, 23, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, S.; Clements, J.D.; Sullivan, J.M.; Oleskevich, S. Automated threshold detection for auditory brainstem responses: Comparison with visual estimation in a stem cell transplantation study. BMC Neurosci. 2009, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Kirby, B.J.; Kopun, J.G.; Tan, H.; Neely, S.T.; Gorga, M.P. Do “optimal” conditions improve distortion product otoacoustic emission test performance? Ear Hear. 2011, 32, 230. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Kasapovi, J.; Stojiljkovi, V.; Todorovi, A.; Rado, L.; Sai, Z.S.; Pajovi, B. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5- fluorouracil, doxorubicin and cyclophosphamide. Clin Biochem. 2010, 43, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Prabasheela, B.; Singh, A.K.; Fathima, A.; Pragulbh, K.; Deka, N.J.; Kumar, R. RRST-Health Science Association between Antioxidant Enzymes and Breast Cancer. Recent Res. Sci. Technol. 2014, 3, 93–95. [Google Scholar]
- Available online: https://clinicaltrials.gov/ct2/results?term=thymoquinone (accessed on 20 November 2021).
| Sl. No. | Types of Cancer | Total Deaths in Percentage |
|---|---|---|
| 1 | Lung cancer | 0.95% |
| 2 | Breast cancer | 0.89% |
| 3 | Stomach cancer | 0.87% |
| 4 | Colorectal cancer | 0.84% |
| 5 | Lip and oral cavity cancer | 0.70% |
| 6 | Other malignant neoplasm | 0.61% |
| 7 | Cervical cancer | 0.48% |
| 8 | Esophageal cancer | 0.41% |
| 9 | Leukemia | 0.36% |
| 10 | Pancreatic cancer | 0.36% |
| 11 | Prostate cancer | 0.34% |
| 12 | Liver cancer | 0.33% |
| 13 | Larynx cancer | 0.32% |
| 14 | Ovarian cancer | 0.24% |
| 15 | Bladder cancer | 0.14% |
| Drug Induced Toxicity | Experimental Model | TQ Dose | Effect of TQ |
|---|---|---|---|
| Doxorubicin induced cardiotoxicity [23,53] | Swiss albino mice/adult male albino rats. | 10 mg/kg p.o and 20 mg/kg, body weight p.o in swiss albino mice. 10 mg/kg/day i.p in albino rats. | ↓ blood serum markers (AST, ALT, LDH, CK-MB, and CPK). ↓ lipid peroxidation levels (MDA). ↑ GSH. ↑ antioxidant enzymes (CAT, SOD, GPx, GR, and GST). ↓ inflammatory Cytokine (IL2). |
| Cyclophosphamide induced cardiotoxicity [54] | Adult male albino wistar rats | 50 mg/L in drinking water (calculated dose of TQ- 4 mg/kg/day) | ↓ CK-MB, LDH, Serum cholesterol, TG, urea, creatinine. ↑ ATP production. ↓ TBARS and NO(x). ↑ GSH, SOD, GPx, and CAT. ↓ proinflammatory mediator (TNF-α). |
| Cisplatin induced cardiotoxicity [55] | Adult male albino wistar rats | 40 mg/kg/day i.p | Restored myocardial damage as observed by histopathological changes. ↑ expression of Bcl-2(anti-apoptotic protein) in myocardial fibers, indicating decreased apoptotic cardiomyocytes. |
| Tamoxifen induced hepatotoxicity [56] | Adult female Sprague-Dawley rats | 50 mg/kg, body weight p.o | ↓ serum enzymes of liver such as AST, ALT, γGT, LDH and ALP and total bilirubin. ↓ liver Lipid peroxidation level and TNF-α. ↑ GSH, SOD. Improved histopathological changes (edema of interstitial tissues and inflammation with decreased size of von kuppfer cells). |
| Cisplatin induced hepatotoxicity [57] | Male Albino wistar rats | 500 mg/kg/day p.o | ↓ serum hepatic biomarkers.(ALT, ALP, AST, γGGT, TB, LDH and ↑ serum albumin levels). ↑ GSH-px, SOD, GST, GSH, CAT activities. ↓ MDA formation. ↓ iNOs, TNFα and IL-1β, NF-Κb-P65 activation. |
| Cyclophosphamide induced toxicity [58] | Male Albino wistar rats | 10 mg/kg, intragastric injection | ↓ AST, ALT, ALP, γ-GT and CPK levels. ↓ elevated levels of urea, creatinine and bilirubin. ↓ TG, cholesterol, and LDL levels. ↑ GSH and decreased MDA levels. |
| Methotrexate induced hepato-renal toxicity [59] | Adult male albino wistar rats | 10 mg/kg/day p.o | Improved renal and hepatic biomarkers (↓ elevated levels of BUN, creatinine, ALT and AST). ↑ GSH, CAT. ↓ renal and hepatic MDA, NO, and TNF-α levels. ↓ expression of iNOs in both kidney and liver. Improved renal and hepatic histology ↓ NF-κB, COX-2 and caspase 3 expressions in kidney and liver. |
| Doxorubicin induced hyperlipidemic nephropathy [60] | Male albino wistar rats | 10 mg/kg/day p.o | ↓ serum urea. ↑ serum proteins and albumin. ↓ Urinary protein, albumin and NAG excretions. ↓ TG and TC in blood and renal tissue. ↓ Renal TBARS levels ↑ renal NPSH content and CAT activity. |
| Cisplatin induced nephrotoxicity [61] | Swiss albino mice, Wistar albino rats | 8 mg/kg/day for mice, 4 mg/kg/day for rats p.o | ↓ serum urea, serum creatinine, and urine volume in both mice and rats. ↑ creatinine clearance. Improved histopathological changes in rats (less degenerative damage and decreased loss of the tubular epithelium). |
| Doxorubicin induced nephrotoxicity [62] | Male Sprague–Dawley rats | 50 mg/kg/day p.o | ↓ creatinine, BUN and albuminuria. ↓ lipid peroxidation in renal cells. ↑ SOD and GST. Restored Nrf2 mRNA and Nrf2 binding activity in kidney. Attenuated renal NOX-4 levels. ↓ IL6 and TNF-α and ↑ IL-10. Improved renal histopathology (almost normal renal tubules and glomeruli). |
| Ifosfamide induced nephrotoxicity [63] | Male wistar albino rats | 50 mg/L p.o | ↓ urea and creatinine levels in the blood. ↑ serum phosphate, albumin content and creatinine clearance. ↓ fractional and total excretion of sodium, potassium, phosphate, glucose and organic acids. ↑ GSH, GST. ↓ Lipid peroxides. |
| Methotrexate induced intestinal toxicity [64] | Adult male rats | 10 mg/kg/day, gastric gavage | Improved intestinal histology (mild shortening of villi present). ↑ intestinal GSH, CAT and ↓ MDA levels. ↓ rise in total nitrite/nitrate levels and iNOS intestinal expression. ↓ TNF-α and ↓ expression of NF-κB and COX-2 in rat intestine. Reversed the up regulation of caspase 3. |
| Cisplatin induced intestinal toxicity [65] | Adult male Wistar rats | 1.5 mg/kg body weight, p.o | ↓ MDA levels and ↑ GSH; total SH levels. ↑ activities of SOD, GSH-Px, CAT, GST, GR and TR and in intestinal mucosa. ↑ ALP, GGTase, LAP, sucrose and decreased ACPase activity. Significantly altered glucose metabolism enzymes in the mucosal homogenate (↓ LDH, HK, ME and increased MDH, G6Pase, FBPase, G6PDH activity) Preserved intestinal histopathology (protected against the damage caused by cisplatin on morphology of intestine) |
| Cyclophosphamide induced hemorrhagic Cystitis [66] | Male Balb/c mice | 5, 10 and 20 mg/kg, i.p | TQ (20 mg/kg) showed complete protection of bladder tissues against inflammatory changes when compared with its low and medium dose. Reversed the Nrf2 suppression and most prominent Nrf2 protein expression was seen in the group receiving 20 mg/kg of TQ. ↓ TNF-α, IL-1β and IL-6 levels in a dose related manner. ↓ MDA level and significantly ↑ GSH, SOD, CAT levels in bladder tissue homogenates. |
| Cisplatin induced ototoxicity [67] | Female Sprague-Dawley rats | 40 mg/kg/day i.p | TQ treatment preserved DPOAE responses and ABR thresholds. |
| Methotrexate induced testicular injury [68] | Male C57BL/6 mice | 10 mg/kg/day i.p | ↓ TAC values and myeloperoxidase activity. Upon microscopic examination of testes, treatment with TQ revealed almost normal seminiferous tubule morphology |
| Cyclophosphamide induced pulmonary toxicity [69] | Male Sprague-Dawley rats | 100 mg/kg/day, p.o. | ↓ serum total protein, TNF-a, TBARS level and SOD activity. Improved histopathological changes (no intralobular necrosis or substantial inflammatory infiltration, indicating minimal lung damage.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, J.; Sultana, R.; Taj, T.; Asdaq, S.M.B.; Alsalman, A.J.; Mohaini, M.A.; Al Hawaj, M.A.; Kamal, M.; Alghamdi, S.; Imran, M.; et al. Insights into the Protective Effects of Thymoquinone against Toxicities Induced by Chemotherapeutic Agents. Molecules 2022, 27, 226. https://doi.org/10.3390/molecules27010226
Farooq J, Sultana R, Taj T, Asdaq SMB, Alsalman AJ, Mohaini MA, Al Hawaj MA, Kamal M, Alghamdi S, Imran M, et al. Insights into the Protective Effects of Thymoquinone against Toxicities Induced by Chemotherapeutic Agents. Molecules. 2022; 27(1):226. https://doi.org/10.3390/molecules27010226
Chicago/Turabian StyleFarooq, Juveriya, Rokeya Sultana, Tahreen Taj, Syed Mohammed Basheeruddin Asdaq, Abdulkhaliq J. Alsalman, Mohammed Al Mohaini, Maitham A. Al Hawaj, Mehnaz Kamal, Saad Alghamdi, Mohd. Imran, and et al. 2022. "Insights into the Protective Effects of Thymoquinone against Toxicities Induced by Chemotherapeutic Agents" Molecules 27, no. 1: 226. https://doi.org/10.3390/molecules27010226












